血栓弹力图在糖尿病肾病维持性血液透析患者抗血小板药物反应性中的评估价值
2020-04-03金钦阳朱勤叶羡云
金钦阳 朱勤 叶羡云


[摘要] 目的 探讨血栓弹力图(TEG)在糖尿病肾病(DN)维持性血透患者行经皮冠状动脉介入治疗(PCI)后抗血小板药物效果的评估价值。 方法 纳入2013年5月~2016年3月于浙江省人民医院就诊,行PCI术后1年内接受阿司匹林联合氯吡格雷双抗治疗的维持性血透患者46例。按照是否为DN所致终末期肾病而开始维持性血透分为DN组(20例)及NDN组(26例),进行TEG检测。血栓最大幅度(MA)、花生四烯酸(AA)途径抑制率、二磷酸腺苷(ADP)受体抑制率评估药物抵抗及分析临床意义。 结果 DN组MA值高于NDN组,差异有统计学意义(P < 0.05)。DN组双联血小板聚集抵抗率高于NDN组,差异有统计学意义(P < 0.05)。两組患者发生阿司匹林抵抗率、氯吡格雷抵抗率比较,差异无统计学意义(P > 0.05)。 结论 与NDN患者比较,DN患者具有更高的PCI术后血栓风险。DN及NDN患者均存在对双联抗血小板药物抵抗,DN患者为甚,提示在此类高危患者可能需要常规进行血小板功能检测以指导调整药物。
[关键词] 糖尿病肾病;血透;血栓弹力图;抗血小板治疗
[中图分类号] R459.5 [文献标识码] A [文章编号] 1673-7210(2020)02(a)-0094-04
[Abstract] Objective To explore the value of thromboelastography (TEG) in evaluating the effect of antiplatelet drugs after percutaneous coronary intervention (PCI) in patients with diabetic nephropathy (DN) maintenance hemodialysis. Methods A total of 46 patients with maintenance hemodialysis who were treated at the People′s Hospital of Zhejiang Province from May 2013 to March 2016 and received aspirin combined with clopidogrel within 1 year after PCI were included. According to whether end-stage renal disease caused by DN started maintenance hemodialysis, all patients were divided into DN group (20 cases) and NDN group (26 cases), and TEG test was performed. The maximum thrombus (MA), arachidonic acid (AA) pathway inhibition rate, and adenosine diphosphate (ADP) receptor inhibition rate were used to evaluate drug resistance and analyze clinical significance. Results The MA value in DN group was higher than that in group B, and the difference was statistically significant (P < 0.05). The resistance rate of double platelet aggregation in DN group was higher than that in NDN group, and the difference was statistically significant (P < 0.05). There were no significant differences in the aspirin resistance rate and clopidogrel resistance rate between the two groups of patients (P < 0.05). Conclusion Patients with DN have a higher risk of thrombosis after PCI compared with NDN patient. Both DN and NDN patients have resistance to dual antiplatelet drugs, and patients with DN are very resistant, suggesting that platelet function to guide the adjustment of the drug may be required in such high-risk patients.
[Key words] Diabetic kidney disease; Hemodialysis; Thrombelastography; Antiplatelet therapy
2013年,我国成人糖尿病患病率为10.9%[1]。而糖尿病肾病(DN)是糖尿病常见而严重的慢性并发症,DN患者占糖尿病总数的67%以上[2]。作为等危症,无论糖尿病或者慢性肾病均相似程度地增加了患者远期心血管病事件风险[3]。血栓弹力图(TEG)可以对包括血小板聚集等凝血全过程变化进行动态监测,在抗栓治疗、评估血小板活性和观察抗血小板聚集效果等方面起到重要的作用[4-6]。本研究通过TEG评估DN血透患者行经皮冠状动脉介入治疗(PCI)后应用双联抗血小板药物反应性,为DN患者术后抗血小板药物的监测和选择提供参考。
1 资料与方法
1.1 一般资料
选择2013年5月~2016年3月于浙江省人民医院就诊,行PCI术后的维持性血透患者共46例,根据是否为DN所致终末期肾病而开始维持性血透,分为DN组(20例)及NDN组(26例)。其中因急性冠脉综合征(ACS)行直接PCI术后患者27例,择期PCI术后患者19例。纳入标准:①患者维持性血透6个月以上;②PCI后1年内且接受双联抗血小板治疗:阿司匹林治疗(德国拜耳制药,批号:BJ37565)100 mg/d+氯吡格雷(法国赛诺菲公司,批号:7A373)75 mg/d,1周以上;③年龄20~75岁;④签署知情同意书。排除标准:①对阿司匹林、氯吡格雷有禁忌證;②纳入1周内使用过糖蛋白Ⅱb/Ⅲa受体拮抗剂及其他具有活血化瘀作用的中草药物及保健品;③应用其他抗血小板及华法林等药物;④血小板计数<100×106 μL,活动性出血及严重凝血异常;⑤妊娠、癌症、严重肝病。
1.2 方法
患者均在血透上机前抽取静脉血,2 h内完成TEG检测。TEG方法:TEG凝血分析仪5000型(美国Haemoscope),使用4个通道进行检测:①高岭土(1% kaolin液);②激活剂F(蝮蛇血凝酶和Xa因子混合而成);③激活剂F+花生四烯酸(AA);④激活剂F+二磷酸腺苷(ADP)。每个通道均由TEG软件根据测试结果自动计算血栓最大幅度(MA)值,包括MA凝血酶(MAThrombin)、MAADP和MA纤维蛋白(MAFibrin)。阿司匹林的血小板聚集抑制率(%)=(MAAA-MAFibrin)/(MAThrombin-MAFibrin)×100%,ADP受体拮抗剂的血小板聚集抑制率(%)=(MAAA-MAFibrin)/(MAThrombin-MAFibrin)×100%。即计算两组患者服用药物后血小板聚集抑制情况。
1.3 药物敏感度判定标准
阿司匹林抵抗[7]:通过TEG方法检测AA诱导的血小板抑制率≤50%;氯吡格雷抵抗[8]:以ADP诱导的血小板抑制率<30%。
1.4 统计学方法
运用SPSS 19.0对所得数据进行统计分析。正态分布计量资料以均数±标准差(x±s)表示,采用t检验。非正态分布计量资料以中位数(四分位数)[M(P25,P75)]表示,采用Wilcoxon秩和检验。计数资料以例数或百分比表示,采用χ2检验。以P < 0.05为差异有统计学意义。
2 结果
2.1 两组患者基线资料比较
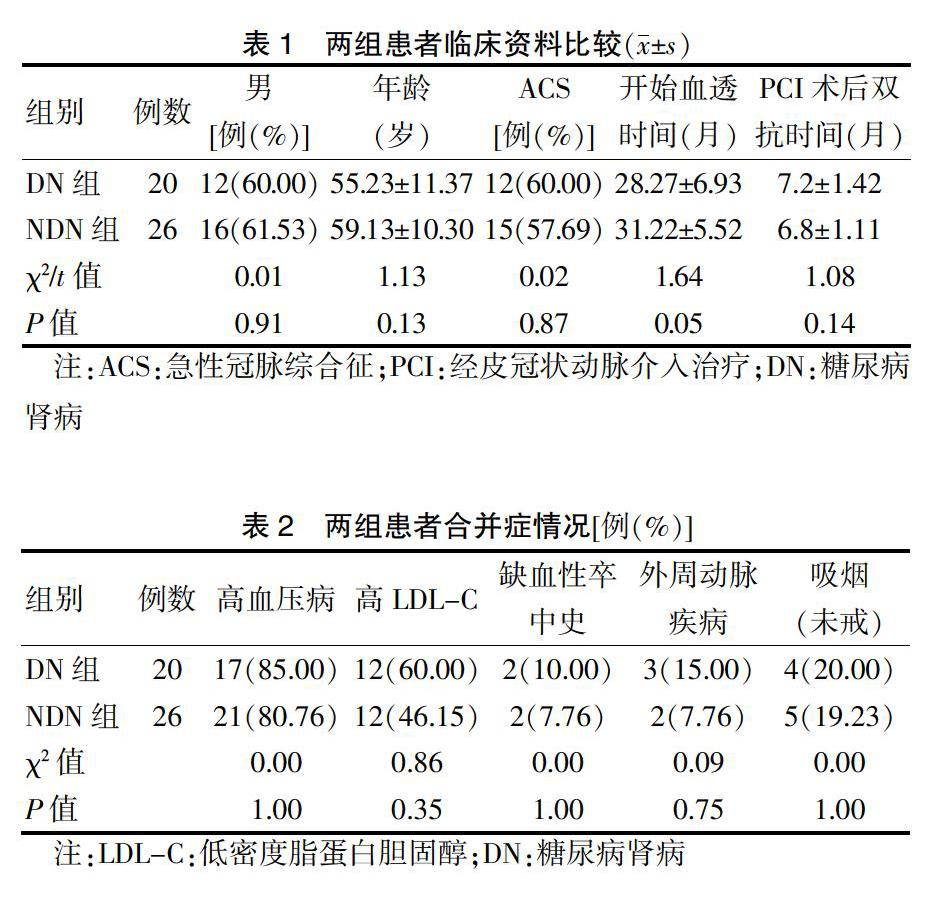
除胰岛素用药,两组患者基线资料比较,差异无统计学意义(P > 0.05),具有可比性。见表1~3。
2.2 两组患者接受抗血小板治疗后MA值比较
DN组MA值显著高于NDN组,差异有统计学意义(P = 0.02)。见图1。
2.3 两组患者抗血小板药物效果比较
两组患者发生阿司匹林抵抗率、氯吡格雷抵抗率比较,差异无统计学意义(P > 0.05)。DN组阿司匹林抵抗及氯吡格雷抵抗显著高于NDN组,差异有统计学意义(P < 0.05)。见表4。
3 讨论
慢性肾病(CKD)是冠心病的等危症,且是高危人群发生心血管疾病的独立危险因素[9]。糖尿病同时合并慢性肾病会进一步增加心血管病事件发生风险[10]。已有临床研究显示[6,10-11]:MA值的升高与冠心病合并糖尿病、PCI术后冠脉缺血事件的发生有关。本研究结果显示,DN组患者MA值高于NDN组。当MA值>68 mm时,患者术后出现血栓事件的比例大大增加[12],提示DN血透患者在PCI术后存在更高的血栓及缺血事件风险。而糖尿病患者血小板功能的改变可能部分由血糖升高所介导。空腹血糖是血小板依赖性冠状动脉血栓形成的独立预测因子[13]。阿司匹林联合一种P2Y12受体拮抗剂的双联抗血小板治疗是目前PCI治疗后的标准抗栓方案。但既往循证研究多数剔除了严重肾功能不全及血透患者。但CKD和/或糖尿病患者PCI后栓塞事件及死亡却时有发生。目前的观点认为,抗栓治疗下的血小板高反应性现象是原因之一[14-15]。然而,多项血小板功能检测指导抗血小板治疗的随机对照研究均为阴性结论,这些研究有一个共同的缺陷在于纳入的研究主要是低心血管风险患者[16]。本研究纳入对象均为可能出现血小板高反应性(HTPR)现象的心血管事件极高危患者,发现两组血透患者中均存在双抗治疗低反应者,而DN组存在更多的HTPR现象。可能因样本量的限制,两组血透患者在阿司匹林及氯吡格雷的TEG检测比较,差异无统计学意义,但两种药物均存在相当比例的低反应患者,尤其是在DN组中氯吡格雷有40%患者存在抵抗现象。提示此类患者可能需要接受血小板功能监测从而发现HTPR及调整治疗。在目前的临床实践中,阿司匹林抵抗仍是存在争议的话题。但因其在支架术后抗血小板治疗的地位不能轻易被其他药物替代或超越,阿司匹林抵抗也更多是以血栓事件的发生而确定。而氯吡格雷在近20年使用总结发现,治疗反应的多样性与基因(CYP2C19)、细胞代谢(P450)及临床合并症(如糖尿病)等因素有关,导致了抗血小板治疗中的HTPR现象,确实造成了临床血栓事件的增加,尤其是冠脉支架内血栓形成[17]。近年来的研究又陆续发现,在肾功能不全的患者中治疗应答差,即使倍量的氯吡格雷也无明显改善[18-19]。目前国外已报道可能有效地调整策略为氯吡格雷换用新型抗血小板药物,如普拉格雷[19]及替格瑞洛[20],较氯吡格雷有更好的血小板抑制作用,且与CYP2C19*2无关。
总之,本研究发现DN组较NDN组有更高的PCI术后血栓风险;两组患者中均存在对双两联抗血小板药物的抵抗,DN患者为甚,提示此类患者可能需要常规进行血小板功能检测以指导调整药物。
[参考文献]
[1] Wang L,Gao P,Zhang M,et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013 [J]. JAMA,2017,317(24):2515-2523.
[2] Lin X,Tao L,Tang D. Gene therapy,a targeted treatment for diabetic kidney disease [J]. Curr Med Chem,2013,20(30):3774-3784.
[3] Saely C,Zanolin D,Vonbank A,et al. Impaired kidney function is a diabetes risk equivalent in patients with established coronary artery disease [J]. J Am Coll Cardiol,2015, 65(10):A1504.
[4] Swallow RA,Agarwala RA,Dawkins KD,et al. Thromboelastography:potential bedside tool to assess the effects of antiplatelet therapy? [J]. Platelets,2006,17(6):385-392.
[5] 王麗丽,李群,康林,等.应用血栓弹力图评估ACS患者替格瑞洛与氯吡格雷抗血小板的疗效[J].中国循证心血管医学杂志,2014,6(3):281-284.
[6] McCrath DJ,Cerboni E,Frumento RJ,et al. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction [J]. Anesth Analg,2005,100(6):1576-1583.
[7] Tantry US,Bliden KP,Gurbel PA. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation [J]. J Ame Coll Cardiol,2005,46(9):1705-1709.
[8] Bliden KP,Tantry U,Zaman K,et al. High platelet reactivity is a risk factor for post-discharge ischemic complications following elective coronary stenting [J]. J Am Coll Cardiol,2005,45:33A-34A.
[9] Sarnak MJ,Levey AS,Schoolwerth AC,et al. Kidney disease as a risk factor for development of cardiovascular disease:a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease,High Blood Pressure Research,Clinical Cardiology,and Epidemiology and Prevention [J]. Hypertension,2003,42(5):1050-1065.
[10] Gurbel PA,Bliden KP,Navickas IA,et al. Adenosine diphosphate-induced platelet-fibrin clot strength:a new thrombelastographic indicator of long-term poststenting ischemic events [J]. Am Heart J,2010,160(2):346-354.
[11] Rafiq S,Johansson PI,Zacho M,et al. Thrombelastographic haemostatic status and antiplatelet therapy after coronary artery bypass surgery(TEG-CABG trial):assessing and monitoring the antithrombotic effect of clopidogrel and aspirin versus aspirin alone in hypercoagulable patients:study protocol for a randomized controlled trial [J]. Trials,2012,13:48.
[12] 王媛媛,李月红,吴英凤,等.血栓弹力图中血栓最大幅度值与急性冠状动脉综合征患者冠状动脉血栓病变的关系[J].中国循环杂志,2016,31(11):1069-1073.
[13] Shechter M,Merz CN,Paul-Labrador MJ,et al. Blood glucose and platelet-dependent thrombosis in patients with coronary artery disease [J]. J Am Coll Cardiol,2000, 35(2):300-307.
[14] Alexopoulos D,Panagiotou A,Xanthopoulou I,et al. Antiplatelet effects of prasugrel vs. double clopidogrel in patients on hemodialysis and with high on-treatment platelet reactivity [J]. J Thromb Haemost,2011,9(12):2379-2385.
[15] Franchi F,Rollini F,Angiolillo DJ. Defining the Link Between Chronic Kidney Disease,High Platelet Reactivity,and Clinical Outcomes in Clopidogrel-Treated Patients Undergoing Percutaneous Coronary Intervention [J]. Circ Cardiovasc Interv,2015,8(6):e002760.
[16] Franchi F,Rollini F,Cho JR,et al. Platelet function testing in contemporary clinical and interventional practice [J]. Curr Treat Options Cardiovasc Med,2014,16(5):300.
[17] Stone GW,Witzenbichler B,Weisz G,et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES):a pro-spective multicentre registry study [J]. Lancet,2013,382(9892):614.
[18] Konishi A,Shinke T,Otake H,et al. Impact of residual platelet reactivity under clopidogrel treatment for lesions and the clinical outcome after drug-eluting stent implantation in patients with hemodialysis [J]. J Cardiol,2016,67(6):531-537.
[19] Alexopoulos D,Panagiotou A,Xanthopoulou I,et al. Antiplatelet effects of prasugrel vs. double clopidogrel in patients on hemodialysis and with high on-treatment platelet reactivity [J]. J Thromb Haemost,2011,9(12):2379-2385.
[20] Jeong KH,Cho JH,Woo JS,et al. Platelet reactivity after receiving clopidogrel compared with ticagrelor in patients with kidney failure treated with hemodialysis:a randomized crossover study [J]. Am J Kidney Dis,2015,65(6):916-924.
(收稿日期:2019-08-28 本文編辑:王晓晔)
