Forniceal deep brain stimulation in severe Alzheimer’s disease: A case report
2020-04-08WeiLinWeiQiBaoJingJieGeLiKunYangZhiPeiLingXinXuJieHuiJiangChuanTaoZuoYuHaiWang
Wei Lin, Wei-Qi Bao, Jing-Jie Ge, Li-Kun Yang, Zhi-Pei Ling, Xin Xu, Jie-Hui Jiang, Chuan-Tao Zuo, Yu-Hai Wang
Wei Lin, Li-Kun Yang, Yu-Hai Wang, Department of Neurosurgery, Joint Logistics Support Unit No. 904 Hospital, Wuxi 214044, Jiangsu Province, China
Wei-Qi Bao, Jing-Jie Ge, Chuan-Tao Zuo, PET Center, Huashan Hospital, Fudan University,Shanghai 200235, China
Zhi-Pei Ling, Xin Xu, Department of Neurosurgery, PLA General Hospital, PLA Postgraduate Medical School, Beijing 100039, China
Jie-Hui Jiang, School of Communication and Information Technology, Institute of Biomedical Engineering, Shanghai University, Shanghai 200444, China
Abstract BACKGROUND Forniceal deep brain stimulation (DBS) has been proposed as an alternative treatment for Alzheimer’s disease (AD). Previous studies on mild to moderate AD patients demonstrated improvements in cognitive functions brought about by forniceal DBS. Here, we report our longitudinal findings in one severe AD patient for whom the activities of daily living (ADL) rather than cognitive function significantly improved after 3 mo of continuous stimulation.CASE SUMMARY In 2011, a 62-year-old Chinese male with no previous history of brain injury or other neuropsychological diseases and no family history of dementia developed early symptoms of memory decline and cognitive impairment. Five years later,the symptoms had increased to the extent that they affected his daily living. He lost the ability to work as a businessman and to take care of himself. The patient was given a clinical diagnosis of probable AD and was prescribed donepezil and subsequently memantine, but no improvement in symptoms was observed. The patient then received DBS surgery. After 3 mo of continuous stimulation, the patient’s ADL score decreased from 65 points to 47 points, indicating the quality of the patient’s daily living improved distinctly. Other scores remained unchanged, suggesting no significant improvement in cognitive function. A follow-up positron emission tomography scan demonstrated perceivable increased glucose metabolism in the classical AD-related brain regions.CONCLUSION Based on this case we hypothesize that forniceal DBS may improve ADL through elevating regional glucose metabolism in the brain.
Key Words: Deep brain stimulation; Alzheimer’s disease; Fluorodeoxy glucose; Positron emission tomography; Activities of daily living; Case report
INTRODUCTION
Alzheimer’s disease (AD), the leading cause of senile dementia, is a neurodegenerative disorder that is characterized neuropathologically by excessive β-amyloid (Aβ)retention and tau-protein accumulation. Extensive synaptic dysfunction and neuronal loss are present in the late stages, leading to consequent memory deficit and cognitive impairment[1]. While therapeutic medication approaches can fail to alter the course of AD[2], deep brain stimulation (DBS), a mature surgical treatment for various neuropsychiatric disorders[3-5], has recently been proposed as an alternative treatment for AD[6].
The fornix is believed to be an important part of the Papez circuit that is responsible for multiple memory functions[7]. Memory improvements induced by forniceal DBS (f-DBS) were first unexpectedly discovered in the treatment of a patient with an eating disorder[8]. Inspired by this phenomenon, a series of phase-I and phase II clinical trials were conducted to explore the effectiveness of f-DBS for AD patients[9-13]. These studies focused on relatively mild AD, with the exclusion criteria of a Clinical Dementia Rating (CDR) greater than 1 or a Mini-Mental State Examination (MMSE) score less than 20. However, whether f-DBS could also benefit severe AD patients has not yet been investigated. To the best of our knowledge, the patient in our case is the first severe AD patient (CDR = 2 and MMSE = 1) to undergo f-DBS.
CASE PRESENTATION
Chief complaints
In 2011, a 62-year-old Chinese male with no previous history of brain injury or other neuropsychological diseases and no family history of dementia developed early symptoms of memory decline and cognitive impairment. Five years later, the symptoms had increased to the extent that they affected his daily living. He lost the ability to work as a businessman and to take care of himself.
History of present illness
The patient’s MMSE[14], Montreal Cognitive Assessment Basic (MoCA-B)[15], CDR[16],and global deterioration scale scores[17]were 1, 0, 2, and 6 points, respectively,indicating that his cognitive function was greatly impaired. The patient’s activities of daily living (ADL) score[18]was 65 points, showing that multiple domains of his daily living were affected. Cerebrospinal fluid tau, ptau, and Aβ1-42levels were all abnormal(Table 1), which was parallel with a distinctively positive [C-11] Pittsburg compound B positron emission tomography (PET) scan and an [F-18] fluorodeoxyglucose PET scan with a typical AD-like hypometabolic pattern (Figure 1A).
FINAL DIAGNOSIS
The patient was given a clinical diagnosis of probable AD according to the National Institute on Aging-Alzheimer’s Association criteria[19].
TREATMENT
The patient was prescribed donepezil 5 mgquaque nocte. However, no apparent alleviation of his symptoms was observed, even when he was subsequently prescribed donepezil 10 mgquaque nocte, and memantine 20 mgquaque die. Therefore, the neurosurgeons decided to accept him as a candidate for DBS at the fornix hoping to improve his impaired cognitive symptoms and quality of daily living. The study was approved by the Ethics Committee of the 101st Hospital of the People’s Liberation Army.
On March 1, 2017, the patient received DBS surgery after signing written informed consent. The electrodes were inserted 2 mm anterior and parallel to the vertical portion of the bilateral post-commissural fornix (Figures 1C and D). Continuous stimulation was delivered by the PINS stimulator system using the following parameters: C+, 1-and 5-, frequency = 130 Hz, voltage = 3.0 V, and pulse = 80 μs[9,12].
OUTCOME AND FOLLOW-UP
After 3 mo of continuous stimulation, the patient returned for a follow-up assessment.Interestingly, only the ADL score decreased (from 65 points) to 47 points, indicating that the quality of the patient’s daily living had improved distinctly (Tables 2 and 3).Both basic and instrumental functions were improved, especially eating meals,dressing, bathing, shopping, and clipping his own toenails, the scores for which each decreased by no fewer than 2 points. Meanwhile, the MMSE, MoCA-B, CDR, and Global Deterioration Scale scores remained unchanged, suggesting that there was no distinct improvement in cognitive function. Since the patient refused to undergo a second lumbar puncture, which he thought was quite invasive, follow-up cerebrospinal fluid tau, ptau, and Aβ1-42results were not available. However, a followup [F-18] fluorodeoxyglucose PET scan demonstrated perceivable increased glucose metabolism in the classical AD-related brain regions, including the posterior cingulate cortices, superior parietal gyri, inferior parietal gyri, supramarginal gyri, angular gyri,and bilateral precuneus (Figure 1B). Semi-quantitative analysis revealed elevation of standardized uptake value ratio in these brain regions (using the global average as a reference) (Table 1).
DISCUSSION
According to previous mild AD studies, patients receiving f-DBS seemed to have a decreased rate of deterioration or even an improvement in cognitive functions[9].However, the MMSE and MoCA-B scores of the patient in our case remained unchanged at 1 point and 0 points, respectively. Considering the patient’s severely impaired baseline cognitive status, the alterations in cognition and memory could have been concealed by the floor effect.
On the other hand, our results showed that the quality of daily living had significantly improved after 3 mo of continuous forniceal stimulation, as demonstrated by the ADL scale, which is consistent with similar findings in a previous f-DBS clinical trial[9]. Since the ADL score is associated with multiple cognitive domains, we believe that the recovery of the ADL scores occurred along with the recovery of hypometabolism in multiple cerebral cortical regions responsible for different cognitive functions[20,21]. Among the previously noted regions, the angular gyrus isassociated with calculation and financial-skill deficit[22], whereas the supramarginal gyrus is linked to object-related sensory integration and manipulation[23]. The precuneus is associated with visuomotor control, attention, and self-processing[24].These structures are also components of the default-mode network, which plays a critical role in executive function, memory, and goal-directed behavior[25]and thus in accomplishing complex daily activities.
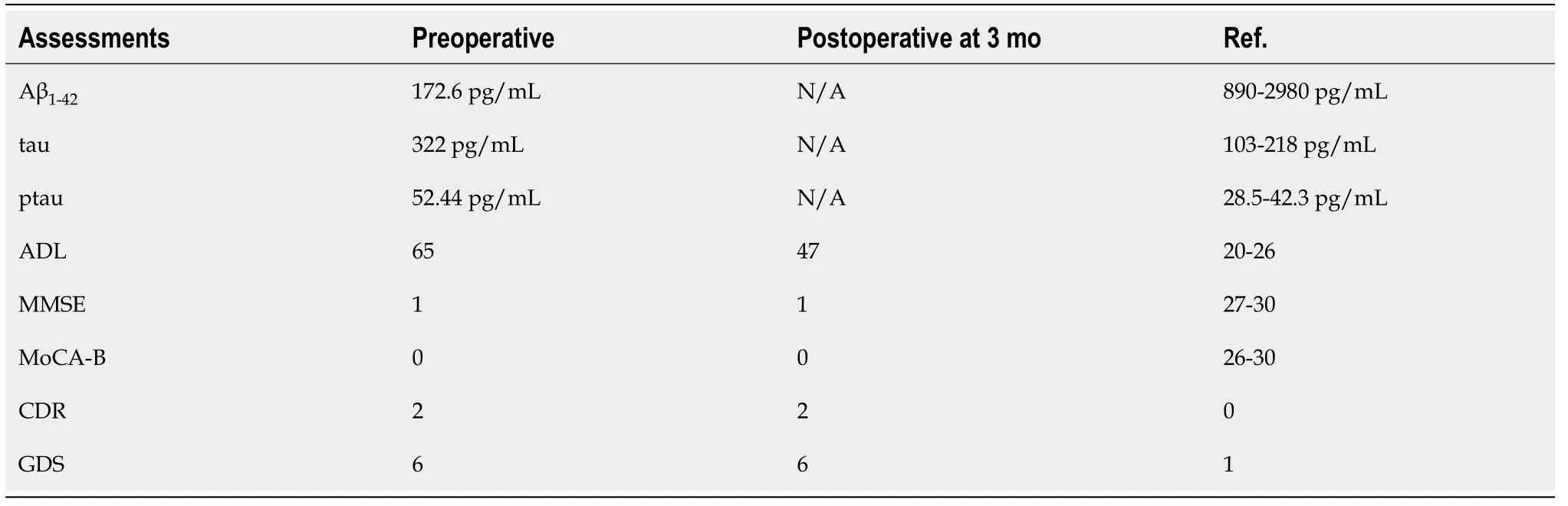
Table 1 Cerebrospinal fluid assessment results (upper three lines) and neuropsychological assessment results (lower five lines) before and after f-deep brain stimulation treatment
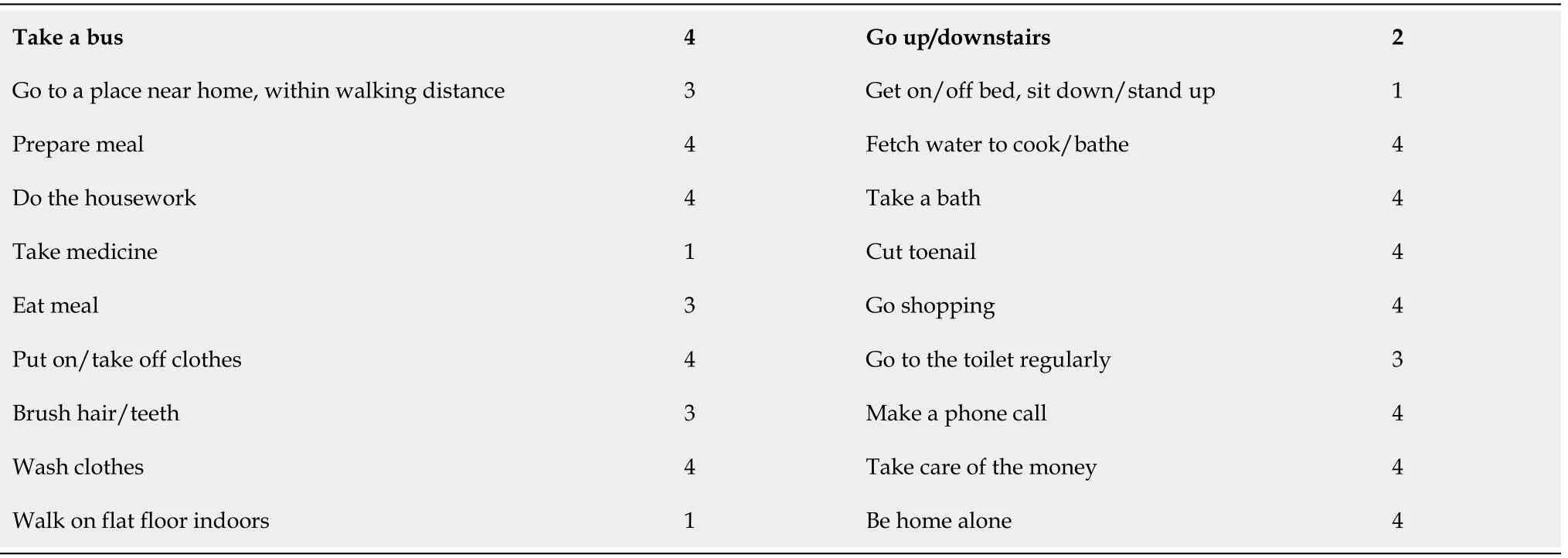
Table 2 Preoperative activities of daily living
CONCLUSION
Because the current discovery is derived from a single case observation, it is undeniable that verification in larger cohorts is required to reach a solid conclusion. It is also notable that all the previous clinical studies conducted a multi-step 1-year follow-up. Whether the alterations in clinical manifestations and the [F-18]fluorodeoxyglucose positron emission tomography of our case would be sustained after a full year of stimulation should be examined further. However, the preliminary findings in this case are promising and provide support for the future clinical application of forniceal deep brain stimulation to severe AD patients.
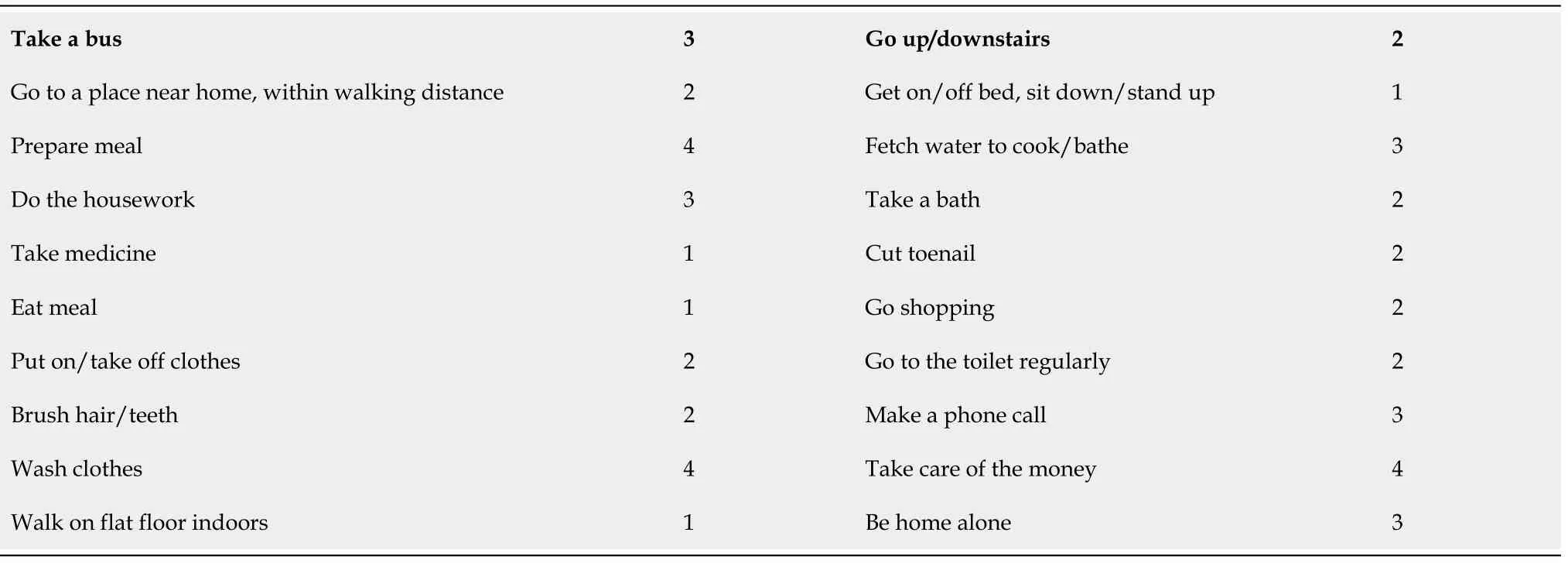
Table 3 Postoperative activities of daily living at 3 mo
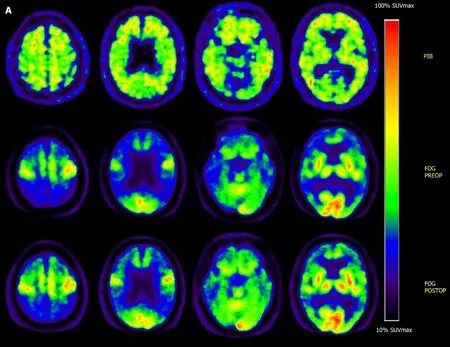
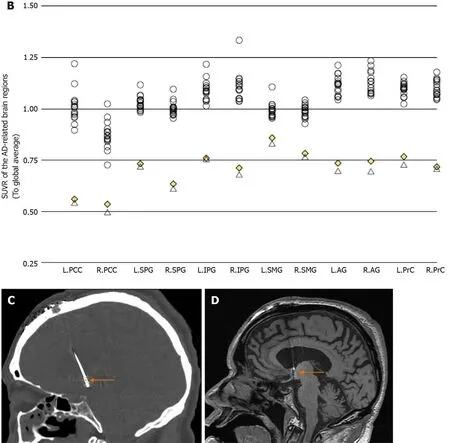
Figure 1 Positron emission tomography and regional standardized uptake value ratio. A: Positron emission tomography (PET) images of the severe Alzheimer’s disease (AD) patient who underwent forniceal deep brain stimulation. Top row: Pre-operative [C-11] Pittsburg compound B PET. Middle row: Preoperative [F-18] fluorodeoxyglucose PET: Hypometabolism is seen in typical AD-affected brain regions. Bottom row: Post-operative [F-18] fluorodeoxyglucose PET:Slight recovery of regional glucose metabolism can be seen after 3 mo of continuous f-deep brain stimulation (DBS); B: Regional standardized uptake value ratio (to global average) of 16 cognitively intact normal controls and the severe AD patient (both pre- and post-operative); C: Coronal computer tomography image (bone window), showing DBS electrode inserting into the post-commissural fornix (orange arrow); and D Coronal T1 magnetic resonance image showing DBS electrode inserting into the post-commissural fornix (orange arrow). L: Left; R: Right; PCC: Posterior cingulate cortex; SPG: Superior parietal gyrus; IPG: Inferior parietal gyrus;SMG: Supramarginal gyrus; AG: Angular gyrus; PrC: Precuneus.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Huiwei Zhang and Dr Ping Wu from PET Center,Huashan Hospital, Fudan University, Shanghai, China for their aid in image processing and Dr Jie Zhu, Dr Yi Feng, and Dr Jirong Dong from the Department of Neurosurgery, Joint Logistics Support Unit No. 904 Hospital, Wuxi, Jiangsu, China and Dr Zhiqi Mao from the Department of Neurosurgery, PLA General Hospital, PLA Postgraduate Medical School, Beijing, China for their aids in DBS surgery and post-op management.
杂志排行
World Journal of Clinical Cases的其它文章
- Effective administration of cranial drilling therapy in the treatment of fourth degree temporal, facial and upper limb burns at high altitude:A case report
- Rare imaging findings of hypersensitivity pneumonitis: A case report
- Successful management of a tooth with endodontic-periodontal lesion: A case report
- Primary chondrosarcoma of the liver: A case report
- Choriocarcinoma with lumbar muscle metastases: A case report
- Monocular posterior scleritis presenting as acute conjunctivitis: A case report
