Celiac disease and Sjögren’s syndrome:A case report and review of literature
2020-04-08DanielVasileBalabanAncutaMihaiAlinaDimaAlinaPoppMarianaJingaCiprianJurcut
Daniel Vasile Balaban,Ancuta Mihai,Alina Dima,Alina Popp,Mariana Jinga,Ciprian Jurcut
Daniel Vasile Balaban,Alina Dima,Mariana Jinga,Internal Medicine and Gastroenterology,Carol Davila University of Medicine and Pharmacy,Dr.Carol Davila Central Military Emergency University Hospital,Bucharest 020021,Romania
Ancuta Mihai,Ciprian Jurcut,Internal Medicine,Dr.Carol Davila Central Military Emergency University Hospital,Bucharest 010825,Romania
Alina Popp,Pediatrics,Carol Davila University of Medicine and PharmacyCarol Davila University of Medicine and Pharmacy,Bucharest 020021,Romania
Alina Popp,Alfred Rusescu Institute for Mother and Child Care,Bucharest 020021,Romania
Alina Popp,Tampere Center for Child Health Research,University of Tampere and Tampere University Hospital,Bucharest 020021,Romania
Abstract BACKGROUND Celiac disease(CD)is a systemic,chronic immune-mediated disease triggered by gluten ingestion in genetically-susceptible individuals,with a prevalence of 1%worldwide.Sjogren's syndrome(SS)is also a systemic autoimmune disease,mainly characterized by ocular and oral sicca symptoms and signs.Sharing a common genetic background,CD and SS are known associated autoimmune diseases,but currently available guidelines are not reporting it.CASE SUMMARY We report the case of a 39-year-old woman,who was in the care of her rheumatologist for 2 years with SS.On routine follow-up she was found to have iron deficiency,without anemia.She had no gastrointestinal complaints and denied any obvious source of blood loss.IgA tissue transglutaminase antibodies were positive and endoscopy with duodenal biopsies revealed crypt hyperplasia and villous atrophy.A diagnosis of CD was set and gluten-free diet was recommended.CONCLUSION We present a review of existing data in the literature regarding the association of the two diseases,summarizing prevalence studies of CD in SS patients and the other way around.Screening recommendations and future research perspectives are also discussed,highlighting clinically relevant unanswered questions with respect to the association of CD with SS.
Key Words:Celiac disease;Sjögren syndrome;Prevalence;Autoimmunity;Screening;Antibodies;Case report
INTRODUCTION
Within the circle of related-autoimmune diseases,celiac disease(CD)and Sjögren’s syndrome(SS)come together with type 1 diabetes mellitus,autoimmune thyroid disease,and primary biliary cholangitis[1].In fact,a major stake when diagnosing one of the above autoimmune diseases is screening for the other ones,especially in the presence of a suggestive clinical scenario.
SS is an autoimmune disease mainly characterized by ocular and oral sicca symptoms and signs,positivity for certain autoantibodies(anti-SS-A/Ro and anti-SSB/La),and suggestive minor salivary gland biopsies.All these items were integrated in classifications criteria,the most recent being the 2016 American College of Rheumatology/European League Against Rheumatism SS’s Classification Criteria[2].In the same paper,the importance of extraglandular manifestations was highlighted by increasing the target population to which the classification criteria can be applied.
Meanwhile,CD is a gluten-dependent,autoimmune enteropathy,affecting 1% of the population with a continuously rising incidence,mainly because of an increase of correct positive diagnosis[3].In the last few decades the paradigm of CD’s clinical spectrum dramatically changed from the classical forms with diarrhea to atypical forms.Moreover,the importance of extraintestinal manifestations and of clinical associated conditions has been increasingly recognized[4-7];hence,CD is now regarded as a chronic,systemic disease triggered by the ingestion of gluten in genetically susceptible individuals.Since the 1960s[8],several publications have been addressing the co-association between SS and CD - first with case reports[9-11],then small series and cohorts.
However,awareness among internal medicine specialists,rheumatologists,and gastroenterologists remains unsatisfactory and currently available guidelines are not reporting this association[2,12].
We herein present the case of a patient with SS who was diagnosed with CD,and summarize the existing data about the association of the two autoimmune disorders,with a focus on clinical application.To the best of our knowledge,this is the first literature review analyzing the existing data related to these two pathologies concomitance.
CASE PRESENTATION
Chief complaints
We report the case of a 39-year-old woman who was in the care of her rheumatologist after being diagnosed with primary SS.Her illness began two years before with bilateral knee arthralgia,debilitating fatigue,xerophthalmia and xerostomia.With positive antinuclear antibodies,anti-Ro[> 200 UI/mL(normal value < 15)]and anti-La[57.5 UI/mL(normal value < 15)]antibodies,positive Shirmer's test,positive Rose Bengal coloration,and salivary gland biopsy demonstrating focal lymphocytic sialadenitis with a focus score of 4,she was diagnosed with SS.She was prescribed hydroxychloroquine and topical treatment,with improvement of sicca and articular symptoms.
History of present illness
Two years later she came into the Rheumatology Department for a routine follow-up visit.She reported good symptom control with therapy without adverse reactions,and her physical examination was unremarkable.
Laboratory examinations
However,routine blood work however revealed lymphopenia-1100/mL(normal range 1170-3170),iron deficiency without anemia[serum iron-23 ug/dL(70-180),ferritin-18 μg/L(20-250),hemoglobin-12.9 g/dL(12-16)]and low vitamin D levels-10 ng/mL(20-160).Except regular menses,the patient's history did not reveal any obvious sources of blood loss.Upon further history taking,she did not report any digestive symptoms.
Further diagnostic work-up
Considering the autoimmune background and the suspicion of associated malabsorption syndrome,screening for CD was recommended.Blood was drawn for serum IgA and IgA tissue transglutaminase(tTG)antibodies.The diagnosis approach was completed with upper gastrointestinal endoscopy which showed mucosal fissures and scalloping in the distal duodenum,with multiple biopsies being taken both from the bulb and distal duodenum(Figure 1 A and B).The histopathology report revealed marked villous atrophy with crypt hyperplasia and intraepithelial lymphocytosis,corresponding to Marsh 3c classification(Figure 2).IgA tissue transglutaminase antibody levels were over 200U(Quanta Lite®h-tTG IgA/Inova,cut-off positivity 20 U).
FINAL DIAGNOSIS
Thus,a diagnosis of CD was made.
TREATMENT
Nutritional therapy,consisting of a gluten-free diet,was recommended.
OUTCOME AND FOLLOW-UP
Upon follow-up,serum iron and ferritin levels normalized,IgA tTG gradually decreased to negative titers and control biopsy revealed restoring of normal villous architecture but with persisting intraepithelial lymphocytosis(Figure 3).
DISCUSSION
In our case presentation,we report a diagnosis of CD in a patient with SS,without any gastrointestinal complaints,starting from mild laboratory changes during a routine follow-up visit.Notably,the subtle alterations in the blood work-up along with a high index of suspicion of the clinician triggered CD testing.
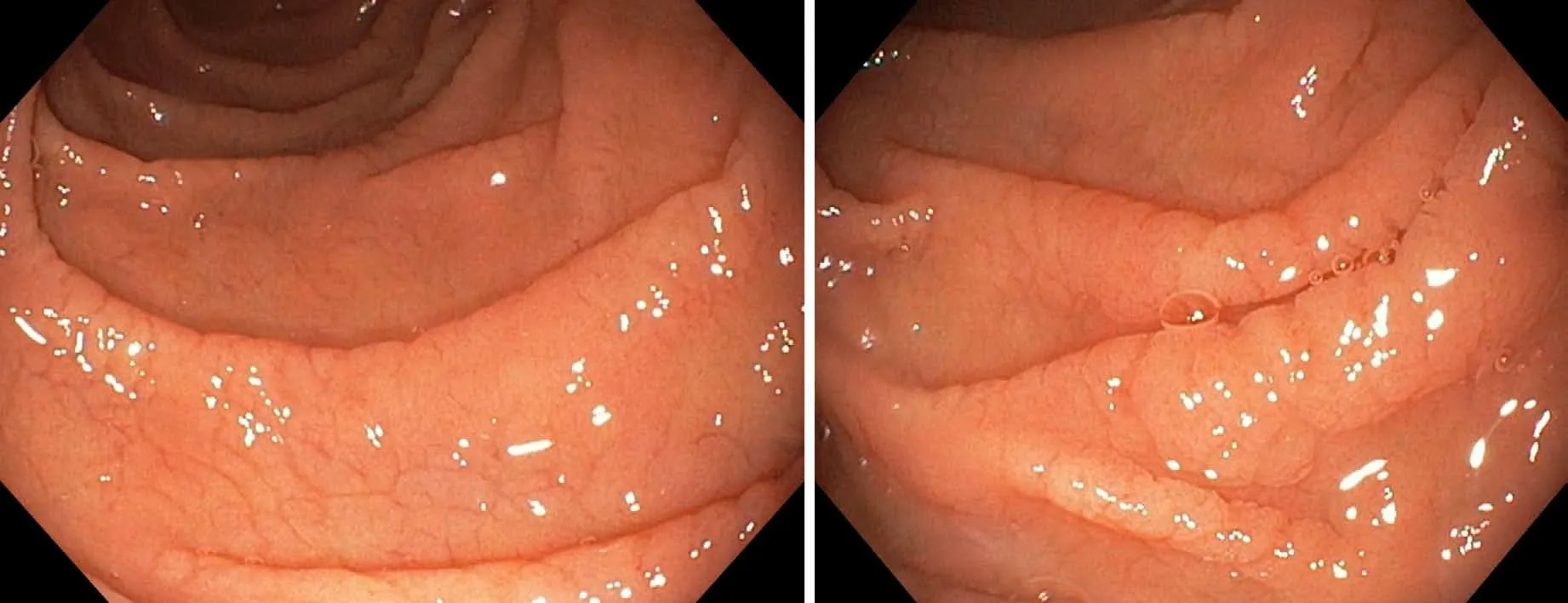
Figure 1 Endoscopic images showing mucosal fissures and scalloping in the distal duodenum.
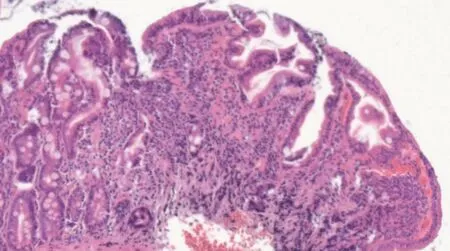
Figure 2 Hematoxilin-eosin stain of duodenal biopsy sample showing marked villous atrophy with crypt hyperplasia and intraepithelial lymphocytosis,corresponding to Marsh 3c classification.
Although the association of CD and SS is well recognized,awareness among practitioners of related medical specialties is far from being satisfactory[13],so opportunities to diagnose CD/SS are often missed.Both disorders are known to be significantly underdiagnosed and this exposes patients to complications and impaired quality of life[14,15].In this setting,searching for CD in SS patients and vice versa could represent an important case-finding tool.
Prevalence of CD in patients with SS
Several papers have looked at the frequency of CD in SS patients.The prevalence of CD among patients with SS ranges from 1% to 14.7%(Iltanenet al[16])- as noted in Table 1[16-22],while the prevalence of CD in general population is about 1%.However,existing data in the literature has important limitations - the studies performed in cohorts of patients with SS included a limited number of patients and the diagnostic criteria used were not similar.The Finnish series by Iltanenet al[16]comprised a small number of patients(34 SS)and reported a high prevalence of 14.7% CD in patients with SS.All five CD patients had atrophic mucosal injury in the biopsy samples,with recovery on a gluten-free diet,while on serology,they were positive for either IgAantiendomysial antibodies(EMA)or the old generation IgA antigliadin antibodies(AGA);the authors acknowledged the low specificity of IgA-AGA as serological screening in SS.As in our case,the clinical picture of CD patients diagnosed by screening was not prominent,and two of them had nutritional deficiencies(iron and folic acid deficiency,respectively).Of note,four SS non-CD patients had normal villous architecture with an increased density of intraepithelial γδ T cells,while two of them were also positive for HLA DQ2,which could represent CD latency.A ratherhigh seroprevalence(12%)was also reported in a small cohort of SS patients(n= 50)in the study by Luftet al[20],five out of the six positive being biopsy-confirmed CD(10%);the authors also found that prevalence of tTG was higher in SS as compared with other rheumatic diseases such as systemic lupus erythematosus(SLE),rheumatoid arthritis or systemic sclerosis.On the other hand,Fasanoet al[22]reported a low prevalence,of 2%,in a cohort of 98 SS patients,and so did Bizzaroet al[19],who found a seroprevalence similar to the one in the general population,of 1%:The one patient in a cohort of 100 SS was IgG tTG positive,but IgA and IgG EMA were negative,as was HLA typing,and according to study protocol,was not biopsied.Other studies such as the one by Caioet al[21]also reported on serology only,lacking histological confirmation;in their study,there was also a SS case with isolated,low titer,IgG antideamidated gliadin peptide antibodies(DGP)positivity,which could raise the CD prevalence to 7.7% if histologically-confirmed.In the largest cohort published,Bartoloniet al[18]reported a prevalence of 7.1% biopsy- proven CD,with 24 out of the 25 confirmed cases being previously diagnosed with CD;screening detected only one
additional subclinical CD.The major issue of the aforementioned studies reporting on prevalence of CD in SS is the heterogeneity of criteria used for CD diagnosis,which partly explains the wide range of prevalence rates.However,when summarizing available data,there seems to be a higher prevalence of CD in SS patients than in general population.Although other larger studies have reported strong associations of SS with other CD-related autoimmune diseases such as ATD or PBC,CD was not looked for in these cohorts[23,24].We hypothesize that overlooking CD in a patient with SS might be due to the gastrointestinal involvement secondary to SS itself,which would be labelled as a disease manifestation and would not trigger testing for CD.

Table 1 Summary of studies reporting prevalence of celiac disease in Sjogren syndrome
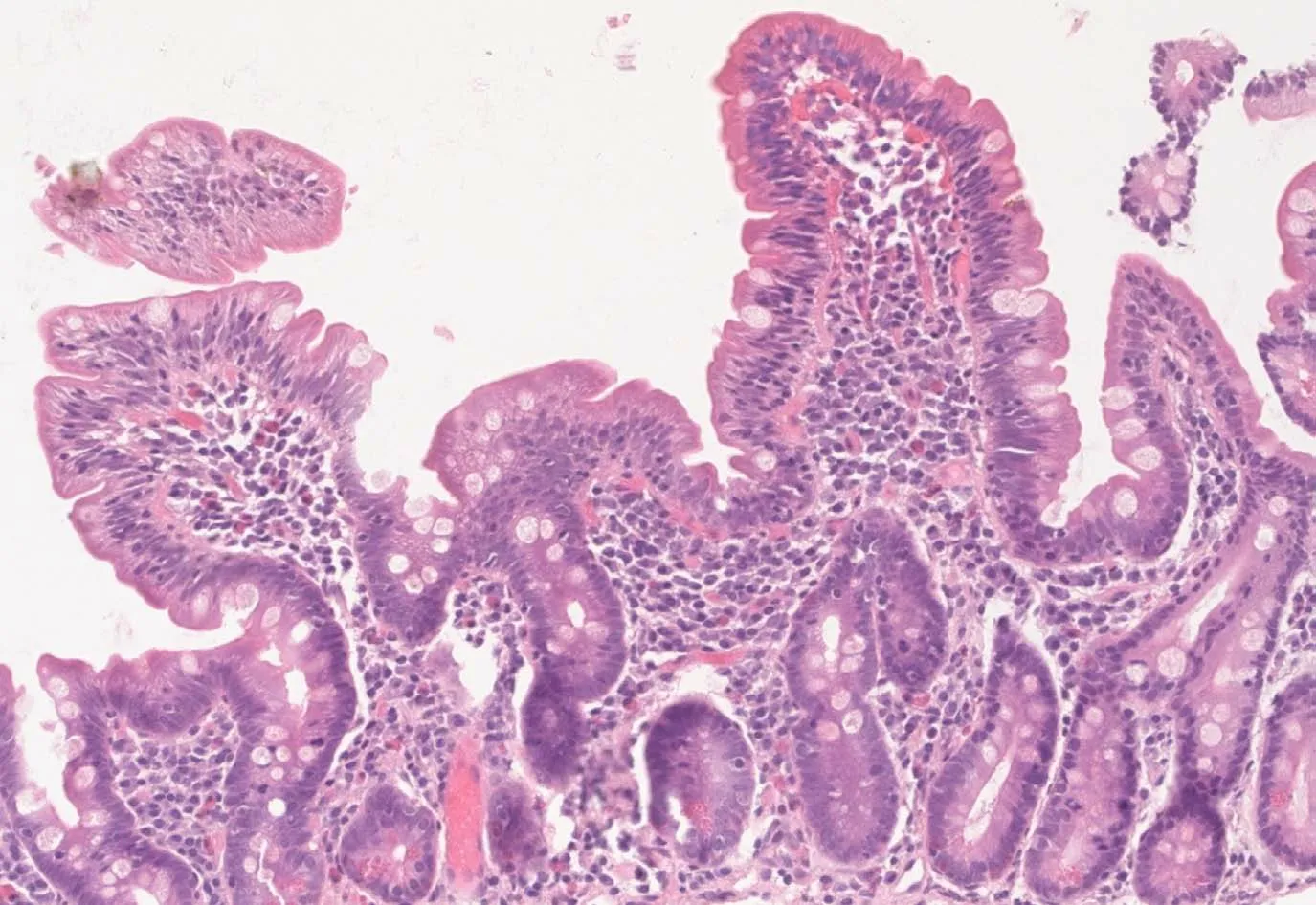
Figure 3 Hematoxilin-eosin stain of follow-up biopsy revealing restoring of normal villous architecture but with persisting intraepithelial lymphocytosis.
Prevalence of SS in patients with CD
Conversely,other authors have also looked at the SS occurrence in CD patients,results of which are summarized in Table 2[25-29].Using the California criteria for SS,Collinet al[28]reported a prevalence of 3.3% for SS in patients with CD,a significant association in contrast with other connective tissue disease[28],while Caglaret al[27]reported a prevalence of 6.5%(SS-A/SS-B antibody)[27].Ayaret al[29]found a high frequency of dry eye/mouth in CD patients,with approximately one in four being symptomatic,but only few had a biopsy-based diagnosis of SS;the frequency of SS classified according to the 2012 ACR or to American-European Consensus Group criteria was 4.9%[29].Similarly,in a small cohort of 82 CD patients,Erbasanet al[25]reported a high prevalence of dry eye(29.3%)and dry mouth(24.4%)symptoms;in this group,the diagnosis of SS was proven by biopsy in 1.2% of CD patients[25].Moreover,four patients(4.9%)were diagnosed with undifferentiated connective tissue disease,two of them being positive for anti-SS-A/La antibodies,but having though negative biopsies.As for the studies analyzing CD prevalence in SS,the ones looking at SS prevalence in patients with CD are also very heterogeneous with respect to diagnostic criteria.
Even if symptomatology like dry mouth or eye is frequently reported in CD patients,biopsy in search for SS is only rarely assessed.Therefore,the available data are incomplete.Even so,the concomitance of SS and CD seems not to be random.
In summary,there is scarce data on SS prevalence in CD,and from the available publications,there is a lack of uniformity with respect to specific classification criteria,study design,and population examined.
Pathophysiology and antibodies in CD and SS
The antibody spectrum in CD and SS has been looked at in a few studies.On one hand,there is data supporting a high prevalence of antinuclear antibodies in patients with CD,ranging from 8.9%(da Rosa Utiyamaet al[30])to 14.6%(Erbasanet al[25]).Additionally,the anti-SS-A/Ro and anti-SS-B/La antibodies were reported to have a prevalence of 6.5% in 31 patients with CD[27].On the other hand,earlier studies using gliadin-based serology have shown significant positivity of CD-related antibodies in SS:Teppoet al[31]found higher values of gliadin,gluten and reticulin antibodies in SS patients compared to rheumatoid arthritis or SLE.Significant false positivity of native AGA has also been reported also in other autoimmune diseases,such as SLE[32,33].A study searching for immune and autoimmune gluten-related phenomenon in SLE patients found higher prevalence for tTG IgA and DGP(3.4% and 4.7%,respectively),but similar to general population prevalence for EMA(0.8%).Moreover,the tTG-IgA were found to be correlated with leucocytes,lymphocytes as well as with the complement levels[33].
To overcome the limitation of gliadin-based serology,clinicians should remember that EMA has the highest specificity for diagnosing CD,and IgA-tTG remains the best test for initial screening with very high sensitivity and specificity[34,35].
The association between CD and SS-related autoantibodies might be based on common genetic background of these two pathologies,related to DR3-DQ2 heterodimer coded by the DQA1*0501 and DQB1*0201 alleles[36,37].Patients possessing these genetic markers had extractable nuclear antigen antibodies,and especially SSB/La antibodies more frequent than patients negative for these alleles[38].Moreover,it is well recognized that CD is associated with specific HLA types in virtually all populations that have been tested,and this very high negative predictive value of HLA-DQ2/DQ8 genotyping can be used to effectively rule out the disease in selected clinical situations[22,39].For SS,the most relevant HLA genes involved in susceptibility are DR3-DQ2,with alleles DRB1*03:01,DQA1*05:01,and DQB1*02:01 being risk factors while,DQA1*03:01,DQA1*05:01 and DQB1*05:01 alleles were found to be protective factors[40].
Besides this common immunogenetic background,the link between SS and CD is also supported by studies researching gluten-challenge and gluten-free diet in SSpatients.Therefore,the study by Patinenat al[41]showed improvement in terms of the autoimmune inflammatory process in the salivary glands of SS patients who were on a gluten-free diet because they had associated CD,while Lidénet al[42]revealed rectal mucosal inflammation in response to gluten challenge in SS patients,thus proving gluten-sensitivity of these patients.Not least,dermatitis herpetiformis,as a cutaneous equivalent of CD,has also been reported in SS[43].
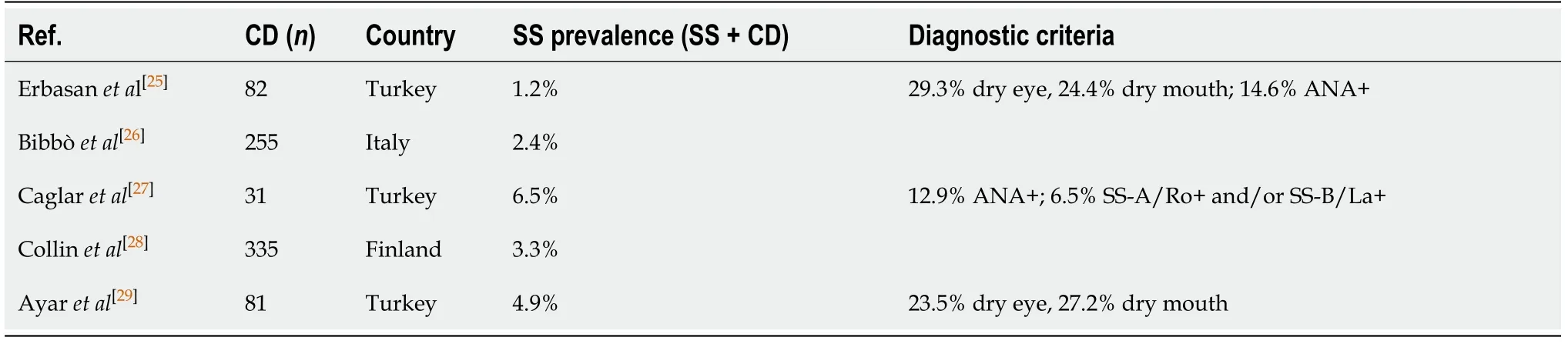
Table 2 Summary of studies reporting prevalence of Sjogren syndrome in celiac disease
Additionally,the histopathologic exam shows lymphocytes infiltrates in both SS and CD,namely a focal lymphocytic sialadenitis,with a focus score of ≥ 1 foci/4 mm2in SS accordingly to the 2016 ACR/ EULAR SS’s classification criteria[2],and intraepithelial lymphocytic infiltration,more than 25 lymphocytes/100 epithelial cell,with associated villous and crypt architectural changes,in CD[12].
Moreover,both SS and CD pathologies are associated with an increased risk of lymphoma occurrence.Initially considered a T cell dysregulation,we recognized that the major derangement and dysregulation of the B lymphocytes in both peripheral circulation and glandular tissues of the SS patients plays a central part in the pathogenic processes[44].The lymphoma risk is increased in SS patients especially for the marginal zone lymphoma,namely the parotid gland mucosa associated lymphoid tissue lymphoma,and the diffuse large B-cell lymphoma[45].In CD,particularly patients with refractory CD,defined as persistent clinical and histological changes after at least 12 months of sustained gluten-free diet,especially in cases with monoclonal rearrangement of T cell receptor(type 2),occurrence of intestinal lymphoma might complicate the clinical course.The risk of both B and T cell lymphoma is increased in CD,and is the highest for the non-Hodgkin T cell intestinal lymphoma(six to nine times higher than in general population)[46].
Screening recommendations and future direction
Despite the strong association between the two conditions,currently available guidelines fail to recommend screening for SS in CD patients and vice versa[2,12],except the 2009 National Institute for Health and Clinical Excellence guideline that recommended that patients with SS may be considered for CD testing[47].However,the updated and most recent NICE guideline on CD does not recommend screening for CD in patients with SS[48].Similarly,the 2017 United States Preventive Services Task Force does not include the SS in the target group for the CD screening[49].
Although data in the literature is not as large as for other associated autoimmune diseases such as type 1 DM or ATD disease[6,50],testing for CD should be thought of in a symptomatic SS patient or upon detection of isolated iron deficiency,alterations of liver functions tests or pancreatic enzymes.However,there are some authors who recommend routine CD screening in patients with SS[51];this would be readily available by IgA tTG testing,which has become increasingly accessible and improved significantly over the last years in terms of standardization of the kits used[52].It is important to remember that IgA deficiency is more frequently found in CD patients,therefore the serum IgA level should also be tested,and IgG-based serology should be done in cases with IgA deficiency[53].In this population of IgA-deficient individuals,DGP testing has been proven to be an accurate tool to identify CD[54].Nevertheless,one should keep in mind the possibility of seronegative CD,meaning villous atrophy(VA)with negative CD-serology,when diagnosis can be supported by celiac-type mucosal injury on histopathology,exclusion of other causes of VA,along with HLA typing and response to gluten-free diet[55-57].
Thus,there are some clinical scenarios needing the search of CD in SS patients or SS in CD patients in Table 3[23].Further studies should focus on indications,screeningmethods,timing of screening and frequency of testing for SS in CD patients and vice versa in Table 4.
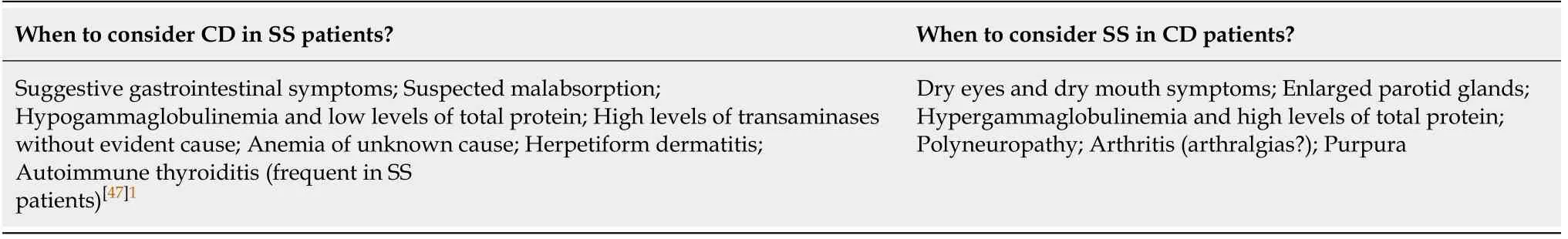
Table 3 Clinical scenarios needing the search of celiac disease in Sjögren syndrome patients or Sjögren syndrome in celiac disease patients
Moreover,another important question that is yet to be answered is about the role of gluten-free diet in non-CD,SS patients.Several papers have reported benefits of the gluten free diet in various non-celiac autoimmune diseases,but results remain controversial[58,59].Considering all the drawbacks of a gluten-free diet in the absence of gluten-intolerance,the role of gluten-free diet in SS remains to be studied and cannot be recommended yet on the basis of current evidence.
CONCLUSION
Sharing also a common genetic background,CD and SS are found to be associated,but evidence to quantify the magnitude of this association is lacking.This concomitance of these two diseases is however easily overlooked in clinical practice and currently available guidelines fail to recognize it.Further research should look at indications for screening,testing methods,as well as the role of the gluten-free diet in non-CD SS patients.

Table 4 Future agenda
ACKNOWLEDGEMENTS
We would like to thank Dr.Vasilescu Florina for her support with the histology slides.
杂志排行
World Journal of Clinical Cases的其它文章
- Lymphoplasmacyte-rich meningioma with atypical cystic-solid feature:A case report
- Left bundle branch pacing with optimization of cardiac resynchronization treatment:A case report
- Arterial embolism caused by a peripherally inserted central catheter in a very premature infant:A case report and literature review
- Gitelman syndrome caused by a rare homozygous mutation in the SLC12A3 gene:A case report
- Massive pulmonary haemorrhage due to severe trauma treated with repeated alveolar lavage combined with extracorporeal membrane oxygenation:A case report
- Successful treatment of encrusted cystitis:A case report and review of literature
