Insulinoma presenting with postprandial hypoglycemia and a low body mass index:A case report
2020-04-08DanaPrdavkovMatejSamoRomanKyinaKatarnaAdamicovMichalKalmanMargitaBelicovMariMok
Dana Prídavková,Matej Samoš,Roman Kyčina,Katarína Adamicová,Michal Kalman,Margita Belicová,Marián Mokáň
Dana Prídavková,Matej Samoš,Margita Belicová,Marián Mokáň,Clinic of Internal Medicine I,Comenius University in Bratislava,Jessenius Faculty of Medicine in Martin,Martin 03601,Slovakia
Roman Kyčina,Clinic of Surgery and Transplant Center,Comenius University in Bratislava,Jessenius Faculty of Medicine in Martin,Martin 03601,Slovakia
Katarína Adamicová,Michal Kalman,Department of Pathological Anatomy,Comenius University in Bratislava,Jessenius Faculty of Medicine in Martin,Martin 03601,Slovakia
Abstract BACKGROUND Insulinomas are the most common type of functioning endocrine neoplasms of the pancreas presenting hypoglycemic symptoms.Patients characteristically develop symptoms while fasting,but some patients have reported symptoms only in the postprandial state.Repeated and prolonged hypoglycemic episodes can reduce the awareness of adrenergic symptoms,and patients may have amnesia,which delays diagnosis.CASE SUMMARY We describe a case of a 24-year-old underweight patient who showed hypoglycemic symptoms for almost 6 years.Although patients with insulinoma characteristically develop symptoms while fasting,this young man had hypoglycemic symptoms up to one hour postprandially,especially after highsugar meals and after physical activity.The fasting tests and imaging methods performed at local hospitals were evaluated as negative for abnormal results.However,brown adipose tissue exhibited increased metabolic activity,and some muscle groups had histological changes as indicated by positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography.Glycogen deficiency was also histologically confirmed.The patient’s symptoms progressed over the years and occurred more frequently,i.e.,several times a month,and the patient had reduced awareness of adrenergic symptoms.The follow-up fasting test was positive,and the imaging results showed a tumor in the head of the pancreas.The patient underwent laparotomy with enucleation of the insulinoma.CONCLUSION Weight gain and fasting hypoglycemia are not necessarily characteristics of insulinoma.In prolonged cases,adrenergic symptoms can be suppressed.
Key Words:Brown adipose tissue;Glycogen deficit;Hypoglycemia;Insulinoma;Underweight;Case report
INTRODUCTION
Insulinomas are characterized clinically by hypoglycemic symptoms resulting from neuroglycopenia and the catecholamine response[1]and are the most common functioning endocrine neoplasms of the pancreas,accounting for 20.9% of all pancreatic endocrine tumors[2].They arise from pancreatic beta cells and are the most common cause of endogenous hyperinsulinemic hypoglycemia.The incidence is 1-4 cases per million persons per year[3].Most patients diagnosed with insulinoma are aged between 30 and 60 years and are predominantly female[4,5].Insulinomas usually present as small,well-demarcated,solitary nodules that may arise in any part of the gland[1,6,7].Of diagnosed insulinomas,approximately 10% involve multiple tumors,less than 10% are malignant,and 5%-10% are associated with multiple endocrine neoplasia syndrome-1[7-10].
The classical diagnosis of insulinoma depends on satisfying the following criteria of Whipple’s triad,which remain the cornerstone of the screening process:(1)Hypoglycemia;(2)Neuroglycopenic symptoms;and(3)Prompt relief of symptoms following the administration of glucose[3].In a consensus report by the United States Endocrine Society[12],the following diagnostic criteria were proposed:Endogenous hyperinsulinism documented by the findings of symptoms,signs,or both with plasma concentrations of glucose Ë‚ 3.0 mmol/L(55 mg/dL),insulin ≥ 3.0 μIU/mL(18 pmol/L),C-peptide ≥ 0.6 ng/mL(0.2 nmol/L),and proinsulin ≥ 5.0 pmol/L.
Patients with insulinoma characteristically develop symptoms while fasting(73%-80%)[2,15],but 6% of patients report symptoms only in the postprandial state,and 21%of patients report symptoms in both the postprandial and fasting states[15].Although fasting hypoglycemia has been considered the main trait of insulinoma,postprandial hypoglycemia has also been occasionally reported as the predominant feature[16-18].Weight gain is found in only 25%-42% of patients[10,19,20],and monthly changes in body weight are significantly correlated with the tumor size and serum insulin concentration[20].Weight gain in insulinoma can be attributed to overeating to treat the hypoglycemia symptoms[21].Furthermore,voluntary weight loss induced by lowering insulin resistance accelerates the time to the clinical presentation of an asymptomatic insulinoma[22],and malnutrition,body fat and muscle depletion can contribute to hypoglycemia due to the limited substrates available for gluconeogenesis and glycogenolysis.
CASE PRESENTATION
Chief complaints
A 24-year-old man was admitted in September 2019 for a preoperative evaluation before surgery for a recently diagnosed symptomatic insulinoma.
History of present illness
The patient’s first episode of hypoglycemia occurred 6 years prior and presented as weakness,sweating and tremor.The symptoms occurred up to one hour postprandially,especially after eating high-sugar meals and after performing physical activity.In addition to the adrenergic hypoglycemic symptoms,the patient also reported the taste of acetone in the mouth.These difficulties progressed over the years,occurred more frequently up to several times a month and involved other neuroglycopenic symptoms,including multiple episodes of loss of consciousness associated with amnesia.
In 2015,emergency medical services confirmed a blood glucose level of 2.0 mmol/L,and the patient was referred to a local hospital to undergo a 72-h fasting test.The test was classified as negative,and further examinations were conducted.Abdominal ultrasonography and multidetector computed tomography(CT)of the chest and abdomen were negative for abnormal findings.CT examination integrated with 2-deoxy-2-[fluorine-18]fluoro-D-glucose did not confirm the presence of tumors but showed that there was enhanced metabolic activity of brown adipose tissue(BAT)bilaterally in the neck,paravertebral,supra- and infraclavicular,axillar,mediastinal,jugular,diaphragmatic and perinephric regions.Increased activity was also found in several muscle groups(e.g.,psoas major muscles)(Figure 1).This finding was attributed to a reactive stress reaction.The presence of autoimmune primary muscle disease was excluded,but a muscle biopsy showed focal glycogen deficits.The episodes of hypoglycemia occurred repeatedly,but the patient became less aware of the decreasing intensity of the adrenergic symptoms.A second fasting test(in August 2019)was performed with a resulting decrease in glycemia to 1.9 mmol/L(34 mg/dL)in the 29thh of the test and an increase in insulin to 5.45 μIU/L(normal range:3.0-25 μIU/L).C-peptide was 0.42 nmol/L(normal range:0.25-0.60 nmol/L).
History of past illness
The patient had no specific history of past illness.
Personal and family history
The patient had no specific personal or family history.
Physical examination
The patient’s weight was 52 kg,and his body mass index was 17.0 kg/m2.His skin,skin adnexa and mucosa showed no pathological changes.The physical examination showed no obvious cardiovascular or respiratory abnormalities.The abdomen was soft without pathological resistance.
Laboratory examinations
Upon hospitalization,the blood glucose level was 4.2 mmol/L,glycated hemoglobin-IFCC was 3.1%(normal range:2.8%-4.8%);the values of C-peptide and insulin,lipid,mineral,protein,acid-base balance,renal and hepatic parameters were in the normal range.The diurnal rhythm of cortisol,function of the thyroid,levels of human growth hormone,adrenocorticotropic hormone,metanephrines,normetanephrines and values of oncomarkers were normal.
Imaging examinations
Endoscopic ultrasonography of the pancreas revealed a well-defined oval lesion(14.7 mm × 13.1 mm)in the head of the pancreas.The lesion was hypervascular with a higher echogenicity and a hyperechoic periphery.A CT scan of the pancreas imaged the homogenous lesion(10.5 mm × 8.0 mm)during the arterial phase of the examination.
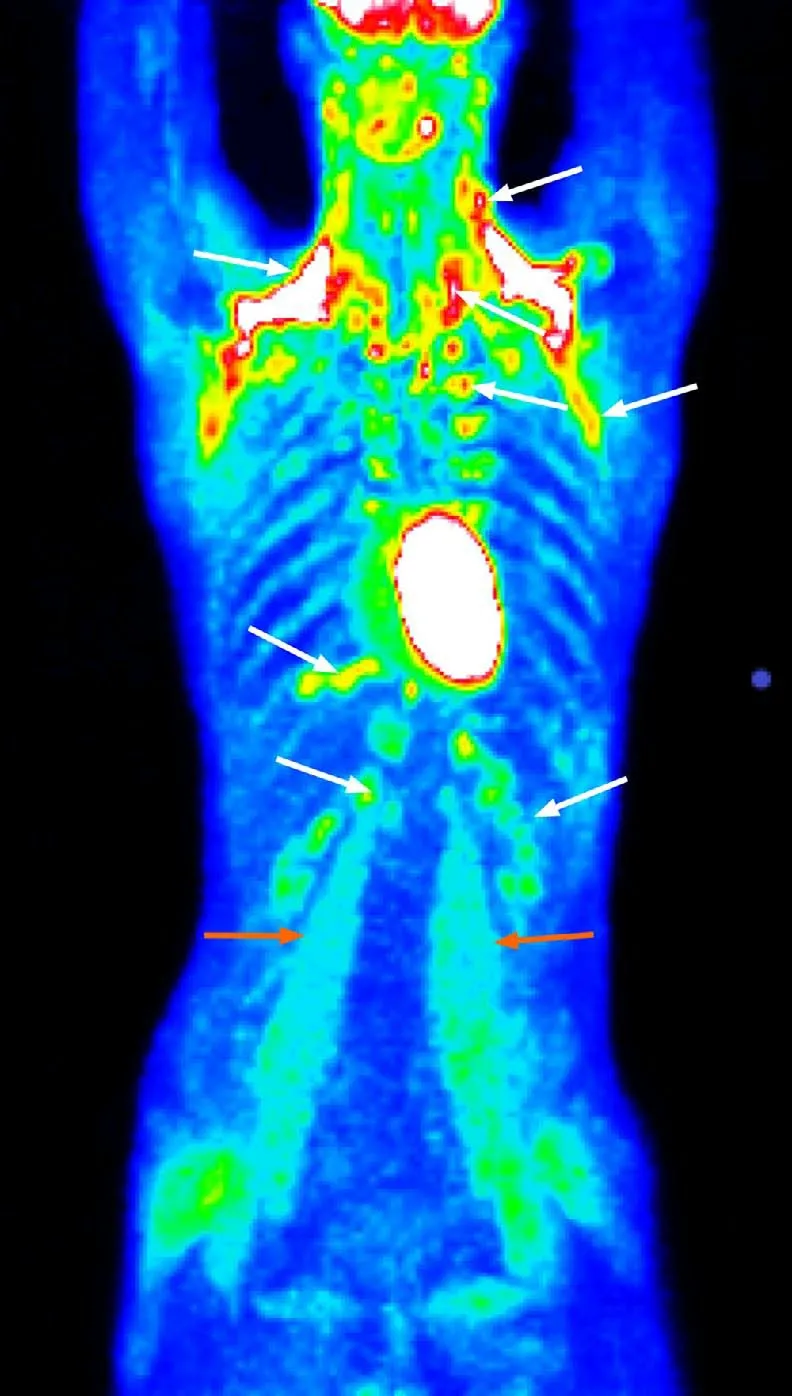
Figure 1 Positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography showing enhanced metabolic activity of brown adipose tissue in the neck,paravertebral,supra- and infraclavicular,axillar,mediastinal,jugular,diaphragmatic,and perinephric regions bilaterally(white arrows)and several muscle groups(e.g.,psoas major muscles)(orange arrows).
FINAL DIAGNOSIS
The patient was finally diagnosed with insulinoma.
TREATMENT
The patient underwent a laparotomy with enucleation of insulinoma(sized 1.0 cm)(Figure 2)and cholecystectomy.The perioperative findings noted a very small amount of visceral fat.The insulinoma was pressing against the common bile duct without any infiltration.Because possible lesion-involvement or sealing of the pancreatic duct by a harmonic scalpel(performed perioperatively during pancreatography,which showed a course of pancreatic duct only 2 cm from the papilla)could not be ruled out,Rouxen-Y anastomosis and nutritive lateral jejunojejunostomy was performed.Significant bleeding occurred from the base of the enucleated tumor,and the patient underwent a second surgical intervention.
OUTCOME AND FOLLOW-UP
The final histological findings(Figure 3)revealed a well-differentiated insulinoma G1(WHO 2017)(33),staging pT1pNxL0,V0,Pn0.The immunohistochemical studies of the lesion confirmed positivity for the neuroendocrine markers synaptophysin and chromogranin A,but the latter was less pronounced.The immunohistochemical studies found that the lesion sections were also positive for insulin,CK8/18 and CD56;the staining for glucagon and vimentin were negative.Lymphatic,vascular and perineural invasion was not confirmed,and the proliferative activity(Ki67)was 2.4%.The borders of the tumor were without neoplastic infiltration.The patient was released with normal levels of plasmatic glycemia.During the 6-mo follow-up period,the patient remained asymptomatic.

Figure 2 Insulinoma(perioperative findings).A:Insulinoma as indicated by the yellow arrows was located in the upper part of the pancreatic head;B:Detailed view of the whole enucleated insulinoma;C:Detailed view of the sectioned insulinoma.

Figure 3 Histological and immunohistochemical findings of the insulinoma and histological findings of muscle biopsy.A:Positive insulin immunostaining;B:Hematoxylin and eosin staining;C:Positive synaptophysin immunostaining;D:Glycogen deficit(light violet areas)in muscle syncytia(musculus quadriceps femoris),PAS stain,20 ×.
DISCUSSION
Delay in the diagnosis of insulinoma is common because the symptoms largely precede the occurrence of an apparent tumor,and there may be misattribution of the symptoms to psychiatric,cardiac or neurological disorders[23].Several studies confirmed that 20%-64% of patients are misdiagnosed with a psychiatric,seizure or other neurological disorder before the final diagnosis of insulinoma is made[24,25].The interval between the onset of symptoms and the diagnosis of insulinoma ranges from 1 mo to 30 years,with a median of 24 mo;only 28% of patients are diagnosed within 1 year of symptom onset,and the diagnosis is delayed beyond 5 years in 19% of patients[19].The patient presented here also initially underwent psychological and psychiatric examinations with a suspicion of eating disorders with the recommendation for psychotherapeutic guidance.The clinical manifestations of hypoglycemia,especially the adrenergic symptoms,are relatively well recognizable.However,patients with long-term illness may develop insufficient awareness of hypoglycemia with the risk of developing neuroglycopenic manifestations without awareness of adrenergic symptomatology[2],and these manifestations also occurred in our patient(episodes of short unconsciousness with amnesia).This phenomenon occurs because the set point of catecholamine secretion in response to hypoglycemia is lowered[26].The prolonged time to the diagnosis of insulinoma in this case was likely also due to a different interpretation of the results of the fasting test performed at the local hospital.Furthermore,it appears that 9% of patients with insulinomas could be overlooked using the older recommended value of insulin ≥ 5 μIU/mL[11].A prolonged fasting test(72 h)may not be diagnostic for 1% of insulinomas[27],which was likely the case in our patient(at the beginning of the diagnostic process).
Classically,insulinomas present with weight gain and fasting hypoglycemia but may also present with postprandial hypoglycemia and weight loss[22].The patient did not have weight gain,which is noted in approximately 25%-42% of insulinoma cases[10,19,20].Voluntary weight loss accelerates the time to clinical presentation of an asymptomatic insulinoma by lowering insulin resistance[22].However,the patient’s diagnosis had long been stagnant,and the patient was assessed in the context of intrapsychic tension and eating disorders.
An increase in 2-deoxy-2-[fluorine-18]fluoro-D-glucose metabolic activity in BAT diffusely and muscle groups to this extent as determined using positron emission tomography/CT examinations is not common,and this phenomenon cannot be explained solely by a reactive stress response.Other factors are involved in the increased activation of BAT,such as a younger age,BAT activation in a previous scan and a lower body mass index[28].BAT is normally present in fetuses and diminishes in adults,accounting for only 1% of the total body mass[29].BAT is richly innervated by sympathetic nervous system efferent fibers,and sympathetic activation is the physiological activator of BAT thermogenesis.BAT is profoundly influenced by insulin sensitivity.The presence of metabolic activity of 2-deoxy-2-[fluorine-18]fluoro-D-glucose-positive BAT is associated with increased plasma catecholamines and is inversely related to central obesity.For example,BAT activation has been observed in cancer cachexia[30].Similarly,our patient’s low body weight and small amount of visceral fat could be associated with increased BAT activity.Although the increased metabolic activity of BAT does not substantially affect glucose utilization(BAT accounts for 1% of the total body glucose utilization compared to 50% of skeletal muscles)during cold exposure in healthy subjects[30],abnormal glucose metabolism with hypoglycemia may contribute to increased metabolic activity of BAT[28]viacounterregulatory elevated catecholamines.The effect of cortisol on BAT remains ambiguous;while some researchers state that cortisol inhibits BAT function,other researchers have demonstrated that cortisol has a stimulatory effect[28].
Primary muscle disease was excluded by muscle biopsy.Muscle biopsy was negative in this sense but showed reduced glycogen stores,which could be associated with prolonged counterregulatory hormonal activity.Skeletal muscles mainly express β2-adrenergic receptors and adrenaline rather than noradrenaline,which stimulates glycogen breakdown.Through the Cori cycle,skeletal muscle deposits of glycogen are broken down during adrenaline stimulation and released as lactate and converted to glucose in the liver.Indeed,the reduced glycogen content in skeletal muscles increases insulin sensitivity.The glycogen content slightly increases by the acute intake of large amounts of carbohydrates[31]and possibly leads to a deepening of hypoglycemia.
CONCLUSION
In summary,although fasting hypoglycemia has been considered a hallmark of insulinoma,postprandial hypoglycemia has also been occasionally reported as the predominant feature similar to weight loss.Repeated and prolonged hypoglycemic episodes can reduce the awareness of neurogenic and neuroglycopenic hypoglycemic symptoms in insulinoma,which delays the diagnosis of the disease.Prolonged but not continuous adrenergic stimulation in the counterregulatory response to hypoglycemia may be indicated by increased metabolic activity of BAT and reduction of muscle glycogen.A low body mass index increases the risk of postoperative complications,and a very small amount of visceral fat can be associated with a lower intraoperative core temperature.Among other things,hypothermia can lead to adverse patient outcomes,including increased blood loss with the need for blood transfer.These complications may lead to higher mortality rates and longer hospital stays,which are positively correlated with the male sex and a younger age[32].
杂志排行
World Journal of Clinical Cases的其它文章
- Special features of SARS-CoV-2 in daily practice
- Gastrointestinal insights during the COVID-19 epidemic
- From infections to autoimmunity:Diagnostic challenges in common variable immunodeficiency
- One disease,many faces-typical and atypical presentations of SARS-CoV-2 infection-related COVID-19 disease
- Application of artificial neural networks in detection and diagnosis of gastrointestinal and liver tumors
- Hepatic epithelioid hemangioendothelioma:Update on diagnosis and therapy
