Neoadjuvant chemoradiotherapy for locally advanced gastric cancer with bulky lymph node metastasis:Five case reports
2020-04-08EijiNomuraHajimeKayanoTakashiMachidaHidekiIzumiSoichiroYamamotoAkitomoSugawaraMasayaMukaiTerumitsuHasebe
Eiji Nomura,Hajime Kayano,Takashi Machida,Hideki Izumi,Soichiro Yamamoto,Akitomo Sugawara,Masaya Mukai,Terumitsu Hasebe
Eiji Nomura,Hajime Kayano,Takashi Machida,Hideki Izumi,Soichiro Yamamoto,Masaya Mukai,Department of Gastroenterological and General Surgery,Tokai University Hachioji Hospital,Hachioji 192-0032,Tokyo,Japan
Akitomo Sugawara,Terumitsu Hasebe,Department of Radiology,Tokai University Hachioji Hospital,Hachioji 192-0032,Tokyo,Japan
Abstract BACKGROUND Neoadjuvant chemoradiotherapy(NACRT)has not been accepted as a general therapy for gastric cancer because of its localized effect and toxicity for radiosensitive organs.However,if radiation therapy could compensate for the limited or inadequate treatment choices available for elderly patients and/or those at high risk,the available therapeutic options for advanced gastric cancer might increase.From this perspective,we present our experiences of five patients with advanced gastric cancer in whom we used NACRT therapy with interesting results.CASE SUMMARY We admitted five patients with clinical Stage III gastric cancer and bulky lymph node metastasis or adjacent organ invasion at the time of diagnosis.A total of 50 Gy of preoperative intensity modulated radiation therapy was delivered to the patients in doses of 2.0 Gy/d,together with a regimen of concomitant chemotherapy comprising two courses of oral tegafur/gimeracil/oteracil(S-1;65 mg/m2 per day)for three consecutive weeks followed by two weeks of rest,starting at the same time as radiotherapy.All patients underwent no residual tumor resection and a pathological complete response of the primary tumors was achieved in two patients.The incidence of hematological toxicity was low,although the digestive toxicities of anorexia and diarrhea developed in three of the five patients,necessitating termination of radiation therapy at 30 Gy and S-1 at three weeks.However,even 30 Gy of irradiation and half the dose of S-1 resulted in sufficient downstaging,indicating that even a reduced amount of NACRT could confer considerable effects.CONCLUSION Slightly reduced NACRT might be useful and safe for patients with locally advanced gastric cancer.
Key Words:Advanced gastric cancer;Neoadjuvant chemoradiotherapy;Intensity modulated radiation therapy;Tegafur/gimeracil/oteracil;Curative resection;Case report;Gastrectomy
INTRODUCTION
Gastric cancer is not very sensitive to radiotherapy;therefore,this therapeutic modality is only rarely applied to gastric cancer with curative intent,but frequently administered in Japan to control pain caused by bone metastasis or cancer invasion[1].On the other hand,according to the 2010 Japanese gastric cancer treatment guidelines[2],neoadjuvant chemoradiotherapy(NACRT)has been evaluated in clinical trials for cardiac and lower esophageal carcinomas in Europe,and for gastric carcinomas in the United States.While the rates of a complete histological response were 20%-30% in phase II trials in the United States[3,4],and the rates of no residual tumor(R0)resection reported in Japan by Inoueet al[5]were 91.7%,the survival benefits of NACRT have not been evaluated in a randomized trial.Furthermore,NACRT is considered to achieve better local control than neoadjuvant chemotherapy(NAC)for gastric cancers[6].However,NACRT has not been accepted as a general therapy for gastric cancer because of its localized effect and toxicity for radiosensitive organs.Although the incidence of gastric cancer in general has tended to decrease in Japan due to a decrease in the prevalence ofHelicobacter pyloriinfection,the number of elderly patients with gastric cancer has increased[7],with a concomitant associated increase in preoperative complications[8].Patients at very high risk are difficult to treat with powerful preoperative chemotherapies,and extensive lymph node dissection might not always be an option.However,if radiation therapy could compensate for limited or inadequate treatment,the availability of therapeutic options for advanced gastric cancer might increase.From this perspective,we present our experiences of five patients with advanced gastric cancer in whom we used NACRT therapy with interesting results.
CASE PRESENTATION
Patients and therapeutic strategy
We encountered five patients with clinical Stage III locally advanced gastric cancer and bulky lymph node metastasis or adjacent tissue invasion at the time of diagnosis,between October 2014 and September 2018.The Human Ethics Review Committee at Tokai University School of Medicine approved our therapeutic strategy(No.13R-237).We obtained written informed consent from all five patients to undergo NACRT and for the publication of their clinical details in accordance with the Declaration of Helsinki(2013 amendment).
Tumors were classified according to the Japanese Classification of Gastric Carcinoma,3thEnglish edition,proposed by the Japanese Gastric Cancer Association[9].The clinical diagnosis in all cases was based on esophagogastroscopy,upper gastrointestinal series and images of 1-mm slices acquired using contrast-enhanced multidetector computed tomography(MDCT).The clinical features common to all of the patients were:Histologically proven adenocarcinoma of the stomach,cT3-cT4b,M0,bulky lymph node metastasis(bulky nodal involvement surrounding the celiac artery and its branches of ≥ 3 cm,or at least two adjacent tumors ≥ 1.5 cm each[10]and adequate organ function.Linitis plastica was not seen in any case.
A total of 50 Gy of preoperative intensity modulated radiation therapy(IMRT)was delivered in doses of 2.0 Gy/d.Concomitant chemotherapy starting at the same time as radiotherapy comprised two courses of oral tegafur/gimeracil/oteracil(S-1;65 mg/m2per day)for three consecutive weeks,followed by two weeks of rest.After two weeks of rest,oral S-1 for three consecutive weeks and residual IMRT were administered until a total of 50 Gy was achieved(Figure 1).Adverse events resulting from chemoradiotherapy(CRT)and surgery were evaluated using the National Cancer Institute Common Toxicity Criteria(version 5.0)[11].Although the target amount of IMRT was 50 Gy,radiotherapy was terminated if symptoms suggestive of side effects appeared and/or laboratory data indicated greater than grade 3 side effects.
Gastrectomy and D2 + dissection[2]proceededvialaparotomy within six to eight weeks after completion of CRT.To prepare for possible postoperative complications,such as anastomotic leakage,pancreatic fistula and malnutrition,each patient received an indwelling enteral tube,and enteral nutrition was started from postoperative day(POD)1[12].Simultaneous oral intake was started from POD 5.Postoperative adjuvant chemotherapy was not administered if the patient opted against it.
Chief complaints and history of present/past illness
Case 1:Anemia was noticed in an 80-year-old male with Alzheimer’s disease,diabetes mellitus,and hypertension.The patient’s past medical history was unremarkable.
Case 2:A 77-year-old male with chronic obstructive pulmonary disease and dementia presented with epigastralgia.The patient’s past medical history was unremarkable.
Case 3:Anemia was identified in a 71-year-old female with a history of total hysterectomy for uterine cancer 26 years previously.
Case 4:A 67-year-old male presented with epigastralgia.The patient’s past medical history was unremarkable.
Case 5:A 73-year-old female with diabetes mellitus and hypertension presented with epigastralgia.The patient’s past medical history was unremarkable.
Personal and family history
In all five cases,their family history was unremarkable.
Physical examination upon admission
Cases 1 and 3 had no special physical signs and did not have any abdominal symptoms.Cases 2,4,and 5 had mild epigastralgia,but did not have any other upper abdominal symptoms,including tenderness.The Eastern Cooperative Oncology Group-Performance Status of cases 1 and 2 was 1,and those of the others was 0.
Laboratory examinations
Serum carcinoembryonic antigen(CEA)levels were 140.4 ng/mL in case 1,37.2 ng/mL in case 2,11.6 ng/mL in case 3,and 29.9 ng/mL in case 5.In case 4,CEA levels were less than the normal range(2.6 ng/mL).
Imaging examinations
Case 1:Gastroscopic examination indicated a diagnosis of two advanced gastric cancers(types 2 and 3 in the lower and upper thirds of the stomach,respectively).MDCT showed enlargement of lymph node No.6 to 3.02 cm in diameter.Furthermore,colonofiberscopic examination identified early transverse colon cancer and MDCT imaging indicated cholecystolithiasis.
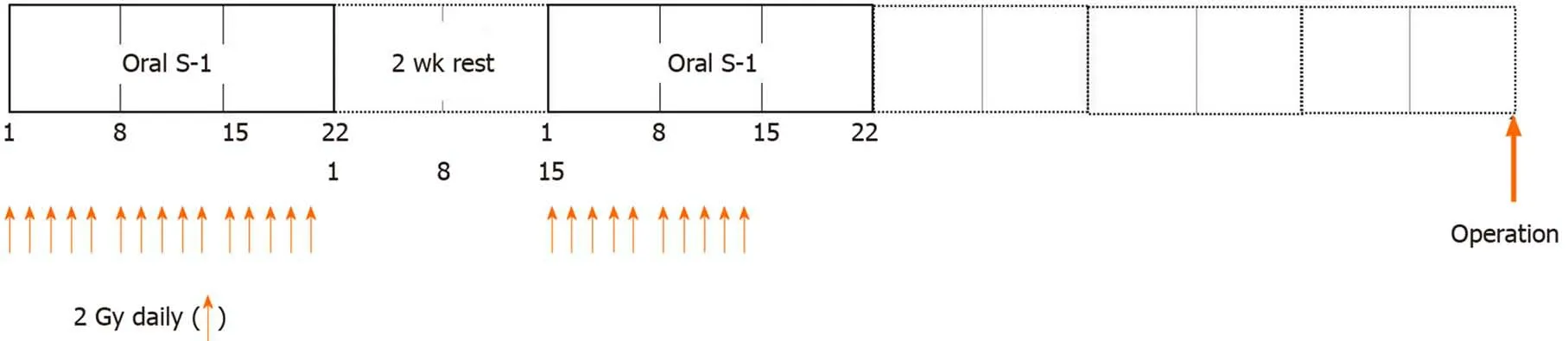
Figure 1 Treatment regimen.A total of 50 Gy of intensity modulated radiation therapy was delivered in doses of 2.0 Gy/d.Concomitant chemotherapy comprised two courses of oral tegafur/gimeracil/oteracil(65 mg/m2)for three consecutive weeks followed by two weeks of rest.S-1:Tegafur/gimeracil/oteracil.
Case 2:Gastroscopic examination revealed type 3 gastric cancer in the lower third of the stomach.MDCT showed enlargement of two adjacent lymph nodes,No.3 to 1.84 cm and 1.73 cm in diameter,respectively.
Case 3:Gastroscopic examination revealed type 3 gastric cancer in the upper third of the stomach.MDCT indicated enlargement of the No.3-11p lymph nodes to 5.26 cm in diameter,with invasion of the pancreatic body.
Case 4:Gastroscopic examination indicated type 3 gastric cancer in the lower third of the stomach.MDCT showed enlargement of the No.8a lymph node to 3.06 cm in diameter.
Case 5:Gastroscopic examination indicated type 2 gastric cancer in the middle to lower third of the stomach.MDCT showed enlargement of two adjacent lymph nodes,Nos.9-11p to 1.82 cm and 1.52 cm in diameter,respectively.
FINAL DIAGNOSIS
Pathological evaluation of the tumors indicated well to moderately differentiated adenocarcinoma of the stomach in all five patients.Table 1 shows the clinical characteristics of all five patients.
TREATMENT
Table 1 shows details of the preoperative treatment in all five patients.Table 2 shows the operative findings of the five patients.
Case 1:The patient underwent 45 Gy irradiation(recommended by a radiotherapist considering the age of the patient and risk factors)and chemotherapy with S-1 at a dose of 100 mg/d for six weeks.Subjective symptoms and hematological toxicity >grade 3 did not occur.Findings of MDCT imaging indicated low signal intensity inside lymph node No.6,although this node was slightly enlarged(3.86 cm),indicating progressive disease(PD;Figure 2).Levels of CEA increased once after CRT(211.8 ng/mL),subsequently decreasing to < 10% of pretherapy values(140.4 ng/mL to 9.8 ng/mL).Six weeks after completing CRT,the patient underwent total gastrectomy with D2 lymph node dissection,partial resection of the transverse colon,and cholecystectomy.
Case 2:The patient received a total of 50 Gy irradiation and 100-80 mg/d of S-1 for six weeks(i.e.,an initial dose of 100 mg/d for 3 wk,followed by 80 mg/d for 3 wk after 2 wk of rest).Thereafter,the size of the No.3 metastatic two adjacent lymph nodes decreased(0.77 cm and 0.72 cm,respectively),indicating a partial response(PR).His CEA value decreased to < 20% of pretherapy levels(37.2 ng/mL to 4.9 ng/mL).Eight weeks after completing CRT,he underwent subtotal distal gastrectomy with D2 lymph node dissection.
Case 3:The primary tumor initially showed bleeding,but settled after starting CRT.The findings of MDCT showed areas of low intensity in the No.3-11p lymph nodes after 30 Gy of irradiation and 80 mg/d of S-1 for three weeks.However,we switchedher regimen to capecitabine(Xeloda),cisplatin and trastuzumab(Herceptin)(XPHER)[13],because of the presence of fatty liver that resembled liver metastasis and an increase in CEA values after CRT(74.9 ng/mL).Thereafter,her CEA decreased to <20% of the pretherapy level(11.6 ng/mL to 2.2 ng/mL).Four weeks after completing two courses of XP-HER,total gastrectomy with resection of the pancreas(body to tail)and spleen was performed.
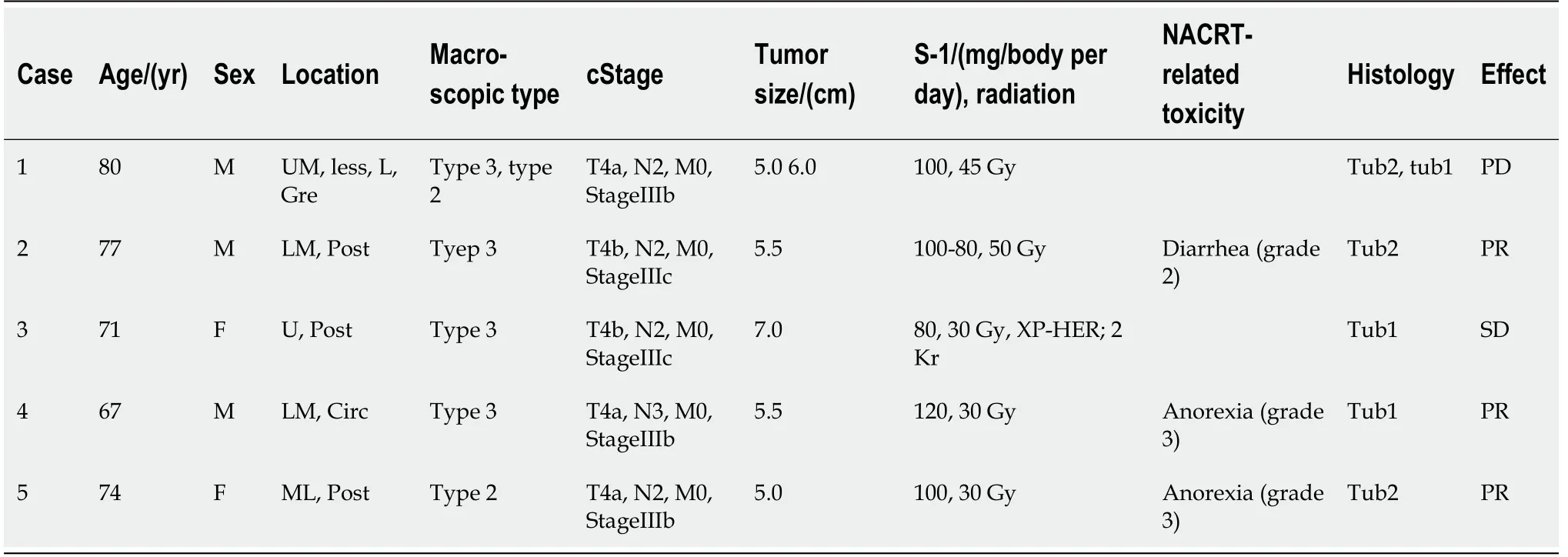
Table 1 Preoperative clinicopathological features
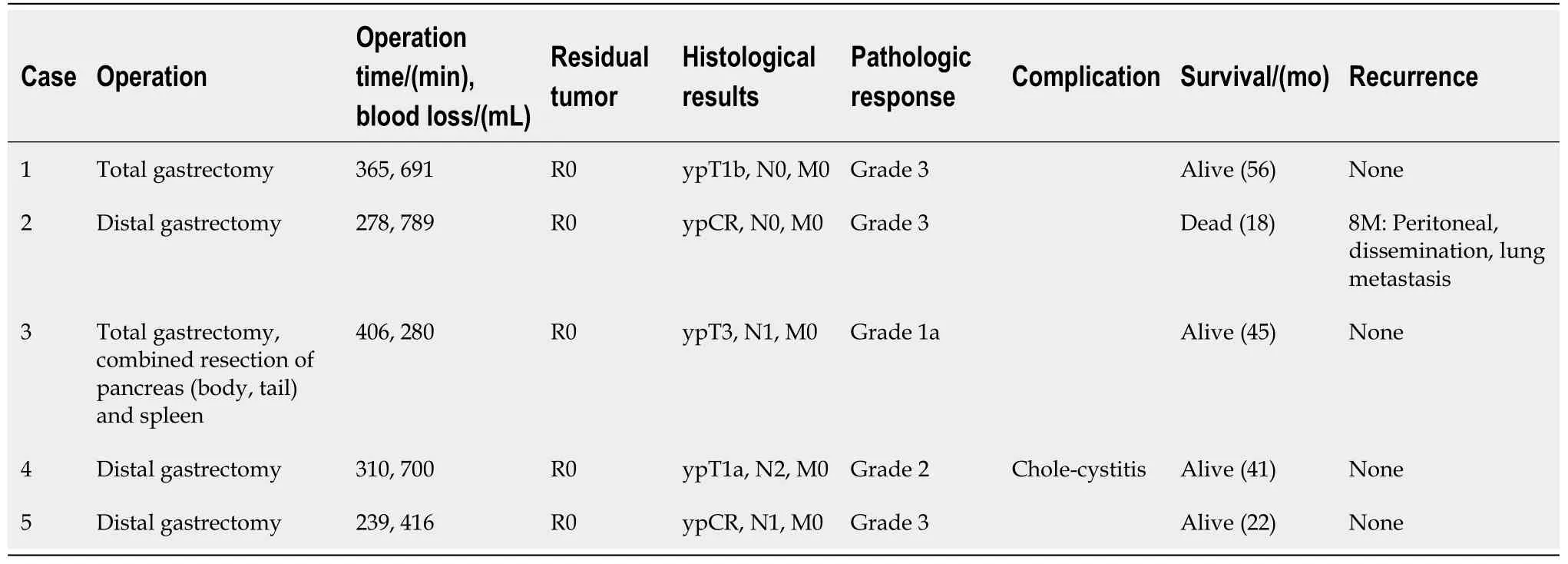
Table 2 Postoperative clinicopathological features
Case 4:The patient developed grade 3 anorexia after 30 Gy of irradiation and 120 mg/d of S-1 for three weeks.MDCT showed that the No.8a metastatic lymph node had shriveled(1.86 cm),indicating PR.Distal gastrectomy with D2 lymph node dissection was performed six weeks after stopping CRT.
Case 5:The patient developed grade 3 anorexia after 30 Gy of irradiation and 100 mg/d of S-1 for three weeks.MDCT showed that metastatic lymph node Nos.9-11p had shriveled(0.97 cm,1.01 cm),indicating PR.Her CEA value decreased after CRT to< 10% of pretherapy levels(29.9 ng/mL to 1.3 ng/mL).Distal gastrectomy with D2 lymph node dissection was performed six weeks after stopping CRT.

Figure 2 Computed tomography findings.Low signal intensity was evident inside the No.6 lymph node,although the lymph node size increased slightly after treatment(arrow).CRT:Chemoradiotherapy.
OUTCOME AND FOLLOW-UP
Table 2 shows the details of postoperative clinicopathological features of the five patients.
Case 1:The histological findings were pathologic classification after preoperative therapy(yp)T1bN0H0P0cy0,Stage 1A.Furthermore,pathologically,colon cancer was not evident.The enlarged lymph nodes with low intensity on computed tomography were cystic lesions,including necrotic tissue,with no cancerous tissue.There was no evidence of recurrence at 56 mo postoperatively.
Case 2:The resected portion of the stomach was free of cancer cells,indicating a pathological complete response(pCR).The other histological findings were ypT0N0H0P0cy0.However,this patient died 18 mo postoperatively due to peritoneal dissemination and multiple lung metastasis.
Case 3:The primary lesion was diagnosed as T3(SS)and disappearance of the pancreatic invasion.The histological findings were ypT3(SS),N1(Nos.3a and 7)H0P0cy0,Stage IIB.This patient opted for postoperative adjuvant chemotherapy with S-1 for more than one year postoperatively.There was no evidence of recurrence at 45 mo postoperatively.
Case 4:The main malignant lesion was mucosal cancer,although metastasis persisted in the No.4d,6,and 8a lymph nodes.The histological findings were ypT1a(M),N2H0P0cy0,Stage IIA.This patient underwent cholecystectomy to treat acute cholecystitis on POD 18.Since anorexia continued for two months,he needed some support of enteral nutrition in the interim.There was no evidence of recurrence 41 mo postoperatively.
Case 5:The resected stomach was free of cancer cells,indicating pCR.Micrometastasis of adenocarcinoma was evident only in fibrous tissues surrounding the No.3b lymph node.The histological findings were ypT0,N1(No.3b)H0P0cy0.This patient opted for adjuvant chemotherapy with S-1 for more than one year after operation.There was no evidence of recurrence 24 mo postoperatively.
DISCUSSION
Schuhmacheret al[14]noted that postoperative chemotherapy has significantly improved survival after gastric resection in Asia,adjuvant chemoradiotherapy is favored in North America,and perioperative chemotherapy is considered as the treatment of choice in Europe.MacDonaldet al[15]reported that postoperative adjuvant CRT was more useful than surgery alone in randomized controlled trials of 556 patients with gastric cancer.However,CRT was not recognized as standard therapy,because D0/D1 lymph node dissection accounted for 90% of all surgical procedures.Furthermore,few reports have described preoperative CRT(NACRT)because of its localized effect and various toxicities.In Japan,in particular,radiation therapy is thought to be one of the causes of damage to radiosensitive organs,such as the liver,pancreas,and kidney.However,advances in irradiation devices have reduced this problem.IMRT prevents delivery of irradiation to unnecessary areas,reduces exposure of radiosensitive organs,and increases exposure of malignant targets.Multidirectional irradiation can also reinforce the therapeutic effects and short-term,stable irradiation can be delivered[16].In addition,S-1 is a standard adjuvant chemotherapy in East Asia[17-20]that has powerful radio-sensitizing effects.While understanding these effects,a stomach damaged by CRT can be reduced and minimized by resecting the damaged part.If radiation therapy can be applied to compensate for limited or inadequate therapeutic choices in high risk patients,the number of available therapeutic options for locally advanced gastric cancer might increase.
All five of our patients underwent R0 resection.Ajaniet al[3]reported an R0 resection rate of 77% and a pCR rate of 26% with triple preoperative chemotherapy and 45 Gy irradiation.Kimet al[21]described 94.4% R0 resection rates and two of 29 patients with unresectable gastric cancer who achieved pCR with doublet or triplet anticancer drugs and 45 Gy of irradiation.In contrast,Inoueet al[5]described 11 patients who underwent R0 resection and one who achieved pCR among 12 patients who received S-1 and 50 Gy of irradiation.Microscopic tumor deposits were not found in the affected organ after combined resection in five(62.5%)of eight patients with T4b tumors;these deposits were replaced by scar tissue with NACRT-related histopathological changes.We found cancer invasion of the pancreatic body during surgery in one patient(Case 3),but microscopic deposits were not evident and histopathological changes included necrosis and inflammatory infiltrates.Furthermore,two of our patients achieved pCR of the primary lesion.However,one of them who received 50 Gy irradiation died due to peritoneal dissemination and multiple hematogenous metastases to the lung.One of the others who received 30 Gy of irradiation(Case 5)had micrometastasis in fibrous tissues surrounding lymph nodes.This suggests that although NACRT is effective,it might only serve as local treatment.However,Inoueet al[5]noted that only one of seven patients who underwent R0 resection developed peritoneal dissemination.This suggests that tumor cells that are peritoneally exposed or have invaded adjacent structures and tumor cells in the vicinity of lymph vessel stumps might become necrotic and covered with fibrotic tissues.This might prevent tumor cell dissemination during surgery,thus contributing to the inhibition of metachronous peritoneal metastasis.
Postoperative complications associated with NACRT,such as anastomotic leakage,did not occur in any of the patients in the present report,and the incidence of hematological toxicity was low,although three of five patients developed anorexia and diarrhea.Only one patient was able to tolerate 50 Gy of irradiation,and three had to stop at 30 Gy.However,even 30 Gy and half the dose of S-1 resulted in sufficient tumor downstaging,indicating considerable positive effects.Although NACRT exerted considerable pathological effects on cancer cells,lymph node size was not always reduced,even though PD or SD was achieved in some patients.Okadaet al[22]reported that 40 or 45 Gy of preoperative CRT with concurrent oral UFT or S-1 for rectal cancer resulted in significantly smaller metastatic lymph nodes inside and outside the radiation field,compared with a group that was treated only with surgery.However,taking our patients into consideration,whether the preoperative size of metastatic lymph nodes can predict a therapeutic effect might be difficult to assess clinically.On the other hand,CEA decreased to < 20% of pretherapy values in four of five of our patients with elevated tumor markers before CRT.Therefore,the effects of NACRT might be determined by observation of reduction in preoperatively elevated tumor markers,such as CEA.
While NACRT seemed to exert beneficial effects on the five patients with advanced gastric cancer described in this report,its utility in this patient population needs to be investigated further in a larger number of patients.Additionally,the fact that radiation therapy has local effects and that all the patients had well to moderately differentiated adenocarcinoma,would have contributed to minimizing peritoneal dissemination[23]and might indicate a bias towards patients with a relatively better prognosis.However,NAC with IMRT did not result in postoperative complications other than cholecystitis in one patient,and downstaging was sufficient,even when the amount of NACRT was reduced due to digestive toxicity.
CONCLUSION
Even when the amount of irradiation and oral S-1 is reduced,NACRT might offer a useful and safe therapeutic option for locally advanced gastric cancer,and compensate for the lack of conventional treatment choices.
ACKNOWLEDGEMENTS
The authors are grateful to Takuma Tajiri,MD,PhD(Department of Pathology,Tokai University Hachioji Hospital)for the pathological diagnoses.
杂志排行
World Journal of Clinical Cases的其它文章
- Special features of SARS-CoV-2 in daily practice
- Gastrointestinal insights during the COVID-19 epidemic
- From infections to autoimmunity:Diagnostic challenges in common variable immunodeficiency
- One disease,many faces-typical and atypical presentations of SARS-CoV-2 infection-related COVID-19 disease
- Application of artificial neural networks in detection and diagnosis of gastrointestinal and liver tumors
- Hepatic epithelioid hemangioendothelioma:Update on diagnosis and therapy
