Role of spleen tyrosine kinase in liver diseases
2020-03-23DhadhangWahyuKurniawanGertStormJaiPrakashRuchiBansal
Dhadhang Wahyu Kurniawan, Gert Storm, Jai Prakash, Ruchi Bansal
Abstract Spleen tyrosine kinase (SYK), a non-receptor tyrosine kinase, is expressed in most hematopoietic cells and non-hematopoietic cells and play a crucial role in both immune and non-immune biological responses. SYK mediate diverse cellular responses via an immune-receptor tyrosine-based activation motifs (ITAMs)-dependent signalling pathways, ITAMs-independent and ITAMs-semidependent signalling pathways. In liver, SYK expression has been observed in parenchymal (hepatocytes) and non-parenchymal cells (hepatic stellate cells and Kupffer cells) and found to be positively correlated with the disease severity. The implication of SYK pathway has been reported in different liver diseases including liver fibrosis, viral hepatitis, alcoholic liver disease, non-alcoholic steatohepatitis and hepatocellular carcinoma. Antagonism of SYK pathway using kinase inhibitors have shown to attenuate the progression of liver diseases thereby suggesting SYK as a highly promising therapeutic target. This review summarizes the current understanding of SYK and its therapeutic implication in liver diseases.
Key words: Spleen tyrosine kinase; Liver diseases; Inflammation; Targeted therapeutics;Spleen tyrosine kinase inhibitors
INTRODUCTION
Spleen tyrosine kinase (SYK) is a cytoplasmic non-receptor protein tyrosine kinase(PTK) that consists of two SYK homology 2 domains (SH2) and a C-terminal tyrosine kinase domain. These domains are linked by two linker regions: Interdomain A between the two SH2 domains and interdomain B between the C-terminal SH2 domain and the kinase domain. SYK is a member of the Zeta-chain-associated protein kinase 70/SYK family of the PTKs, with the estimated molecular weight of 70 kDa[1,2](Figure 1). SYK is highly expressed in hematopoietic cells including mast cells,neutrophils, macrophages, platelets, B cells and immature T cells, and is important in signal transduction in these cells[2,3]. In Immune cells, SYK mainly functionsviainteraction of its tandem SH2 domains with immunoreceptor tyrosine-based activation motifs (ITAMs). In mast cells, SYK mediates downstream signalingviahigh-affinity IgE receptors, FcεRI and in neutrophils, macrophages, monocytes and platelets downstream signalling is mediatedviahigh affinity Igγ receptors, FcγR[4-6].SYK plays a key role in signaling downstream of the B and T cell receptors, hence also play a crucial role in early lymphocyte development[4,7-10]. Upon activation, SYK modulates downstream signaling events that drive inflammatory pathways of both the innate and adaptive immune systems[11]. Besides ITAM-dependent signalling pathway, SYK also mediates ITAM-independent signalingviaintegrins and C-type lectins. For instance, SYK induces β2 integrin-mediated respiratory burst, spreading,and site-directed migration of neutrophils towards inflammatory lesions[12].
The multifactorial role of SYK in the immune system has attracted attention in the past years. SYK is recognized as a potential target for the treatment of inflammatory diseases such as rheumatoid arthritis, asthma, allergic rhinitis, renal disorders, liver fibrosis and autoimmune diseases[3,7,13-23]. In particular, the prevention of activation of cellsviaimmune complexes or antigen-triggered Fc receptor signaling and prevention of B cell receptor-mediated events are believed to have increasing therapeutic potential of SYK[24,25].
Besides hematopoietic cells, SYK has also been shown to be expressed in nonhematopoietic cells including fibroblasts, epithelial cells, hepatocytes, neuronal cells,and vascular endothelial cells[7,26]. Here, SYK has shown to be involved in signalling steps leading to mitogen activated protein kinase activation by G-protein-coupled receptors in hepatocytes[26,27]. Besides being implicated in hepatocytes, SYK is also expressed in hepatic macrophages, hepatic stellate cells (HSCs) and hepatic sinusoidal endothelial cells in liver[28]. However, studies investigating SYK signalling pathway in liver diseases are still limited, hence this review highlights and discusses the opportunities and challenges of SYK as a potential target for the treatment of liver diseases.
SPLEEN TYROSINE KINASE SIGNALLING MECHANISMS
Immunoreceptor signalling through SYK requires the SYK kinase activity as well as both SH2 domains[29]. The SYK kinase domain is inactive in the resting state of the protein but can be activated by interaction of both SH2 domains to dual phosphorylated ITAMs[30]. Phosphorylation of tyrosine residues within the linker regions (interdomain A or B) also results in kinase activation even in the absence of phosphorylated ITAM binding[29,30]. Binding of the SH2 domains of SYK to phosphorylated ITAMs is a critical step in SYK activation and downstream signalling[31]. SYK itself can catalyse the autophosphorylation of its linker tyrosine’s,leading to sustained SYK activation after a transient ITAM phosphorylation. In addition, SYK itself can phosphorylate ITAMs, suggesting the existence of a positivefeedback loop during initial ITAM-mediated SYK activation[32]. Tsanget al[33]showed that SYK can be fully triggered by phosphorylation or binding of its SH2 domains to the dual-phosphorylated immune-receptor tyrosine based activity motif (ppITAM)(Figure 2)[33,34]. Recently, Slomiany and Slomiany demonstrated lipopolysaccharides(LPS)-induced SYK activation through protein kinase Cδ-mediates SYK phosphorylation on serine residues that is required for its recruitment to the membrane-anchored TLR4, followed by SYK subsequent activation through tyrosine phosphorylation. Hence, the intermediate phase of protein kinase Cδ-mediated SYK phosphorylation on serine residues affects the inflammatory response[35]. The activated SYK binds to a number of downstream signalling effectors and amplifies the inflammatory signal propagation by affecting transcription factors activation and their assembly to transcriptional complexes involved in proinflammatory genes expression[36].
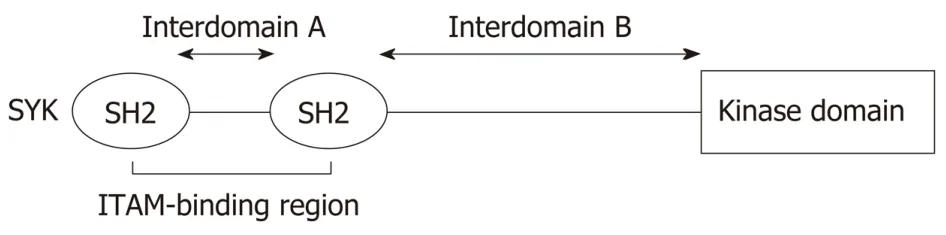
Figure 1 Structure of spleen tyrosine kinase. Spleen tyrosine kinase contains tandem pair of spleen tyrosine kinase homology 2 which connected by interdomain A and separated by interdomain B from the catalytic (kinase)domain. SYK: Spleen tyrosine kinase; SH2: Spleen tyrosine kinase homology 2; ITAM: Immune-receptor tyrosinebased activation motifs.
SPLEEN TYROSINE KINASE IN LIVER FIBROSIS
Liver fibrosis, triggered by hepatitis B/C viral infection (viral hepatitis), alcohol abuse(alcoholic liver disease) or non-alcoholic steatohepatitis (NASH)etc., is characterized by an excessive deposition of extracellular matrix (ECM) proteins[37], leading to tissue scarring that further progresses to end-stage liver cirrhosis and hepatocellular carcinoma[38].
Lately, liver fibrosis poses a major health problem accounting for more than 1 million people deaths every year worldwide because of this disease[39]. Moreover,there is no therapeutic treatment available to date[40]. The central player that produces ECM resulting in liver fibrosis is HSCs[41]. HSCs are no9rmally localized in the perisinusoidal area, termed as space of Disse, as quiescent cells in healthy liver and functions as retinoid storage cells[42]. Owing to hepatic injury, quiescent HSCs phenotypically transdifferentiate into activated, contractile, highly proliferative and ECM-producing myofibroblasts[43].
SYK has been documented to play a critical role in the activation of HSCs and its upregulation is evidenced in hepatic fibrosis/cirrhosis in hepatitis B and C patients,alcoholic hepatitis as well as in NASH patients[28,44]. Upregulated SYK further aggravate fibrosis by augmenting trans-communication between hepatocytes and HSCs[28]. Blockage of SYK pathway using SYK inhibitors abrogated HSCs activation,thereby ameliorated liver fibrosis and hepatocellular carcinoma (HCC) developmentin vivoin animal models[28]. SYK has shown to mediate its functionviaexpression of transcription factors associated with HSCs activation (cAMP response elementbinding protein, CBP; myeloblastosis proto-oncogene, MYB and myelocytomatosis proto-oncogene, MYC) and proliferation (MYC and cyclin D1, CCND1)[28].Furthermore, two isoforms of SYKi.e., the full-length SYK (L) and an alternatively spliced SYK (S) have been suggested whereby SYK (L) but not SYK (S) found to play a major role in liver fibrosis while SYK (S) has been associated with increased tumorigenicity, HCC invasiveness and metastases[28].
Interestingly, the crosstalk between SYK and Wnt (portamanteau of int and wg,wingless-related integration site) signalling pathways also mediates activation of HSCs and accumulation of immune cells at the site of fibrosis[28]. Wnt signalling has been shown to be upregulated in activated HSCs and blockade of canonical Wnt pathway by adenoviral mediated transduction of Wnt antagonist (Dickkopf-1) orviaselective inhibitors reinstates quiescent phase of HSCs in cultured cells[45,46]. In-depth investigation at a genetic level revealed overexpression of certain transcriptional factors (MYB, CBP and MYC) which plays a vital role in the activation of HSCs[47,48].Notably, both the canonical Wnt pathway and SYK has shown to regulate the expression of MYC and CBP[23,49]highlighting SYK-Wnt crosstalk during liver fibrogenesis. SYK has also shown to promote expression of several target genes including Wnt in activated macrophages in a similar manner as in HSCs and this potential crosstalk between SYK and other signalling pathways warrants further investigation. Dissection of the trans-communication between signalling pathways is of great importance in order to highlight prominent therapeutic targets to hinder inflammation and fibrogenesis. SYK is the major signalling pathway and is also shown to be expressed in recruited macrophages, besides HSCs, in the hepatic fibrosis[20,28]. Selective blocking of SYK or its deletion in macrophages has been correlated with the diminished activation of macrophages, which is indicated by a reduction in the expression of Fc gamma receptors, monocyte chemoattractant protein 1 (MCP-1), tumour necrosis factor α (TNF-α) and interleukin 6 (IL-6)[5]. In summary,activation of HSCs under the influence of SYK signalling leads to the secretion of soluble factors in the form of cytokines and chemokines. These elements not only facilitate the recruitment of resident and migrated macrophages but also arbitrates their activation to further worsen the site of fibrosis.

Figure 2 Basis of spleen tyrosine kinase activation. In the resting state, spleen tyrosine kinase is autoinhibited, because of the binding of interdomain A and interdomain B to the kinase domain. This auto-inhibited conformation can be activated by binding of the two spleen tyrosine kinase homology 2 domains to dually phosphorylated immune-receptor tyrosine-based activation motifs or by phosphorylation of linker tyrosine’s in interdomain A or B. This is a logic “OR” relationship between those two activation mechanisms. SH2: Spleen tyrosine kinase homology 2; ITAM: Immune-receptor tyrosine-based activation motifs.
SPLEEN TYROSINE KINASE IN VIRAL HEPATITIS
In the recent study, SYK expression was found to be highly induced in the liver tissues of HBV and HCV infected patients. Furthermore, markedly increased expression of SYK was observed in HCV-infected hepatocytes which in turn promoted reciprocal higher SYK expression in HSCs thereby inducing HSCs activation and disease development[28,50]. Furthermore, the preliminary study analysing gene expression profiles in Egyptian HCC patients associated with HCV,showed that SYK is one of the most up-regulated genes out of 180 of genes were up regulated[51].
HCV is also associated with B lymphocyte proliferative disorders, as evidenced by the binding of HCV to B-cell surface receptor CD81[52]. CD81 (cluster of differentiation 81, also known as TAPA1), is identified as a target of an antibody that controlled Bcell proliferation. Engagement of CD81 with HCV[53,54], leads to ezrin and radixin phosphorylation through SYK activation[55,56]. Ezrin and radixin are members of the ERM (ezrin, radixin, moesin) family of actin-binding proteins[56]. Hence, ezrin-moesinradixin proteins and SYK are important therapeutic host targets for the development HCV treatment[57].
SYK is also an important regulator and therapeutic target against HCV infection in hepatocytes[55]. SYK expression has been observed near the plasma membrane of hepatocytes in HCV-infected patients[57,58]. HCV non-structural protein 5A has been shown to physically and directly interact with SYK thereby promoting the malignant transformation of HCV-infected hepatocytes[58]. These studies suggests that the strategies blocking SYK activation before HCV-CD81 interaction, and/or modulating HCV post-entry and trafficking within target cells involving SYK, F-actin, stable microtubules and EMR proteins provide novel opportunities for the development of anti-HCV therapies[55].
SPLEEN TYROSINE KINASE IN ALCOHOLIC LIVER DISEASE
The pathogenesis of alcoholic liver disease (ALD) is multifactorial involving many complex processes including ethanol-mediated liver injury, inflammation in response to the injury, and intestinal permeability and microbiome changes[59-61]as shown in Figure 3. Alcohol and its metabolites generate reactive oxygen species (ROS) and induce hepatocyte injury through mitochondrial damage and endoplasmic reticulum(ER) stress[62-64]. Damaged hepatocytes release pro-inflammatory cytokines and chemokines resulting in recruitment and activation of immune cells. Central cell types involved in ALD progression are macrophages that have an important role in inducing liver inflammation[65]by stimulating infiltration of immune cells (mainly monocytes) and activation of Kupffer cells (KCs, resident macrophages)[59]. The early communication of hepatocyte damage is mediated by KCs through damageassociated molecular patterns released by dying hepatocytes or pathogen-associated molecular patterns including lipopolysaccharides (LPS)viapattern recognition receptors (PRRs) such as TLRs (Toll-like receptors), and NF-κB (nuclear factor kappalight-chain-enhancer of activated B cells) signalling and inflammasome activationetc.In ALD, resident and recruited macrophages in the liver are activated by TLR4 (Tolllike receptor 4) signalling pathway regulated by bacterial endotoxin (LPS) that is elevated in the portal and systemic circulation owing to increased intestinal permeability after excessive alcohol intake[66,67]. However, there are also other mechanisms that regulate macrophage activation, such as hepatocyte injury and lipid accumulation, histone acetylation in ethanol-exposed macrophages and complement system[68]. SYK plays an important role in TLR4 signalling, and SYK phosphorylation in neutrophils and monocytes has been correlated with pro-inflammatory cytokine secretion including TNF-α and MCP-1[69]. Interestingly, SYK phosphorylation has been shown to be regulated by LPS/TLR adaptor molecules MyD88/IRAKM (IL-1Rassociated kinase M)-mincle axis linking LPS-induced hepatocyte cell death with inflammation during ALD disease pathogenesis. Zhouet al[70]has shown that damaged hepatocytes releases endogenous Mincle ligand spliceosome-associated protein 130 as a danger signal that synergistically with LPS drives inflammation including inflammasome activation during ALD[70].
SPLEEN TYROSINE KINASE IN NON-ALCOHOLIC STEATOHEPATITIS
NASH is characterized by increasing accumulation of so-called toxic lipids in hepatocytes, that can develop into cirrhosis and primary liver cancer[74]. NASH is the more severe and clinically significant form of NAFLD (non-alcoholic fatty liver disease)[75], characterized by hepatic cell injury, steatosis together with inflammation,resulting into fibrosis signified by deposition of extracellular matrix mainly composed of collagen/fibrin fibrils[76]. The progression of NASH is associated with a progressive build-up of danger signals particularly PRRs including TLRs, and nucleotide oligomerization domain-like receptors (NLR)[77]that engage multiple receptors during immune response[78].

Figure 3 Role of spleen tyrosine kinase in alcoholic liver disease and non-alcoholic steatohepatitis pathogenesis. Excessive alcohol consumption andIncreased fat accumulation due to an increased fat biogenesis and reduced metabolism, causes hepatocellular injury that generates reactive oxygen species, release of pro-inflammatory cytokines and chemokines leading to activation of resident macrophages (Kupffer cells), and recruitment of circulating immune cells includingneutrophils and monocytes. Overconsumption of alcohol also trigger the production of lipopolysaccharides due to increased intestinal permeability. Increased levels of pathogen-associated molecular patterns (Lipopolysaccharides) and damage-associated molecular patterns (released from dying hepatocytes) that in turn interacts with toll-like receptors e.g., toll-like receptor 4 resulting in the activation of spleen tyrosine kinase signaling pathway, NF-κB signaling pathway, and inflammasome activation. These processes develop into liver inflammation and fibrosis via increased infiltration and activation of immune cells and hepatic stellate cells, respectively.SYK: Spleen tyrosine kinase; LPS: Lipopolysaccharides; PAMPs: Pathogen-associated molecular patterns; DAMPs: Damage-associated molecular patterns; TLRs:Toll-like receptors; HSCs: Hepatic stellate cells; ROS: Reactive oxygen species.
Activated KCs instigates TLR4 and recruit an activated SYK, which is also expressed in HSCs, hepatocytes, and cholangiocytes[77,84-86]. SYK plays a role in IL1-induced chemokine releaseviaassociation with TRAF-6 (TNF receptor activating factor 6), which is a shared molecule in multiple signalling pathways and is recruited through interactions of adaptor MyD88 and IRAK-1 (IL1 receptor-associated kinase 1)with TLR4[87-89]. Likewise, TLR4 transduces signalsviathe B-cell receptor (BCR)leading to activation of SYK, which is important for B-cell survival, proliferation[90],and BCR-mediated immune response[5]. Lipid peroxidation products, derived from phospholipid oxidation are one of the sources of neo-antigens that are able to promote an adaptive immune response in NASH[91]. The involvement of T and B cells in the progression of NASH automatically implicate role of SYK in this process.
Recently, we have shown the positive correlation of SYK expression with the increasing NAS score (NAFLD activity score) in livers from NASH patients as compared to normal livers[44]. As aforementioned, the role of SYK in NASH is not onlyviaPRR pathways, but also through NLR pathways. The role of several NLRs have been crucial in the formation of inflammasomes and the nomenclature of inflammasomes is hence based on the NLR[92]. SYK is required for NLRP3 (NLR protein 3) inflammasome activation[93], that forms an IL-1β-processing inflammasome complex. Inflammasome activation has been shown to be associated with the late stages of NASH, and not in early steatosis in mice[94]. Inflammasome activation can be induced by free fatty acids and these free fatty acids can also induce apoptosis and the release of danger signals in hepatocytes[94,95]. Consequently, pharmacological inhibition of NLRP3 inflammasomein vivohas been demonstrated to reduce liver inflammation, hepatocyte injury, and liver fibrosis in NASH[44,96].
SPLEEN TYROSINE KINASE IN HEPATOCELLULAR CARCINOMA
On the other hand, checkpoint kinase 1 (CHK1) was found to be overexpressed and correlated with poor survival of HCC patients. CHK1 phosphorylate tumor suppressor SYK isoform, [SYK (L)] at Ser295 and inducing its proteasomal degradation. However, non-phosphorylated mutant form of SYK (L) has been shown to suppress proliferation, colony formation, migration and tumor growth in HCC lines. Therefore, a strong inverse correlation between the expression levels of CHK1 and SYK (L) was observed in patients with HCC[101]. Interestingly, Honget al[102]showed that another SYK isoform, SYK (S) promotes tumor growth, downregulate apoptosis, enhances metastasis and counteract the opposing effects of SYK (L). These studies suggest that SYK (L) downregulation or SYK (S) upregulation are the strong predictors of poor clinical outcome in patients with HCC.
SMALL MOLECULES SPLEEN TYROSINE KINASE INHIBITORS
Over the past decade, SYK signalling pathway has been recognized as a promising target for the therapeutic intervention in different disease including autoimmune and inflammatory disorders, fibrotic diseases and tumour. However, specificity and selectivity remain the major concern for the development of drugs targeting ubiquitously expressed kinases. Hence, debate about the specificity of SYK inhibitors has been a major point of discussion and has still not reached an appropriate conclusion since the first SYK inhibitors entered into medicinal chemistry optimization[25,103,104]. Over the past few years, several SYK inhibitors have been designed while many are still in development, and the molecular structures of some of these SYK inhibitors are depicted in Figure 4. Several SYK inhibitors are been evaluated in preclinical and clinical studies in different diseases[103,105], as highlighted in Table 1[106-127].
Some of the above mentioned SYK inhibitors have been explored in liver diseases and are presented in Table 2. R406 has been shown to reduce SYK expression and phosphorylation in macrophages, and other hepatic cells and has been shown to ameliorate non-alcoholic and alcoholic steatohepatitis by inhibiting steatosis,inflammation and fibrosis suggesting multi-faceted effects of this highly selective SYK inhibitor[20,44]. GS-9973 is a new emerging, selective and potent inhibitor of SYK that was evaluated in activated HSCs and showed anti-fibrotic effects in rodent liver fibrosis models[28]. Very recently, two new inhibitors PRT062607 and Piceatannol have been investigated in myeloid cells to reveal their protective effect against liver fibrosis and hepatocarcinogenesisin vivo. Both inhibitors selectively blocked SYK phosphorylation, significantly reduced the infiltration of inflammatory cells and HSCs trans-differentiation, and inhibited malignant transformation in fibrotic livers[128].
Despite the encouraging results with SYK inhibitors, some issues remain unsolved(e.g., their long-term safety has not yet been demonstrated). Moreover, due to the ubiquitous expression of SYK in different cells, concerns have been raised about the possibility of side-effects owing to the overall inhibition of the multiple cellular functions[2,127]. A major challenge therefore is how to inhibit pathological processes without disrupting physiological cell functions[129]. Nanotechnology is an interesting and promising alternative to improve the efficacy and therapeutic effect of the SYK inhibitore.g., using poly lactic-co-glycolic acid nanoparticles, we have demonstrated improved therapeutic effectivity of R406 in MCD-diet induced NASH[44]. In this study,we have shown that R406, encapsulated in poly lactic-co-glycolic acid nanoparticles,reduced expression of SYK in macrophagesin vitro, and attenuated steatosis,inflammation and fibrosis in vivo in NASH mouse model[44].

Table 1 Summary of pre-clinical and clinical studies using spleen tyrosine kinase inhibitors

Figure 4 Molecular structure of several spleen tyrosine kinase inhibitors. R406, GS-9973, PRT062070, andPiceatannol have been studied in liver diseases, while R788 and TAK-659 are being investigated in other diseases.
CONCLUSION
In this review, we have highlighted the implication of SYK signaling pathways in different diseases, more importantly in liver diseases. SYK plays a multifaceted role in liver diseases such as liver fibrosis, alcoholic liver disease, non-alcoholic steatohepatitis, viral hepatitis, and hepatocellular carcinoma. Furthermore, several SYK-related mechanisms have been understood in the past decade which led to the development of numerous small-molecule inhibitors that have been and are currently evaluatedin vitro,in vivoin different animal models and in clinical trials in patients for different indications. These inhibitors have shown highly potent effects in the tested models and therefore is a promising therapeutic target that should be explored further. To improve the therapeutic efficacy and clinical use of SYK inhibitors with improved safety profile and reduce the side effects, nanotechnology approaches, such as polymeric nanoparticles, liposomal-mediated delivery, or micelles, and finally organ (tumor)-targeted drug delivery could be explored.
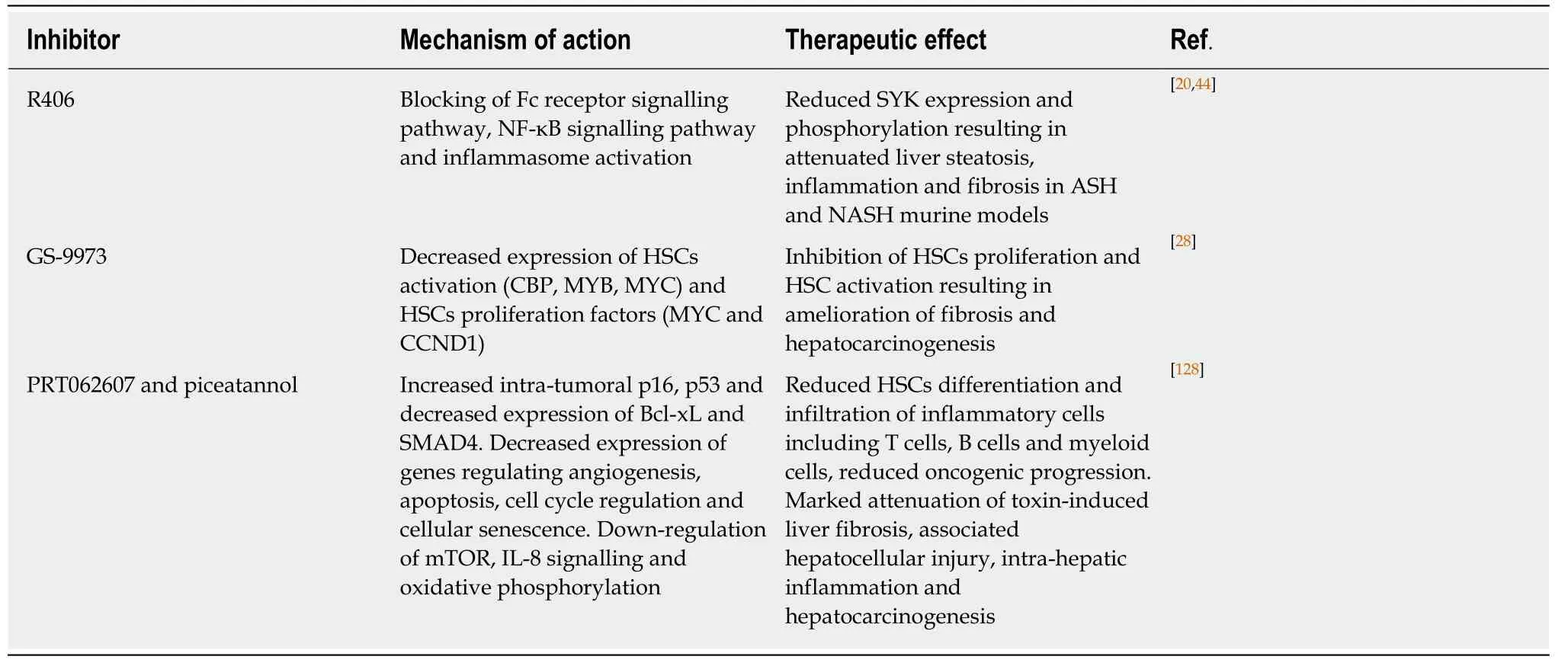
Table 2 Spleen tyrosine kinase inhibitors implicated in liver diseases
38Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues.Mol Aspects Med2019; 65: 37-55 [PMID: 30213667 DOI: 10.1016/j.mam.2018.09.002]
43Nishikawa K, Osawa Y, Kimura K. Wnt/β-Catenin Signaling as a Potential Target for the Treatment of Liver Cirrhosis Using Antifibrotic Drugs.Int J Mol Sci2018; 19 [PMID: 30308992 DOI:10.3390/ijms19103103]
46Akcora BÖ, Storm G, Bansal R. Inhibition of canonical WNT signaling pathway by β-catenin/CBP inhibitor ICG-001 ameliorates liver fibrosis in vivo through suppression of stromal CXCL12.BiochimBiophys Acta Mol Basis Dis2018; 1864: 804-818 [PMID: 29217140 DOI: 10.1016/j.bbadis.2017.12.001]
杂志排行
World Journal of Gastroenterology的其它文章
- Is aggressive intravenous fluid resuscitation beneficial in acute pancreatitis? A meta-analysis of randomized control trials and cohort studies
- Technetium-99m-labeled macroaggregated albumin lung perfusion scan for diagnosis of hepatopulmonary syndrome: A prospective study comparing brain uptake and whole-body uptake
- Predictors of outcomes of endoscopic balloon dilatation in strictures after esophageal atresia repair: A retrospective study
- Serum N-glycan markers for diagnosing liver fibrosis induced by hepatitis B virus
- Double-balloon endoscopic retrograde cholangiopancreatography for patients who underwent liver operation: A retrospective study
- Prognostic factors and predictors of postoperative adjuvant transcatheter arterial chemoembolization benefit in patients with resected hepatocellular carcinoma
