Influencing Factors of Determination of Total Arsenic in Soil by Atomic Fluorescence (AFS-9760) Method
2020-03-18TingtingMENG
Tingting MENG
Shaanxi Provincial Land Engineering Construction Group Co., Ltd., Xi’an 710075, China; Institute of Shaanxi Land Engineering and Technology Co., Ltd., Xi’an 710021, China; Key Laboratory of Degraded and Unused Land Consolidation Engineering, Ministry of Land and Resources, Xi’an 710075, China; Shaanxi Provincial Land Consolidation Engineering Technology Research Center, Xi’an 710064, China
Abstract In this paper, the atomic fluorescence (AFS-9760) method used for the determination of total arsenic in soil was verified based on three national quality control samples GSS-8, GSS-25, and GSF-3. Under the conditions of negative pressure of 270 V, carrier gas flow of 300 mL/min, and shield flow of 1 000 mL/min, the detection limit of the method was 0.009 2 mg/kg; the precision of GSS-8, GSS-25, and GSF-3 was 3.96%, 1.47% and 1.08%, and the accuracy was 3.07%, 2.13%, 2.93% respectively. In addition, the matters needing attention during this experiment, such as reagent factor, instrument conditions, calibration curve, etc., were summarized. It provides certain reference for the determination of total arsenic content in soil by atomic fluorescence spectrometry.
Key words Atomic fluorescence, Soil total arsenic, Influencing factors
1 Introduction
Arsenic is an element widely distributed in nature. The background value of arsenic in soil mainly comes from soil parent material, generally not exceeding 15 mg/kg[1]. With the mining and smelting of arsenic-containing metal minerals, the burning of fossil fuels, the use of arsenic-containing chemicals and pesticides, and the discharge and illegal dumping of industrial wastewater,etc., the arsenic concentration in soil has increased day by day, which has caused different levels of soil arsenic pollution in the world, so soil arsenic pollution and its serious consequences can not be ignored[2-3]. Soil arsenic pollution has the characteristics of poor mobility, long residence time, and not being degraded by microorganisms, and arsenic can enter water, crops,etc. to ultimately affect human health[4-5].
In recent years, research on arsenic pollution has received more and more attention. Arsenic is a highly toxic metal and is an important parameter for detailed investigation of soil pollution. The current laboratory methods for determining soil arsenic include atomic fluorescence spectrometry (AFS), inductively coupled plasma mass spectrometry (ICP-MAS), and atomic absorption spectrometry (AAS),etc[6]. Atomic fluorescence spectrometry (AFS) is widely used to measure arsenic content in water, soil, food, pharmaceuticals, cosmetics, the atmosphere, and ores due to its high degree of automation, recovery and detection sensitivity[7-8]. From the results of total arsenic content in soil in a previous study, it is found that there are many factors affecting the determination of arsenic in soil by atomic fluorescence spectrometry (AFS), and all of them will lead to inaccurate experimental results. In this paper, the national quality control samples GSS-8, GSF-3, and GSS-25 were used to verify the atomic fluorescence (AFS-9760) method used for the determination of total arsenic in soil. In addition, the optimal parameters for atomic fluorescence spectrophotometer were determined through experiments, and the influencing factors during the experiment were also analyzed to provide certain reference for the measurement of total arsenic in soil by atomic fluorescence spectrometry.
2 Method principle
2.1 EquipmentMain equipment is AFS-9760 atomic fluorescence photometer (Beijing Haiguang Instrument Co., Ltd.).
2.2 Measurement principleSoil arsenic was detected according to the determination method of soil arsenic in theSoilQuality—AnalysisofTotalMercury,ArsenicandLeadContents—AtomicFluorescenceSpectrometry(GB/T 22105.1-2008)[9]. After the arsenic in the sample was digested by heating, thiourea was added to the sample to reduce the pentavalent arsenic to trivalent arsenic, and then potassium borohydride was added to the sample to reduce it to hydrogen arsenide. It was introduced into the quartz atomizer with argon to be atomized into an atomic state. It generated atomic fluorescence under the excitation of emission light of a special arsenic hollow cathode lamp. The intensity of fluorescence generated was proportional to the content of the measured element in the sample. Compared with the standard series, the arsenic content in the sample was obtained.
2.3 Instrument parametersIn the experiment, the optimal parameters for the determination of total arsenic by the AFS-9760 atomic fluorescence spectrophotometer was determined by the determination of quality control samples. They are shown as follows: negative high voltage: 270 V; atomizer pre-heating temperature: 200 ℃; A lamp current: 0 mA; carrier gas flow: 300 mL/min; B lamp current: 60 mA; shield flow: 1 000 mL/min; observation height: 10 mm; measurement method: calibration curve; reading method: peak area; reading time: 20 s; delay time: 1 s; number of repeat measurements: 2.
2.4 FormulaThe total arsenic contentωin the soil samples was calculated by mass fraction, and the unit of a value is mg/kg. The formula is as follows:
ω=(c-c0)×V2×Vtotal/V1m×Wdm×1 000
(1)
In the formula,cis arsenic content found from the calibration curve, ng/mL;c0is determination concentration of reagent blank liquid, ng/mL;V2is the constant volume of the diluted sample solution during the measurement, ml;Vtotalis the constant volume of the sample solution after digestion, mL;V1is the volume of digestion solution of the taken sample during the measurement, mL;mis the mass of samples, g;Wdmis the content of dry matter in soil samples, %; 1 000 is the coefficient that converts "ng" to "μg".
3 Results and Analysis
In accordance with the above methods and conditions, the atomic fluorescence (AFS-9760) method used for the determination of total arsenic in soil was verified. National quality control samples GSS-8, GSF-3, and GSS-25 were selected to measure the detection limit, precision and accuracy of soil total arsenic. As shown in Table 1, the detection limit of this experiment was 0.009 2 mg/kg, less than the detection limit of 0.01 mg/kg specified by this method. The precision of the three national quality control samples GSS-8, GSS-25, and GSF-3 was 3.96%, 1.47%, and 1.08%, and the accuracy was 3.07%, 2.13%, and 2.93% respectively(Table 1), which meet the requirements of this method. That is, under repeated conditions, the relative deviation of the two independent determination results shall not exceed 7%, and the absolute value of relative error shall not exceed 5%.
4 Conclusions and Discussion
4.1 ConclusionsThe national quality control samples GSS-8, GSF-3, and GSS-25 were used to verify the determination of total arsenic in soil by atomic fluorescence (AFS-9760) method. The optimum parameters for atomic fluorescence spectrophotometer were selected in the experiment, and the detection limit, precision and accuracy of the method were determined. The results show that it is feasible and accurate to use this method to determine total arsenic in soil. In order to improve the determination accuracy and precision of total arsenic in soil, matters needing attention during the experiment will be discussed, and corresponding solutions will be proposed.
4.2 Discussion
4.2.1Reagents used. Reducing agent: The reducing agent was the mixed solution of potassium borohydride and potassium hydroxide. It is worth noting that potassium borohydride was added to the potassium hydroxide solution when this mixed solution was made. When potassium borohydride was selected, it is needed to note whether potassium borohydride absorbed water to be agglomerated and became hard. Once potassium borohydride was agglomerated,a new bottle was needed. The concentration of the reducing agent also affected the experimental results. A high concentration would dilute atomic vapor to reduce the fluorescence intensity of arsenic and make the experimental results small. A low concentration would cause incomplete reduction of the solution and result in small results. In the experiment, 1% was as the common concentration of potassium borohydride. Under this condition, arsenic had a high fluorescence intensity, a stable signal, a complete reduction, and good values. The reducing agent was made when it was used.
Table 1 Detection values of quality control samples
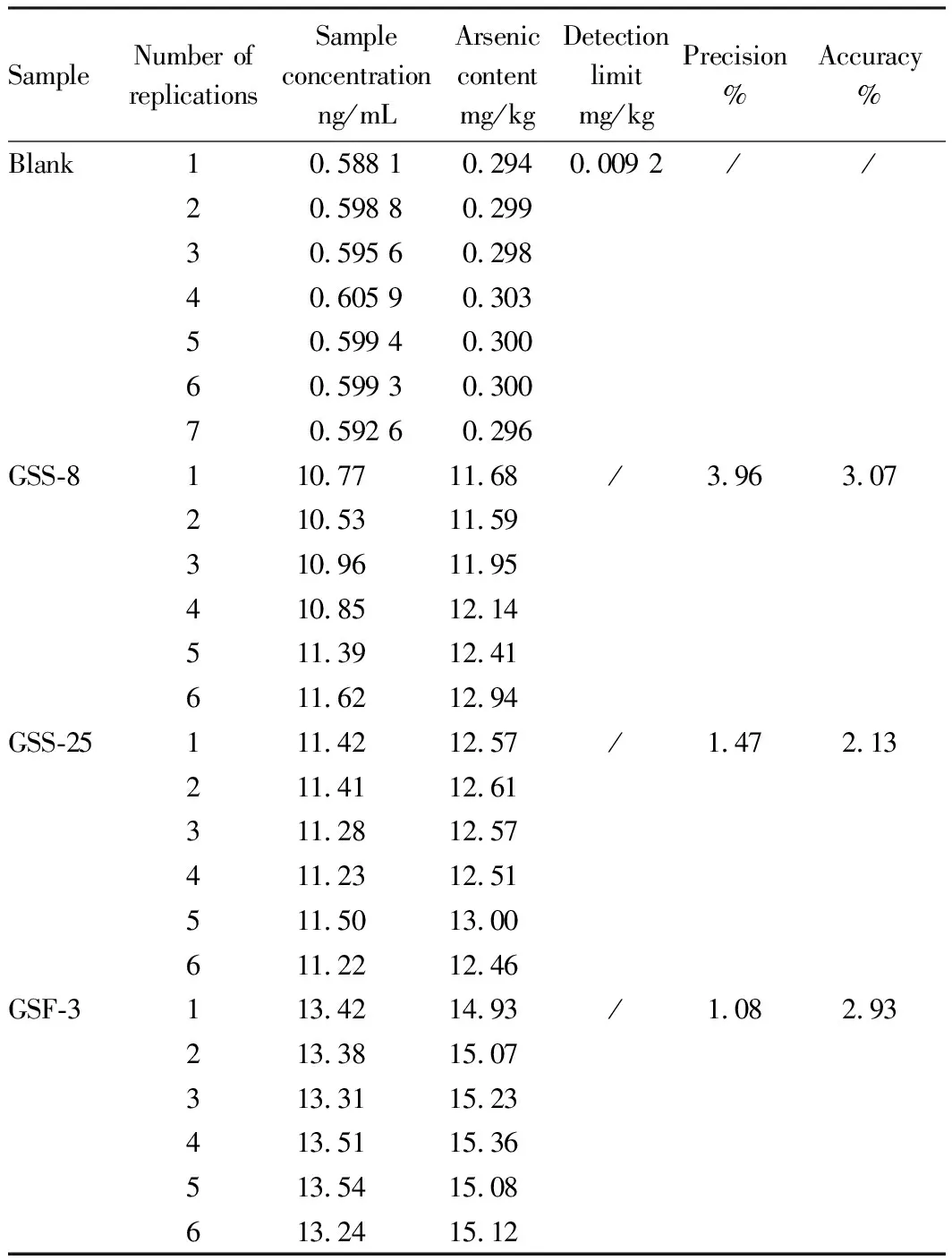
SampleNumber ofreplications Sampleconcentrationng/mLArseniccontentmg/kgDetectionlimitmg/kgPrecision% Accuracy%Blank10.588 10.2940.009 2//20.598 80.29930.595 60.29840.605 90.30350.599 40.30060.599 30.30070.592 60.296GSS-8110.77 11.68 /3.963.07210.53 11.59 310.96 11.95 410.85 12.14 511.39 12.41 611.62 12.94 GSS-25111.42 12.57 /1.472.13211.41 12.61 311.28 12.57 411.23 12.51 511.50 13.00 611.22 12.46 GSF-3113.42 14.93 /1.082.93213.38 15.07 313.31 15.23 413.51 15.36 513.54 15.08 613.24 15.12
Carrier gas flow: In this experiment, 5% nitric acid was as the carrier gas. It is found that too high or too low concentration of nitric acid would affect the detection results, and the detection results were stable when 5% nitric acid was as the carrier gas.
Pure water: First-grade pure water was used to compound the reagent solution during the experiment.
4.2.2Instrument conditions. Among the instrument conditions, the negative high voltage had the most significant influence on experimental values. Negative high voltage had a positive correlation with the standard blank value. That is, as negative high pressure increased, the standard blank value also rose; as negative high pressure decreased, the standard blank value also reduced. The test proved that negative high voltage should be 260-280 V. In continuous experiments, the stability of the standard blank is sometimes maintained by adjusting negative high voltage.
4.2.3Calibration curve. Since total arsenic content in soil, which was detected by atomic fluorescence spectrophotometry, was calculated based on the regression equation established by its standard curve, the concentration of arsenic in the sample solution in the experiment should be equivalent to the standard curve concentration. In general, the measured value should fall in the middle of the standard curve to make the measured value more accurate. Therefore, choosing the appropriate standard curve concentration in the experiment is also an important means to ensure the accuracy of the test results.
4.2.4Standard value of national quality control samples. The standard value of national quality control samples is a important parameter for testing the stability of the instrument, the accuracy of reagents (carrier gas and reducing agent), and the correctness of the standard curve during the experiment. If the measured value of the national quality control samples deviated far from the standard value, three problems should be considered firstly. The first one is the stability of the instrument. The standard blank should be seen when the needle was in the air. If the standard blank was very small (30-60), the problem of instrument stability can be eliminated. The second one is the accuracy of the reagent. It is needed to check whether the standard blank was stable in the presence of carrier gas and reducing agent. If it was stable, the carrier gas and the reducing agent are no problem at this time. Finally, the accuracy of the standard curve was checked. If the measured value deviated too much from the national quality control standard value, the standard curve value was too small; if it deviated too little from the national quality control standard value, the calibration curve value was too large.
4.2.5Utensil and instrument cleaning. Glassware was soaked with 30% excellent grade nitric acid for 24 h before use, and was rinsed with ultrapure water several times to reduce the memory effect of arsenic. The adsorption effect of arsenic would cause arsenic accumulation in the instrument pipeline, so the instrument was cleaned after each experiment. 10% excellent pure nitric acid and 1% reducing agent were used for long-term measurement of the blank (20-30 times), and then it was washed with pure water. If the blank was too high due to the experimental environment, argon was used to clean the atomization chamber to achieve the purpose of cleaning.
4.2.6Sample weighing. Cross-contamination should be avoided in the process of weighing samples, especially for soil heavily contaminated with arsenic. In the experiment, a piece of weighing paper was changed, and the weighing spoon was wiped after a sample was weighed. In addition, it should be noted that cuvette plugs cannot be mixed in use during the entire experiment to avoid cross-contamination.
杂志排行
Asian Agricultural Research的其它文章
- Land Use Regionalization in Poor Counties in Mountainous Areas Based on Targeted Poverty Alleviation
- Effects of Cultivation Method on Seed Yield and Quality of Bitter Gourd
- Progress of Land Engineering Discipline and Comparative Study on Teaching Methods
- Effects of Straw Utilization Methods on Dry Matter Production and Yield of Japonica Rice in Northern China
- Changes in Utilization of Contracted Land in Xundian County of Yunnan Province in the Background of Poverty Alleviation
- Impacts of Nanchang Metro on Accessibility of Public Transport Network and Fairness of Travel for Indemnificatory Housing Residents
