Prognostic value of preoperative weight loss-adjusted body mass index on survival after esophagectomy for esophageal squamous cell carcinoma
2020-03-12HanLuZhangYuShangYangJiaNanDuanQiXinShangSongLinHeYiMinGuWeiPengHuWenPingWangYangHuYunWangYongYuanLongQiChen
Han-Lu Zhang, Yu-Shang Yang, Jia-Nan Duan, Qi-Xin Shang, Song-Lin He, Yi-Min Gu, Wei-Peng Hu,Wen-Ping Wang, Yang Hu, Yun Wang, Yong Yuan, Long-Qi Chen
Abstract BACKGROUND The impact of body mass index (BMI) on survival in patients with esophageal squamous cell carcinoma (ESCC) undergoing surgery remains unclear. Therefore,a definition of clinically significant BMI in patients with ESCC is needed.AIM To explore the impact of preoperative weight loss (PWL)-adjusted BMI on overall survival (OS) in patients undergoing surgery for ESCC.METHODS This retrospective study consisted of 1545 patients who underwent curative resection for ESCC at West China Hospital of Sichuan University between August 2005 and December 2011. The relationship between PWL-adjusted BMI and OS was examined, and a multivariate analysis was performed and adjusted for age, sex, TNM stage and adjuvant therapy.RESULTS Trends of poor survival were observed for patients with increasing PWL and decreasing BMI. Patients with BMI ≥ 20.0 kg/m2 and PWL < 8.8% were classified into Group 1 with the longest median OS (45.3 mo). Patients with BMI < 20.0 kg/m2 and PWL < 8.8% were classified into Group 2 with a median OS of 29.5 mo. Patients with BMI ≥ 20.0 kg/m2 and PWL ≥ 8.8% (HR = 1.9, 95%CI: 1.5-2.5),and patients with BMI < 20.0 kg/m2 and PWL ≥ 8.8% (HR = 2.0, 95%CI: 1.6-2.6),were combined into Group 3 with a median OS of 20.1 mo. Patients in the three groups were associated with significantly different OS (P < 0.05). In multivariate analysis, PWL-adjusted BMI, TNM stage and adjuvant therapy were identified as independent prognostic factors.CONCLUSION PWL-adjusted BMI has an independent prognostic impact on OS in patients with ESCC undergoing surgery. BMI might be an indicator for patients with PWL <8.8% rather than ≥ 8.8%.
Key words: Esophageal neoplasms; Body mass index; Body weight change; Survival;Surgery; Nutrition status
INTRODUCTION
Esophageal cancer ranks the sixth cause of malignancy-related deaths worldwide[1,2].The main histological type of esophageal cancer is esophageal squamous cell carcinoma (ESCC). Radical surgical resection remains the mainstay of treatment for early and locally advanced esophageal cancer, but it is a highly invasive operation.Significant efforts have been made to reduce postoperative morbidity and mortality,and improve quality of life[3], but long-term survival remains low[2,4]. Therefore, an investigation into the efficacy of independent prognostic factors may help surgeons settle upon an optimal treatment option for patients with esophageal cancer.
Body mass index (BMI) is a simple and objective nutritional parameter that is easily available to physicians, and studies in patients with cancer have suggested that low BMI may be associated with poor long-term outcomes[5]. The relationship between BMI and survival after esophagectomy has been described in many studies, but the results are controversial[6-8]. Of note, Martin et al[9]demonstrated that the impact of BMI on survival varied with different levels of preoperative weight loss (PWL) in patients with cancer, which was validated in a prospective cohort of patients by Vagnildhaug et al[10]. Therefore, combined analysis of PWL and BMI may provide accurate information about nutritional status, and assist in preoperative risk stratification for surgery rather than a single index (PWL or BMI). The present study is the first to investigate the relationship between PWL-adjusted BMI and long-term surgical outcomes in patients with ESCC.
MATERIALS AND METHODS
Patients
This was a retrospective study of 1545 patients undergoing esophagectomy for ESCC in the Department of Thoracic Surgery, West China Hospital, Sichuan University between August 2005 and December 2011. All patients were preoperatively diagnosed with ESCC by upper gastrointestinal endoscopy and biopsy. The tumor was staged to be resectable according to contrast computed tomography (CT) scans of the chest and upper abdomen, esophageal barium swallow, and endoscopic ultrasound. In selected cases, integrated fluorodeoxyglucose positron emission tomography was performed to exclude the presence of metastatic disease. The exclusion criteria were: (1) Patients with non-squamous cell carcinoma; (2) Patients with an incidental finding of M1 stage during surgery (pathological stage IV cancer); (3) Patients receiving neoadjuvant therapy; and (4) Patients with incomplete data.
Surgical procedure
Patients without distant metastasis or definitive evidence of extensive adjacent organ invasion underwent surgical resection. All patients underwent curative transthoracic subtotal esophagectomy with two-field lymphadenectomy, including the Sweet, Ivor-Lewis and McKeown approaches. Patients with a tumor in the middle or lower thoracic esophagus with no evidence of lymph node involvement in the superior mediastinum or in the neck received esophagectomy via Sweet or Ivor-Lewis esophagectomy. Patients with a tumor in the middle or upper thoracic esophagus, or with possible lymph node metastasis in the superior mediastinum or neck, were operated upon via McKeown esophagectomy. Gastroesophageal anastomosis was created in the chest or neck, depending on the location of the tumor. Esophagogastrostomy was created between the proximal esophageal remnant and the gastric conduit using a circular stapler or a two-layer hand-sewn procedure, based on the surgeon's own technical expertise.
Data collection and follow-up
Clinical data of all patients were obtained retrospectively, including patient demographics, surgical procedures, treatment details, pathological stage of disease,and survival. The primary outcome was overall survival (OS). OS was the time from surgery to the date of death or last clinic visit. Patients alive at the last follow-up were censored for OS.
Patients' current body height and weight were routinely measured before surgery.Stable weight 3 mo before admission was provided by the patient, and was defined as the control weight. PWL was calculated as: [(stable weight 3 mo before admission -current weight) / stable weight 3 mo before admission] × 100.
All patients were seen in follow-up at our outpatient department every 3 mo in the first 2 years after resection, and every 6 mo thereafter. The follow-up protocol included history-taking, physical examination and chest abdominal CT scans. Upper gastrointestinal endoscopy, radionuclide bone scan, positron emission tomography CT scans, and abdominal ultrasound were arranged if clinically indicated. All patients were observed until death, or were censored at their last follow-up.
Statistical analysis
Continuous data are presented as mean and standard deviation. Categorical variables are shown as frequency and percentage. The Kaplan-Meier method was used to estimate survival. The log-rank test was used to compare survival. Univariate analysis was used to examine the association between potential predictors and survival.Hazard ratios (HRs) with 95% confidence intervals (CIs) were used to quantify the association between predictors and survival. Univariate factors with P < 0.25 and believed to be associated with cancer-related deaths were entered into a multivariate Cox proportional hazards regression model. A backward stepwise elimination of variables was used to construct the final model.
To define the prognostic significance of PWL and BMI, these two categories were divided into deciles as suggested by Martin et al[9](i.e., dividing the distribution of continuous variables into 10 equal groups). Thus, the patients were divided into 11 groups (a weight stable group and 10 weight loss groups) according to PWL values,and 10 groups according to BMI values. To determine the optimal cut-off points for BMI and PWL, we compared the OS HR between groups with different deciles of BMI and PWL using a Cox proportional hazards regression model. The highest χ2value was deemed the cut-off point[11-13]. Two categories of PWL and BMI were then created.Based on the combination of PWL and BMI categories, patients were classified into corresponding groups according to median survival and prognostic significance. P <0.05 was considered statistically significant. Data analysis was performed with SPSS version 24.0 (SPSS Inc., Chicago, IL, United States).
RESULTS
Demographics and clinical characteristics
With the exception of 156 patients with non-squamous esophageal carcinoma, 1572 patients were initially considered. According to the exclusion criteria, 27 patients were then excluded. In detail, five patients had an incidental finding of M1 stage during surgery, thirteen patients received neoadjuvant therapy, and nine patients had incomplete data. Finally, we included 1545 patients (1288 males and 257 females,mean age 59.8 ± 8.4 years) in this retrospective analysis. Of these, 986 (63.8%) patients were weight stable, while 559 (36.2%) experienced varying degrees of PWL. Two hundred and twelve (13.8%) patients were underweight (BMI < 18.5 kg/m2), 1144(74.0%) were normal weight (18.5-24.9 kg/m2), 172 (11.1%) were overweight (25.0-29.9 kg/m2) and 17 (1.1%) were obese (≥ 30.0 kg/m2). The clinicopathological characteristics of the entire cohort are summarized in Table 1.
Categorical assignment of PWL and BMI
To explore the impact of increasing PWL and decreasing BMI on OS, PWL and BMI were divided into deciles. First, patients were divided into two groups according to preoperative body weight change: Patients with stable weight (body weight change =0) and patients with PWL (body weight change > 0). Ten subgroups of patients with PWL were classified according to the order of body weight change (with equal cases in each subgroup). Second, patients were divided into ten equal groups according to BMI (with equal cases in each subgroup). Trends of poor survival were observed for patients with increasing PWL and decreasing BMI (Figure 1).
Optimal cut-off values of BMI and PWL
To determine the optimal cut-off points of PWL and BMI that could yield the maximum differential in survival, we constructed a Cox's proportional hazards regression model using age, sex, TNM stage and adjuvant therapy. χ2scores were calculated for the model for threshold ranging from the lowest to highest BMI and the lowest to highest PWL (Tables 2 and 3). For the entire group, the threshold of BMI with 20 kg/m2had the maximum χ2score, and was therefore the best cut-off point (χ2score, 24.5; HR, 1.4; 95%CI: 1.2-1.6). The threshold of PWL with 8.8% had the maximum χ2score, and was therefore the best cut-off point (χ2score, 48.4; HR, 1.8;95%CI: 1.5-2.2) (Figure 2). Based on the PWL threshold, the patients were categorized as no or limited (< 8.8%) and severe (≥ 8.8%) PWL. Based on the BMI threshold, the patients were categorized as low (< 20.0 kg/m2) and normal or high (≥ 20.0 kg/m2)BMI.
Combined analysis of PWL and BMI for postoperative survival
Based on the optimal cut-off values of PWL and BMI, two categories of PWL and BMI were created. Four different combinations of PWL and BMI were presented, and patients were subsequently allocated into four groups for statistical analysis. Sample size, median OS and unadjusted HRs for the combinations are shown in Table 4, Table 5 and Table 6, respectively. Combining groups with similar HRs yielded three distinct grades with significantly different survival (P < 0.05, Figure 3). Patients with BMI ≥20.0 kg/m2and PWL < 8.8% were classified into Group 1 with the longest median OS(45.3 mo). Patients with BMI < 20.0 kg/m2and PWL < 8.8% were classified into Group 2 with a median OS of 29.5 mo. Similar HRs were observed for patients with BMI ≥20.0 kg/m2and PWL ≥ 8.8% (1.9, 95%CI: 1.5-2.5) and patients with BMI < 20.0 kg/m2and PWL ≥ 8.8% (2.0, 95%CI: 1.6-2.6), so these patients were combined into Group 3 with a median OS of 20.1 mo. Patients in Group 1 had the lowest risk, while patients in Group 3 had the highest risk. The PWL-adjusted BMI grades were entered into a multivariate analysis controlled for age, sex, TNM stage and adjuvant therapy (Table 7), which demonstrated that combined analysis of PWL and BMI was an independent prognostic factor for patients with ESCC undergoing surgery.
DISCUSSION
Due to the obstructive nature and catabolic effect of cancer, PWL is frequent in patients with ESCC[14,15]. However, nutrition assessment is generally not a routine part of the preoperative assessment, although malnutrition is frequently seen in patients with ESCC undergoing surgery[14]. Preoperative malnutrition is associated with poorsurvival in cancer patients[16-18]. BMI and PWL are simple and objective nutritional parameters easily available to physicians, and may be valuable predictors to riskstratify patients prior to surgery. However, the effect of BMI and PWL on postoperative survival in ESCC has not been well-studied. The present study is believed to be the first to investigate the relationship between PWL-adjusted BMI and long-term surgical outcomes of patients with ESCC. The authors demonstrated that low BMI and significant PWL have a negative effect on surgical outcomes for patients with ESCC. Intriguingly, BMI might be an indicator for OS in patients with slight (0-8.8%) PWL rather than severe (≥ 8.8%) PWL.
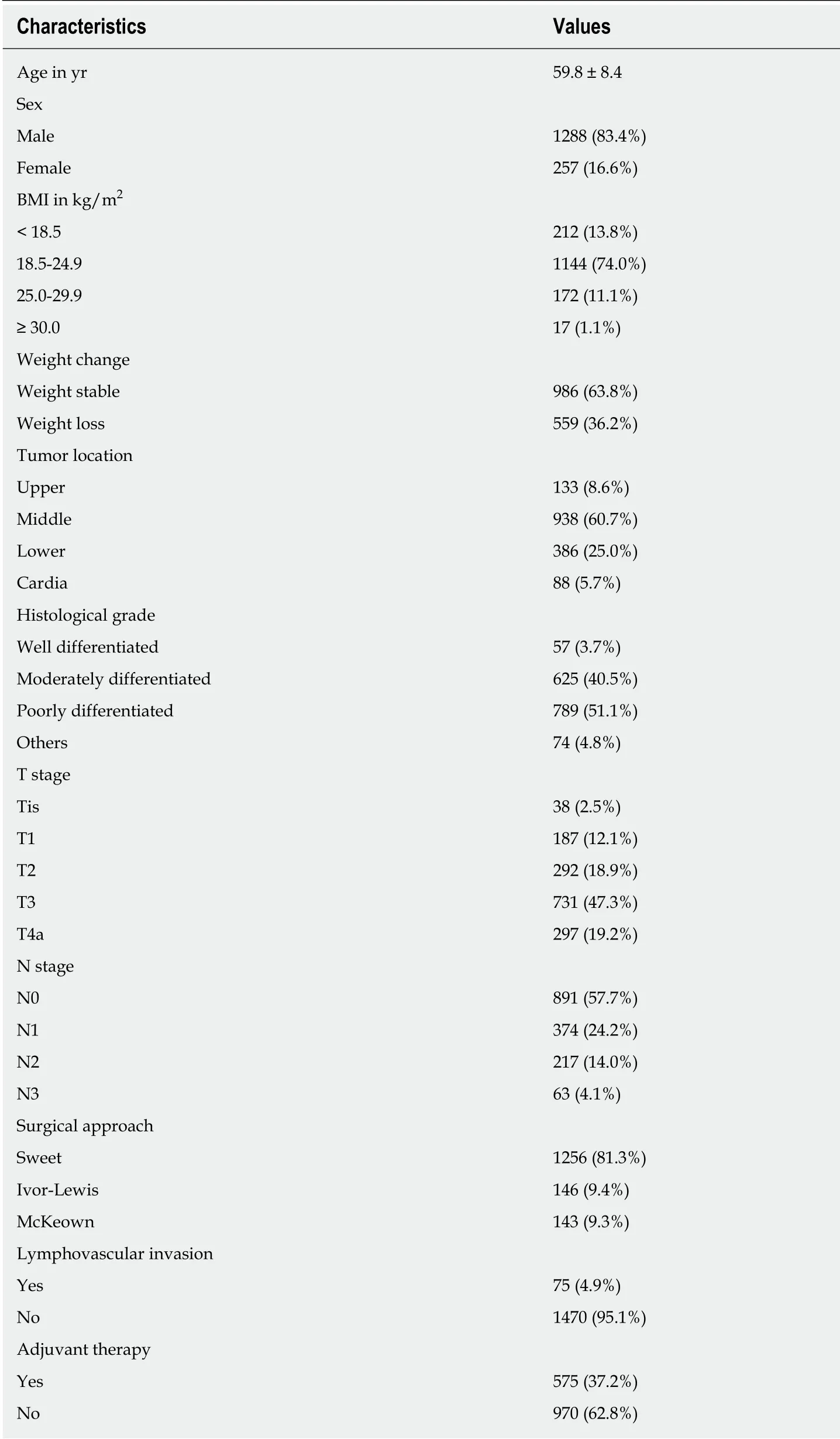
Table 1 Clinical characteristics of the entire patient cohort
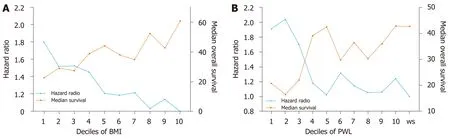
Figure 1 Trends of poor survival were observed for patients with increasing preoperative weight loss and decreasing body mass index. A, B: Risk of reduced survival (blue line) increases with decreasing BMI (A) and increasing PWL (B). Median OS (golden line) decreases with decreasing BMI (A) and increasing PWL (B). PWL: Preoperative weight loss; BMI: Body mass index.
A previous study by Skipworth et al[19]found that combined BMI and PWL analysis failed to predict survival after esophagogastric resection for cancer. Unlike the cut-off point of PWL defined by Skipworth et al[19], van der Schaaf et al[20]demonstrated that a PWL > 10% was followed by decreased 5-year survival after esophagectomy for esophageal cancer. For patients with BMI < 25 kg/m2, Martin et al[9]indicated that there were significantly different subgroups of patients, with median survival times as long as 15.7 mo and as short as 3.7 mo. Therefore, arbitrarily defining a single threshold of a PWL of 0 and BMI of 25 kg/m2by Skipworth et al[19]might have the pitfall of subgrouping patients with disparate degrees of risk, and underestimate the real prognostic value of PWL and BMI for patients with esophagogastric carcinoma,resulting in a nonsignificant effect of BMI-adjusted PWL on the survival of patients with esophagogastric carcinoma.
Although studies in patients with cancer have suggested that low BMI may be associated with poor long-term outcomes[5], there is no general consensus on the influence of BMI on survival in esophageal cancer[22]. We speculate that heterogeneous definitions of clinically important BMIs in different studies may result in an unclear impact of BMI on postoperative survival after esophagectomy[9]. Hasegawa et al[21]classified patients into three categories, < 18.49, 18.50-24.99 and ≥ 25.0 kg/m2, defined as low, normal and high BMI, respectively, according to the World Health Organization criteria. They held the view that overweight or underweight alone should not contraindicate surgical resection for patients with ESCC, because there were no significant differences in overall and relapse-free survival among patients with low, normal and high BMI status in univariate and multivariate analyses[21].Zhang et al[22]classified patients according to Asian-specific BMI cutoff values[23], and demonstrated that patients with higher BMIs had a significantly favorable OS.
To investigate the relationship between BMI and the prognosis of ESCC patients, a traditional BMI value defined by the World Health Organization criteria[24]was not used in the current study. Patients were only classified into the low BMI group and normal/high BMI group because the incidence of obesity was low. Cutoff values of 20 kg/m2for BMI was selected, and subsequently yielded the maximum differential in survival. We found a trend of poor survival for patients with decreasing BMIs.Through depletion of the body's energy and protein reserves, PWL is considered to be an index of severity for nutritional status[9]. In the present study, a cutoff value of 8.8%for PWL was selected to investigate the relationship between PWL and the prognosis of ESCC patients. We found a trend of poor survival for patients with increasing PWL.
Previous studies did not take into account the potential influence of severe PWL in the risk assessment of patients with low or high BMI. Since excessive weight and obesity are now prevalent worldwide[25], a number of patients with extreme weight loss by the time of treatment have normal BMIs. Therefore, combination analysis of PWL and BMI is needed. We evaluated the prognostic significance of BMI in patients with low and severe PWL, respectively. Multivariate analysis showed that the combination analysis of PWL and BMI had an independent impact on OS for patients with ESCC undergoing surgery.
The prognostic advantage for high BMI and low PWL patients might be attributed to the fact that patients with high BMI and less weight loss have a better nutritional status. When PWL was < 8.8%, patients with high BMI had a significantly better prognosis than patients with low BMI for OS. Intriguingly, no significant difference was observed between patients with low BMI and patients with high BMI for OSwhen PWL was > 8.8%. ESCC patients with high BMI are often malnourished owing to severe weight loss, so excessive PWL patients had a poor OS irrespective of the BMI levels[20]. Our study suggested that patients with excessive PWL (> 8.8%) had the worst survival, so adequate selection of such patients for surgery is crucial.

Table 2 Optimal cut-off point for body mass index
The study had some limitations. Firstly, it was a retrospective and single-center study. Secondly, the included patients were at an operable stage, and patients in late cachectic stages were not scheduled for surgical resection and were not included.Thus, the present result may only be applicable to patients with ESCC undergoing surgery. Thirdly, we evaluated BMI and weight loss, which were easily available to physicians and may be available in all patients, while biochemical markers were not considered in the current study. Fourthly, neoadjuvant therapy prior to surgery might result in further weight loss, so patients receiving neoadjuvant therapy were excluded. The prognostic value of PWL-adjusted BMI on patients undergoing neoadjuvant followed by surgery needs to be investigated further.
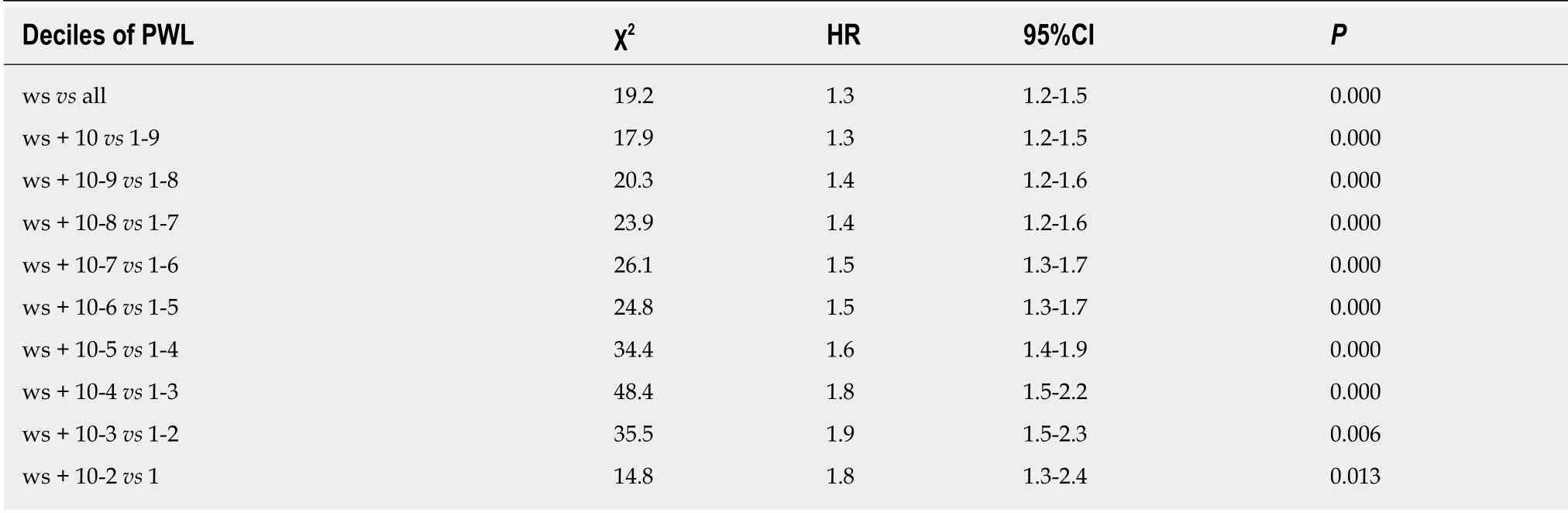
Table 3 Optimal cut-off point for preoperative weight loss

Table 4 Combined analysis of preoperative weight loss and body mass index (sample size)

Table 5 Combined analysis of preoperative weight loss and body mass index (median overall survival, mo)

Table 6 Combined analysis of preoperative weight loss and body mass index (unadjusted estimated hazard ratios)
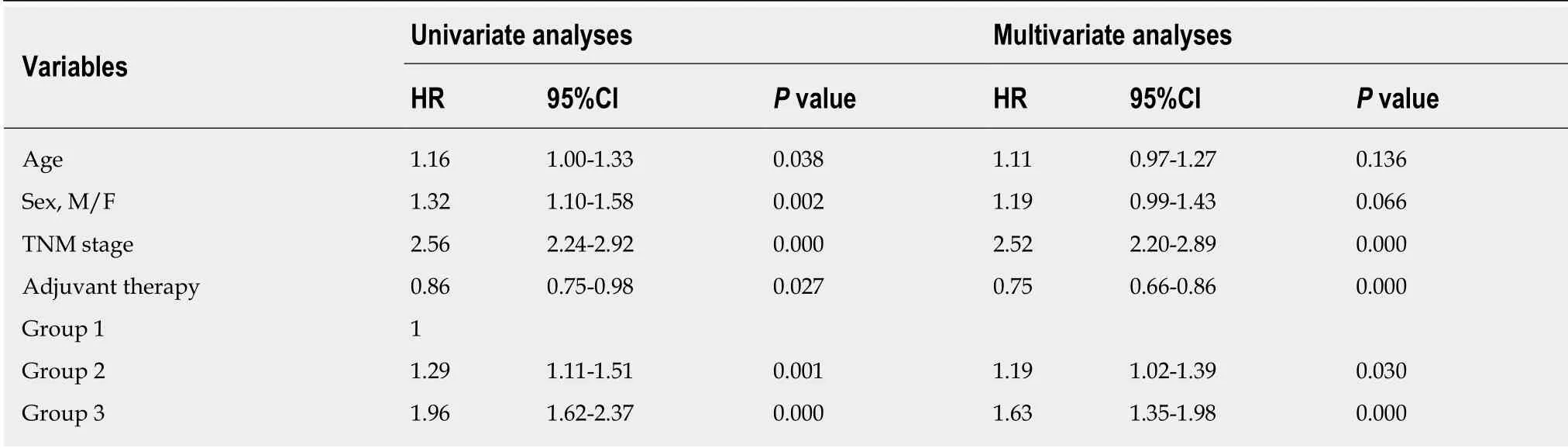
Table 7 Predictors of overall survival in univariable and multivariable analyses
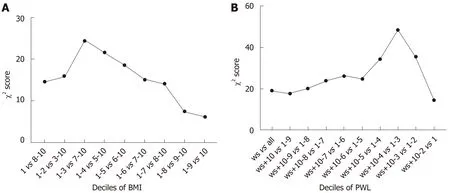
Figure 2 The threshold of BMl with 20.0 kg/m2 had the maximum χ2 score, and was therefore the best cut-off point (A). The threshold of PWL with 8.8% had the maximum χ2 score, and was therefore the best cut-off point (B). ws: weight stable; PWL: Preoperative weight loss; BMI: Body mass index.
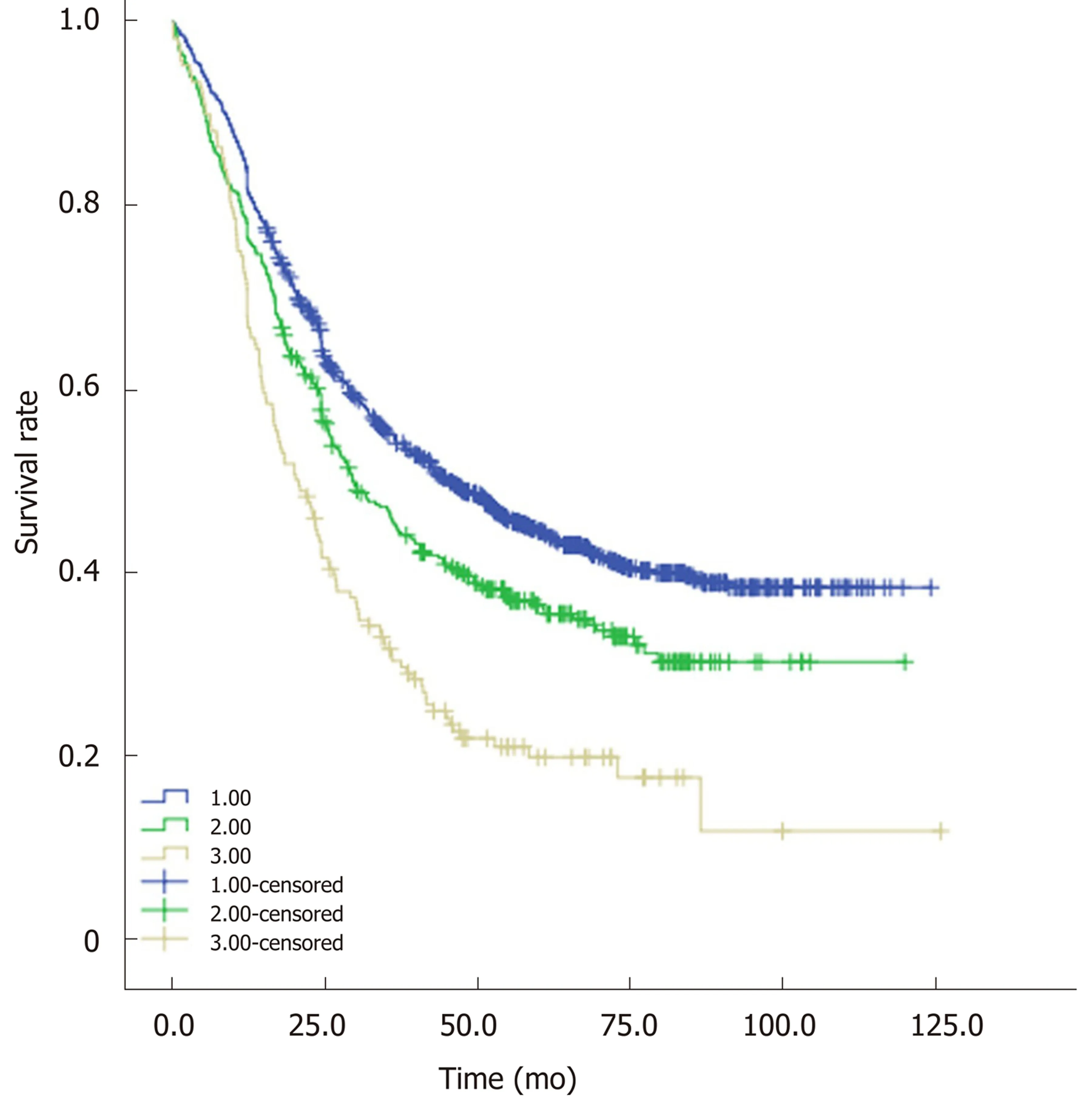
Figure 3 Patients in the three groups were associated with significantly different overall survival (blue line: Group 1; green line: Group 2; golden line:Group 3).
ARTICLE HIGHLIGHTS
Research background
Lower than average weight and preoperative weight loss (PWL) are common for patients with esophageal squamous cell carcinoma (ESCC). To our knowledge, these are also thought to be important prognostic factors in many cancers. However, various levels of body mass index (BMI)and PWL are used to define clinically-significant BMI and PWL.
Research motivation
Definitions of clinically-significant BMI and PWL in patients with ESCC are unclear.
Research objectives
The aim of this study was to explore whether BMI and PWL are valuable predictors to riskstratify esophageal squamous cell cancer patients prior to surgery.
Research methods
We retrospectively analyzed the data of 1545 ESCCs who underwent curative surgical resection.To define the prognostic significance of PWL and BMI, patients were divided into three groups:Patients with BMI ≥ 20.0 kg/m2and PWL < 8.8% (Group 1), patients with BMI < 20.0 kg/m2and PWL < 8.8% (Group 2), and patients with PWL ≥ 8.8% (Group 3). Then, a multivariate analysis was performed and adjusted for age, sex, TNM stage and adjuvant therapy.
Research results
Trends of poor survival were observed for patients with increasing PWL and decreasing BMI.Patients in Group 1 had the longest median overall survival (OS) with 45.3 mo, patients in Group 2 had a median OS of 29.5 mo, and patients in Group 3 had the worst median OS with 20.1 mo.When PWL was < 8.8%, patients with high BMI had a significantly better prognosis than patients with low BMI. When PWL was higher than 8.8%, no significant difference was observed between patients with low BMI and patients with high BMI.
Research conclusions
Both PWL and BMI are prognostic factors for patients with ESCC.
Research perspectives
Both BMI and PWL are simple and objective nutritional parameters that are easily available to physicians, and may be valuable predictors to risk-stratify patients prior to surgery.
杂志排行
World Journal of Gastroenterology的其它文章
- Diagnosis and management of a solitary colorectal juvenile polyp in an adult during follow-up for ulcerative colitis: A case report
- Surgical outcome of laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass for resolution of type 2 diabetes mellitus: A systematic review and meta-analysis
- Diverting colostomy is an effective and reversible option for severe hemorrhagic radiation proctopathy
- Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer
- Neoadjuvant chemotherapy vs upfront surgery for gastric signet ring cell carcinoma: A retrospective,propensity score-matched study
- Downregulation of orosomucoid 2 acts as a prognostic factor associated with cancer-promoting pathways in liver cancer
