基于AMPK/GLUT4/GSK3β/PPARα信号通路研究地骨皮水提物改善2型糖尿病大鼠胰岛素抵抗的实验研究
2020-04-02姚欢欢陈吉陈思思周迪夷
姚欢欢 陈吉 陈思思 周迪夷
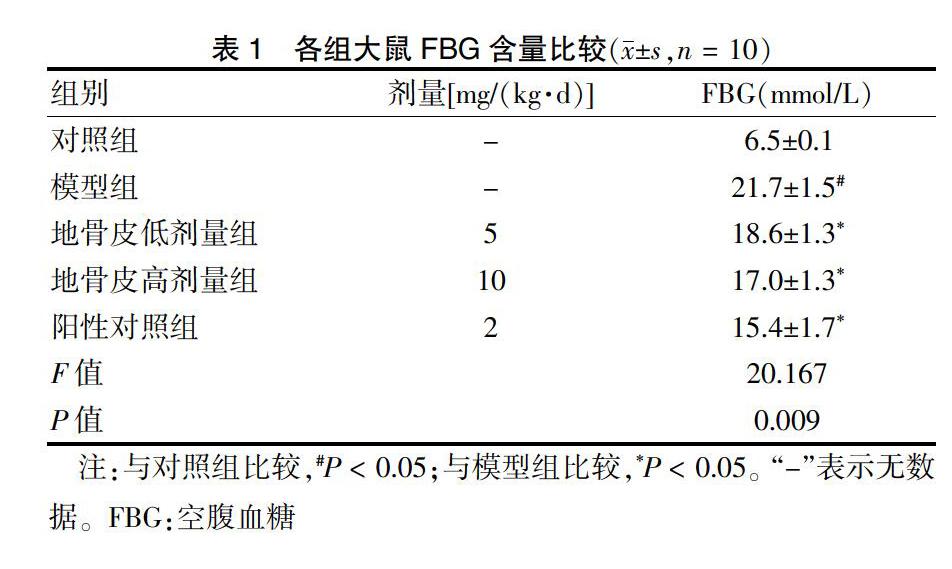
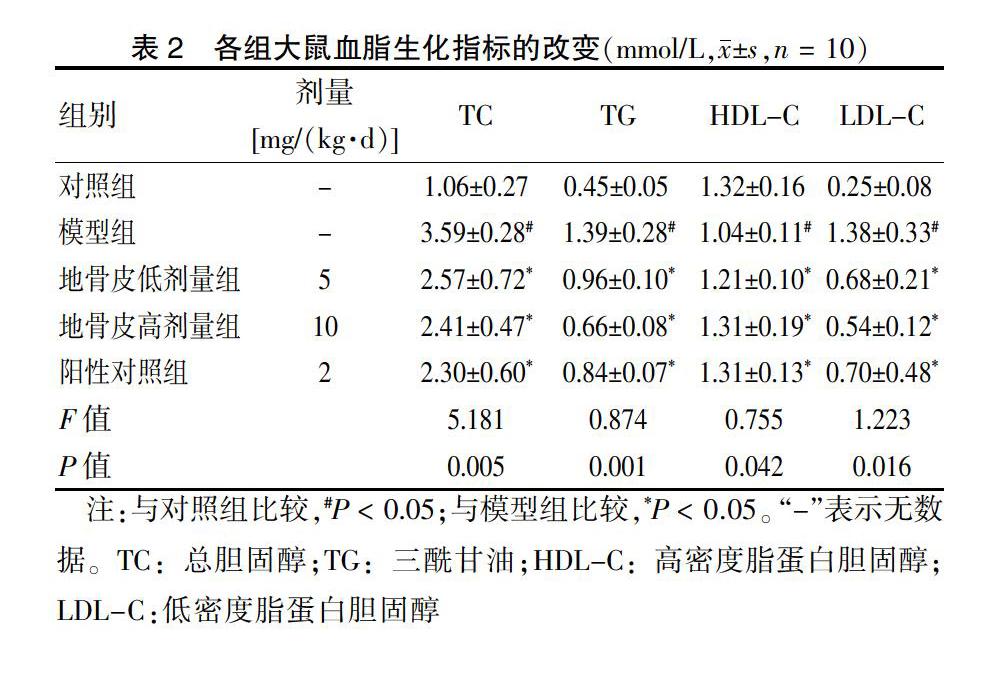
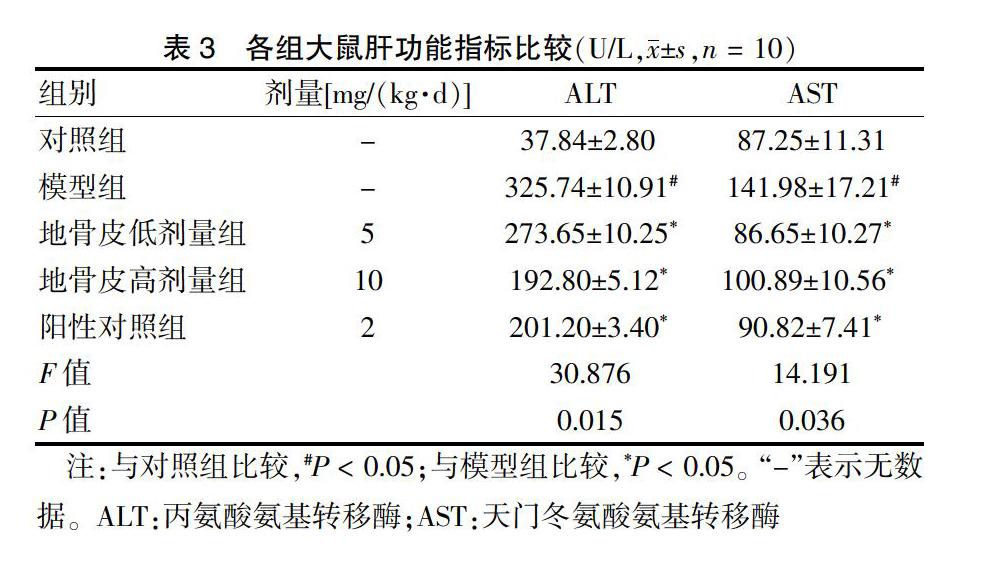
[摘要] 目的 基于AMPK/GLUT4/GSK3β/PPARα信號通路探讨地骨皮对2型糖尿病(T2DM)大鼠胰岛素抵抗(IR)的影响。 方法 60只6周龄Wistar大鼠按随机数字表法分为对照组(n = 10)和高脂实验组(n = 50),采用随机数字表法将高脂实验组中造模成功且符合模型要求的40只大鼠分为模型组,阳性对照组[罗格列酮2 mg/(kg·d)],地骨皮低剂量组[5 mg/(kg·d)]、高剂量组[10 mg/(kg·d)],每组10只。连续给药6周后,比较各组大鼠空腹血糖(FBG)、总胆固醇(TC)、三酰甘油(TG)、低密度脂蛋白胆固醇(LDL-C)、高密度脂蛋白胆固醇(HDL-C)、天门冬氨酸氨基转移酶(AST)、丙氨酸氨基转移酶(ALT)水平,并观察肝脏形态。BCA法测定肝脏中蛋白质质量浓度。结果 模型组大鼠FBG高于对照组,地骨皮低、高剂量组FBG含量均显著低于模型组,差异有统计学意义(均P < 0.05)。模型组TC、TG、LDL-C水平高于对照组,HDL-C水平低于对照组,差异均有统计学意义(均P < 0.05);干预6周后,地骨皮低、高剂量组TC、TG、LDL-C水平低于模型组,HDL-C水平高于模型组,差异均有统计学意义(均P < 0.05)。模型组ALT、AST水平高于对照组,干预6周后,地骨皮低、高剂量组ALT、AST水平低于模型组,差异均有统计学意义(均P < 0.05)。苏木精-伊红染色显示:对照组肝小叶结构清晰;模型组肝细胞排列紊乱,脂质空泡明显可见;地骨皮高剂量组大鼠的肝脂肪空泡明显减少,肝细胞结构病变和脂肪变性程度显著改善。油红O染色显示:模型组大鼠的肝脏有明显累积的脂质液滴,地骨皮高剂量组脂质累积减少。模型组大鼠肝脏中p-AMPK、PPARα和GLUT4的水平低于对照组,GSK3β水平高于对照组,差异均有统计学意义(均P < 0.05);地骨皮高剂量组p-AMPK、PPARα和GLUT4蛋白水平上调显著,GSK3β水平显著下调(P < 0.05)。 结论 地骨皮可显著降低T2DM大鼠的FBG,改善血脂异常和肝脏病理变化,其可能通过AMPK信号通路途径上调GLUT4和PPARα蛋白表达水平,下调GSK3β蛋白表达来改善IR。
[关键词] 地骨皮;2型糖尿病模型大鼠;胰岛素抵抗;磷酸腺苷活化蛋白激酶
[中图分类号] R285 [文献标识码] A [文章编号] 1673-7210(2020)02(b)-0008-05
Experimental study on the improvement of insulin resistance in rats with type 2 diabetes mellitus with Cortex Lycii Radicis based on AMPK/GLUT4/GSK3β/ PPARα signaling pathway
YAO Huanhuan1 CHEN Ji1 CHEN Sisi1 ZHOU Diyi2
1.Department of Pharmacy, Huzhou Third People′s Hospital, Zhejiang Province, Huzhou 313002, China; 2.Department of Endocrinology, Zhejiang Hospital of Integrated Traditional Chinese and Western Medicine, Zhejiang Province, Hangzhou 310003, China
[Abstract] Objective To study on the improvement of insulin resistance (IR) in rats with type 2 diabetes mellitus (T2DM) was conducted based on AMPK/GLUT4/GSK3β/PPARα signaling pathway. Methods Sixty Wistar rats were divided into control group and high-fat experimental group (n = 50), and 40 rats which were successfully modeled and met the requirements of the model in the high-fat experimental group were divided into, model group, positive control group [Rosiglitazone 2 mg/(kg·d)], Cortex Lycii Radicis low dose group [5 mg/(kg·d)] and high dose group [10 mg/(kg·d)] by the random number table method, with 10 rats in each group. Each groups were given continuous administration for 6 weeks. Fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), aminotransferase (AST) and alanine aminotransferase (ALT) levels of rats in each group were compared and liver morphology was observed. Protein mass concentration in liver was determined by BCA method. Results FBG levels in model group were higher than those in control group, while FBG levels in Cortex Lycii Radicis low dose group and Cortex Lycii Radicis high dose group were significantly lower than those in model group, with statistically significant differences (all P < 0.05). The levels of TC, TG and LDL-C in model group were higher than those in control group, while the levels of HDL-C were lower than those in control group, with statistically significant differences (all P < 0.05). After 6 weeks of intervention, the levels of TC, TG and LDL-C in Cortex Lycii Radicis low dose group and Cortex Lycii Radicis high dose group were lower than those in model group, while the levels of HDL-C were higher than those in model group, with statistically significant differences (all P < 0.05). The levels of ALT and AST in model group were higher than those in control group. After 6 weeks of intervention, the levels of ALT and AST in Cortex Lycii Radicis low dose group and Cortex Lycii Radicis high dose group were lower than those in model group, with statistically significant differences (all P < 0.05). HE staining showed clear hepatic lobule structure in the control group. In model group, the arrangement of hepatocytes was disordered and lipid vacuoles were obvious. The hepatic fat vacuoles of rats in Cortex Lycii Radicis high dose group were significantly reduced and the structural changes of hepatocytes and steatosis were significantly improved. Oil red O staining showed liver was significant accumulation of lipid droplets in the liver of rats in model group and was decreased lipid deposition in Cortex Lycii Radicis high dose group. The levels of p-AMPK, PPARα and GLUT4 of liver in model group were lower than those in control group, and the GSK3β levels were higher than those in control group, with statistically significant differences (all P < 0.05). The levels of p-AMPK, PPARα and GLUT4 of liver were significantly up-regulated and GSK3 levels were significantly down-regulated in Cortex Lycii Radicis high dose group (P < 0.05). Conclusion The FBG of T2DM rats can be significantly reduced by Cortex Lycii Radicis, improve dyslipidemia and liver pathological changes. It may up-regulate the expression of GLUT4 and PPARα protein and down-regulate the over-expression of GSK3β protein through AMPK signaling pathway to improve IR.
[Key words] Cortex Lycii Radicis; Type 2 diabetes mellitus model rats; Insulin resistance; AMP-activated protein kinase
我国为全球排名首位的糖尿病大国[1-2]。2型糖尿病(T2DM)的主要发病机制是胰岛素抵抗(IR),改善IR是治疗T2DM的一个主要目标。目前临床上治疗T2DM的药物主要是口服降糖药及胰岛素制剂[3-5],但其并不能全面有效改善T2DM及其他并发症[6]。因此,从天然药物中寻找能够改善IR的药物,具有重要的临床意义。
地骨皮为茄科落叶灌木枸杞(Ly cium Chinense Mill.)或宁夏枸杞(Lycium Barbarum L.)的干燥根皮。现代药理学研究显示[7-10],地骨皮具有降血糖、调血脂、改善IR的作用。本实验通过建立T2DM大鼠模型研究地骨皮水提物对AMPK/GLUT4/GSK3β/PPARα蛋白表达的影响[11-12],地骨皮的肝脏毒性和安全性,探讨地骨皮改善IR的可能机制。
1 对象与方法
1.1 实验动物
6周龄Wistar大鼠60只,雄性,SPF级,体重(160±20)g。购自上海斯莱克动物实验有限公司,实验动物生产许可证号SCXK(沪)2017-0015,实验动物合格证号11400700346241。实验动物的饲养、造模、给药、取材方案均取得浙江中医药大学科研伦理委员会批准。动物饲养于动物实验研究中心,SPF饲养环境:室温22℃,相对湿度65%,昼夜交替时间12 h,自由摄食和饮水。
1.2 主要仪器与试剂
地骨皮(浙江中医药大学中药饮片有限公司,批号:180101);链脲佐菌素(STZ)(CALBIOCHEM公司,Cat#5762201、Lot#B56981);高脂饲料和普通饲料(江苏协同生物科技有限责任公司);总胆固醇(TC)生化试剂盒(Lot#5115250)、三酰甘油(TG)生化试剂盒(Lot#5110359)、低密度脂蛋白胆固醇(LDL-C)测定试剂盒(Lot#11334601)、高密度脂蛋白胆固醇(HDL-C)测定试剂盒(Lot#1162018)、天门冬氨酸氨基转移酶(AST)测定试剂盒(Lot#1130191)、丙氨酸氨基转移酶(ALT)测定试剂盒(Lot#1170052),均購自南京建成生物科技有限公司;p-AMPK抗体(Lot#2531)、AMPK抗体(Lot#5832)、GSK3β抗体(Lot#2531)、GAPDH抗体(Lot#75581),均购自Cell Signaling Technology公司;PPARα抗体(Abclonal公司,Lot#6951);GLUT4抗体(Santa Cruz 公司,Lot#0006);简易血糖仪、血糖试纸(美国罗氏公司,型号:卓越型);低温高速离心机(美国Beckman公司,型号:Beckman 64R);Multiskan Sky 全波长酶标仪(美国Thermo Fisher Scientific公司);日立全自动生化分析仪(HITA-CHI Automatic Analyzer,型号:7020);光学显微镜(日本Olympus公司,型号:BX53)。
1.3 地骨皮水提液的制备
地骨皮加水煎煮3次,加水量分别为生药体积的10倍量、10倍量及8倍量,煎煮时间分别为1.5、1.5 h 及1 h,合并滤液,浓缩至每毫升含生药3 g,4℃保存。
1.4 实验分组与处理
1.4.1 T2DM大鼠模型的建立 60只大鼠按随机数字表法分成对照组(n = 10)和高脂实验组(n = 50)。对照组饲以普通饲料,高脂实验组饲以高脂饲料。4周后高脂实验组大鼠尾静脉注射STZ 30 mg/kg,诱导T2DM模型。注射STZ 72 h和2周后,分别测定空腹血糖(FBG)含量,2次FBG超过11.1 mmol/L视为造模成功[13]。
1.4.2 分组及给药 剔除不符合模型要求的10只大鼠,将造模成功的40只大鼠按随机数字表法分为4组,每组10只,分别为模型组、地骨皮低剂量组[5 mg/(kg·d)]、地骨皮高剂量组[10 mg/(kg·d)]、阳性对照组[罗格列酮2 mg/(kg·d)]。给药组每日灌胃给药1次,模型组灌服等体积生理盐水,给药6周。
实验第6周末,大鼠禁食8 h,麻醉后腹主动脉采血,3500 r/min离心15 min,离心半径22 cm,取血清,-20℃保存。取血完成后迅速分离肝脏,一部分冻结在液氮中,其余存储于甲醛(山东科源制药,批号:20180401)中。
1.5 观察指标
1.5.1 FBG含量检测 实验第6周末,各组大鼠禁食8 h,尾静脉采血检测。
1.5.2 血清生化指标检测 各组大鼠血清二次离心后取上清液,全自动生化分析仪检测ALT、AST、TC、TG、HDL-C和LDL-C水平。
1.5.3 肝功能指标检测 各组大鼠血清二次离心后取上清液,全自动生化分析仪检ALT、AST水平。
1.5.4 形态学观察 取各组大鼠肝脏,常规苏木精-伊红(HE)染色和常规油红O染色,观察地骨皮水提物对T2DM大鼠肝脏病理改变和脂滴累积的影响。
1.5.5 蛋白表达水平检测 BCA法测定肝脏中蛋白质质量浓度,Image J(1.51j8)软件进行相关蛋白的半定量分析。
1.6统计学方法
采用SPSS 20.0统计学软件进行数据分析,计量资料用均数±标准差(x±s)表示,多组间比较采用单因素方差分析,组间两两比较采用LSD-t检验。以P < 0.05为差异有统计学意义。
[9] 卫琮玲,石渊渊,任艳彩,等.地骨皮的降血糖机制研究[J].中草药,2005,36(7):1050-1052.
[10] 周晶,孟林,黄建安,等.地骨皮对四氧嘧啶糖尿病小鼠的降糖作用[J].中成藥,2001,23(6):424-425.
[11] Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome [J]. Nature,2001,414(6865):821-827.
[12] 王薪宁,徐斌,周金培,等.基于新靶点的抗糖尿病药物研究进展[J].中国药科大学学报,2015,46(2):141-152.
[13] Huang JX,Chen ZS,Zhang Y,et al. Establishment of rat model of type 2 diabetes complicated with hypertension [J]. Zhongguo Ying Yong Sheng Li Xue Za Zhi,2017,33(4):329-333.
[14] Goldberg IJ. Clinical review 124:Diabetic dyslipidemia:causes and consequences [J]. J Clin Endocrinol Metab,2001,86(3):965-971.
[15] 卓雅芬,孙志纯.沙格列汀与利拉鲁肽治疗初发肥胖型2型糖尿病的效果[J].中外医学研究,2019,17(35):54-56.
[16] 付顺昆,顾燕红,乔青燕,等.碳酸镧诱导人骨骼肌细胞胰岛素抵抗模型构建[J].临床和实验医学杂志,2018, 17(6):583-586.
[17] 高雪,安至超,何其英,等.高脂饲料喂养时间对2型糖尿病肾病大鼠模型的影响[J].中国实验动物学报,2018, 26(1):114-119.
[18] 万芳,曹玲玲,孙斐,等.2型糖尿病患者25羟维生素D与胰岛素抵抗的相关性研究[J].中国现代医生,2018, 56(33):44-46.
[19] 黄彩艳,谢冠聪,陆文松,等.糖尿病前期合并肥胖患者血脂水平与胰岛素抵抗的关系[J].中国医药科学,2018, 8(10):214-216.
[20] 李慕白,陈靖馨,王婷婷,等.多囊卵巢综合征子宫内膜胰岛素抵抗的研究进展[J].中国医药导报,2019,16(29):49-52.
[21] 张瀚丹,宋天章,杨柳萌,等.葡萄糖代谢异常导致SIVmac239感染北平顶猴急性期体重变化[J].中国实验动物学报,2018,26(6):693-699.
[22] 赵水平.高密度脂蛋白的研究现状[J].中国动脉硬化杂志,2005,13(6):673-675.
[23] Xiao B,Sanders MJ,Underwood E,et al. Structure of mammalian AMPK and its regulation by ADP [J]. Nature,2011,472(7342):230-233.
[24] Kahn SE,Hull RL,Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes [J]. Nature,2006,444(7121):840-846.
[25] Szrejder M,Piwkowska A. AMPK signaling:Implications for podocyte biology in diabetic nephropathy [J]. Biol Cell,2019,111(5):109-120.
[26] 苏椿淋,徐雯,陈敏.睾酮对胰岛素诱导的肝细胞糖原合成和Akt/GSK3β磷酸化水平的影响[J].生殖与避孕,2016,36(6):439-445.
[27] Otunctemur A,Besiroglu H,Dursun M,et al. The comparison of GLUT-4 and nNOS expression in diabetic and non-diabetic patients with BPH/LUTS [J]. Int Urol Nephrol,2015,47(6):899-904.
(收稿日期:2019-09-02 本文编辑:刘明玉)
