Triterpene-Enriched Olive Extract as an Immunopotentiator in Black Sea Bream (Acanthopagrus schlegelii)
2020-03-10RONGJiahuanHANYuZHAShanjieTANGYuSHIWeiGUANXiaofanDUXueyingHEMaolongandLIUGuangxu
RONG Jiahuan, HAN Yu, ZHA Shanjie, TANG Yu, SHI Wei, GUAN Xiaofan,DU Xueying, HE Maolong, and LIU Guangxu,
1) College of Animal Science, Zhejiang University, Hangzhou 310058, China
2) Innovation Division, Lucta (Guangzhou) Flavours Co. Ltd., Guangzhou 510530, China
Abstract Recently, the use of natural immunopotentiators, such as plant extracts, is predicted to offer high disease-prevention potential in aquaculture. However, few reports on the immunomodulatory impacts of olive extract (OE) on teleost are available. Therefore,the effects of dietary intake of triterpene-enriched OE on black sea bream (Acanthopagrus schlegelii) were investigated in this study.Our data showed that total blood cell counts, in vivo content of lysozyme, activities of antiprotease and myeloperoxidase, and contents of IL-4 and IL-6 were significantly up-regulated by dietary intake of triterpene-enriched OE. Additionally, the hampered immune response induced by cadmium exposure was significantly mitigated by the administration of OE as were indicated by partially or completely rescued immune-relating parameters. Furthermore, the expressions of immune-related genes encoding NF-κB inhibitor alpha (IkBα), tumor necrosis factor α (TNFα), cyclooxygenase-2 (COX-2), and proto-oncogene protein c-fos (FOS) were found to be significantly up-regulated by the dietary intake of OE. In general, the results suggested that the dietary intake of triterpene-enriched OE has immune enhancing effect in black sea bream. Such effect may be realized by 1) increasing the total counts of diverse blood cells; 2) activating nonspecific immune biomolecules; and 3) affecting signaling pathways such as IKK and ERK and subsequently inducing IL-4 and IL-6 biosyntheses.
Key words black sea bream; olive extract; immunopotentiator; gene expression
1 Introduction
With the intensification of aquaculture and the aggravation of environmental contamination, the outbreaks of pathogen caused diseases significantly threat the aquaculture industry (Harikrishnan et al., 2011). According to the statistics, the annual economic loss in aquaculture due to these diseases has exceeded 400 million USDs in China even available preventive measures have met partial success (Harikrishnan et al., 2011). In recent decades, methods of either combating pathogens or enhancing the immunity of the cultured species such as the use of antibiotics, vaccines and natural immunopotentiators have been increasingly developed and widely used to prevent the outbreak of pathogen caused diseases (Abd-El-Rhman,2009; Cerezuela et al., 2009; Hai, 2015). In comparison with other methods, natural immunopotentiators such as propolis (Abd-El-Rhman, 2009), Citrus sinensis extract(Acar et al., 2015), Cotinus coggyria (Bilen et al., 2011),Rhodomyrtus tomentosa (Na-Phatthalung et al., 2017; Na-Phatthalung et al., 2018) and Phoenix dactylifera fruit extracts (Hoseinifar et al., 2015) are not only effective in protecting various fish species against a broad spectrum of pathogens, but also cost-saving and user- and environment-friendly (Raa et al., 1992; Bilen et al., 2011; Acar et al.,2015; Hoseinifar et al., 2015; Na-Phatthalung et al., 2017).Therefore, in recent years, more and more interests have been drawn to the immune enhancement characteristics and working mechanism of natural immunopotentiators(Diedrich et al., 2002; Covas, 2007).
Among natural immunopotentiators, plant extracts are believed to be most prospective in preventing pathogens due to their intrinsic merits in easy availability, low cost and huge diversity (Zedlitz et al., 2002; Immanuel et al.,2009; Baba et al., 2015; Gobi et al., 2016; Soltanian and Fereidouni, 2016). Recently, olive extract (OE), either as the byproduct of olive oil production or leave extract, has been proved as a natural product with different functions(McDonald et al., 2001; Sedef and Karakaya, 2009; Liu et al., 2017). It has been demonstrated that such extracts possess antithrombotic (Dub and Dugani, 2013), immune enhancement (Zari and Alattar, 2011; Baba et al., 2018),anti-inflammatory (Khalatbary and Zarrinjoei, 2012), antimicrobial (Liu et al., 2017) and antioxidant (McDonald et al., 2001) activities in both terrestrial and aquatic animals including rabbit, rat and rainbow trout. Though OE has been indicated to be a latent natural immunopotentiator, only a few studies have carried out to investigate the immune modulatory impact of OE on teleost to our best knowledge.
Phenols, flavonoids, terpenoids and carbohydrates have been demonstrated to be the main bioactive components of OE (Heimler et al., 1992; Goulas et al., 2009; Stit and Hartmann, 2012). However, only the immunomodulatory effects of phenols and flavonoids of OE have been elucidated to date (Benavente-Garcı́a et al., 2000; McDonald et al., 2001; Lee and Lee, 2010), leaving the function of terpenoids such as triterpenes largely less looked. In fact,triterpenes have been regarded as the main anti-inflammatory, anti-viral, anti-bacterial, anti-tumor and immunomodulatory components in many herbal medicines (Rios,2010). The immunomodulatory impact has been demonstrated in model species such as mice and rats (Chang et al.,2012; Duggina et al., 2015). However, whether triterpenes extracted from olive exert similar immunomodulatory effects on fish needs to be determined.
Teleost possess both specific and non-specific immunities against pathogens (Magnadóttir, 2006). It has been indicated that lysozyme, antiproteases and myeloperoxidase are the main biomolecules against pathogens in the nonspecific immune response of fish (Fearon and Locksley, 1996; Dalmo et al., 1997; Palic et al., 2005). Moreover,cytokines, such as interleukins, especially the interleukin 4 (IL-4) and 6 (IL-6), are signaling molecules which play important roles in both specific and non-specific immunities (West et al., 1996; Lin et al., 2016). Though molecules and pathways participating in immune response are well studied, little is known about the impact of OE on these immune-related parameters of teleost.
Data obtained from model species support that immune-relating molecules and pathways are the main targets of triterpenes exerting species-specific immunomodulatory effect (Behboudi et al., 1997; Sethi et al., 2007). Brinker et al. (2007) found that oleanolic acid, a type of triterpenes, isolated from Tripterygium wilfordii, inhibits the production of immune-relating cytokines such as IL-6 and TNF-α. Similarly, the inhibitory effects of avicins, a type of saponins, as well as celastrol isolated from Celastrus orbiculatus, on the activation of NF-κB have been detected, suggesting these triterpenes suppress immune response (Haridas et al., 2001; Lee et al., 2006; Sethi et al.,2007). In contrast, a mixture of triterpenes obtained from Quillaja saponaria (Rosaceae) has been found to up-regulate immune response by activating antigen-presenting cells (APC) and promoting the production of IL-1 and IL-6 (Behboudi et al., 1996, 1997). Additionally, immune enhancement as was indicated by the activation of extracellular signal regulated protein kinase (ERK) and up-regulation of cyclooxygenase-2 (COX-2) by triterpenes have also been documented (Ji et al., 2003). These findings indicated that triterpenes from different sources have different immunomodulatory effects due to their differential impacts on cytokines, NF-κB and ERK signaling pathways(Passos et al., 2013). The effects of triterpenes extracted from olive on immune-relating molecules and pathways, if any, are still unclear.
Black sea bream, Acanthopagrus schlegelii, a euryhaline omnivorous species, widely distributes in the Asian Pacific (Ma et al., 2008; Rong et al., 2018). Due to its fast growth, high feed utilization efficiency and high meat quality, it is one of the most important commercial fish species in China (Hong and Zhang, 2003; Shao et al.,2008). According to the incomplete statistics, the annual fry production of black sea bream exceeds 1 million individuals in China alone (Hong and Zhang, 2003). Despite the commercial importance of black sea bream, little is known about the immunomodulatory effects of natural immunopotentiators on this fish species. To improve current limited understanding of the natural immunopotentiators and the associating mechanisms, the impacts of dietary intake of triterpene-enriched OE on the blood cell counts, in vivo contents of lysozyme and the activities of lysozyme, antiprotease and myeloperoxidase in black sea bream were investigated. In addition, the expressions of key immune-relating genes encoding NF-κB inhibitor alpha (IkBα), tumor necrosis factor α (TNFα), cyclooxygenase-2 (COX-2) and proto-oncogene protein c-fos (FOS)were also analyzed.
2 Materials and Methods
2.1 Experiment Animals and Acclimation
Black sea bream individuals (length, 9.00 cm ± 0.32 cm;weight, 21.69 g ± 0.21 g) were purchased from the Dongtou Fish-breeding Farm and immediately transferred to the Qingjiang Station of Zhejiang Mariculture Research Institute, Wenzhou, China, in June, 2018. Individuals were acclimated for two weeks in a 1000 L tank filled with 800 L aerated seawater, and fed with pellet feed without olive extract (diameter, 2 mm) to satiation twice a day at 9 AM and 5 PM. Healthy individuals without physical injury were used for the following experiments. This study was performed in accordance with the Animal Ethics Committee in the School of Medicine, Zhejiang University (ETHICS CODE Permit NO. ZJU2011-1-11-009Y, issued by the Animal Ethics Committee in the School of Medicine, Zhejiang University).
2.2 Exposure Experiment
CdCl2at 0.5 mg L-1with reported immunotoxicity to fish was used to induce immune damage to black seabream(negative control) (Guardiola et al., 2013; Zhang et al.,2017). The stock solution (1 mol L-1) was prepared by dissolving CdCl2(analytical grade, > 99% purity, Aladdin,China) in deionized water, which is sufficient to prevent weighing error and salinity fluctuation (Shi et al., 2018,2019). In order to investigate the effect of dietary intake of triterpene-enriched OE on both healthy and CdCl2damaged black sea breams, fish individuals treated with or without CdCl2were fed with diets containing 0, 0.5 and 5 g kg-1of OE, respectively, for 10 days. The commercial OE used in this study was provided by Lucta (Guangzhou)Flavours Co. Ltd., which contains 7.8% terpenes (5.5%maslinic acid), 7.4% oil, 5.2% protein, 44.1% carbohydrate and 35.5% salt and unidentified components. After 2-week acclimation, 108 healthy black sea bream individuals were randomly divided into 18 white plastic tanks filled with 200 L aerated filtered seawater, 6 each. During the experiment, the fish individuals were fed with diets containing corresponding concentrations of OE to satiation (about 5% of the body weight) twice a day at 9 AM and 5 PM, respectively. Except for the OE component,the experimental diets were formulated following previous experiment (Zhou et al., 2011). During experiment,the seawater in each tank was replaced with freshly filtered seawater with desired concentrations of Cd2+everyday. As described in our previous study (Shi et al., 2016),the working concentration of Cd2+in each treatment (Table 1) was measured using the graphite furnace atomic absorption spectrophotometer at a detection limit of 0.01 μg L-1before and during (every 2 days) the experiment.No individual mortality was detected during the experiment.

Table 1 Working concentrations of Cd2+ for different treatments
2.3 Blood Sample Collection and Total Count Analysis
After 10-day corresponding treatment, 9 individuals were randomly selected from each experimental group and anaesthetized with eugenol (60 mg L-1) in a bucket. According to the method described previously (Baba et al., 2018),blood was obtained from the caudal vasculature with a plastic disposable syringe. The blood with prefilled edathamil in syringe was used for blood cell counting. Those without edathamil was transferred into centrifuge tubes and kept at 4℃ overnight, which was centrifuged at 3500 g for 15 min for serum collection. Red blood cell (RBC) and white blood cell (WBC) were counted using a veterinary hematology analyzer (BC-5000Vet, Mindray, China) following the manufacturer’s procedure (Xiang et al., 2015).
2.4 Concentration and Activity of Lysozyme in Serum
The concentration and activity of lysozyme were determined using commercial ELISA kits (FK-97442 and FK-97441, respectively, FKBIO, Shanghai, China) following manufacturer’s instructions (Su et al., 2018). In brief, 10 μL serum was mixed with 40 μL diluent in a microwell plate and then incubated at 37℃ for 30 min. After washing with detergent, 50 μL enzyme-labeling reagent was added, mixed and incubated. After washing, 50 μL chromogenic reagents A and B were added sequentially and mixed in dark for 15 min before adding 50 μL terminator. The concentration and activity of lysozyme were subsequently determined by reading the absorption value at 450 nm on a microplate reader (Thermo Multiskan Go,United States).
2.5 Activities of Serum Antiprotease and Myeloperoxidase
The activities of serum antiprotease and myeloperoxidase were determined using commercial ELISA kits 69-22426 and 69-53387 (MSKBIO, Wuhan, China), respectively, following the manufacturer’s instructions and the methodological details described previously (Vjf et al.,2018). Briefly, after mixing 10 μL serum with 40 μL diluent in the microwell plate, the mixture was incubated at 37℃ for 30 min. After being washing with detergent, 50 μL enzyme-labeling reagent was mixed with the diluted serum and incubated at 37℃ for a further 30min, and washed again with detergent. To the labeled serum, 50 μL chromogenic reagents A and B were added sequentially and mixed in dark for 15 min, and then 50 μL terminator was added and mixed. The absorption value was read on a microplate reader (Thermo Multiskan Go, United States)set at 450 nm. The activities of antiprotease and myeloperoxidase were subsequently calculated by referring the standard curves.
2.6 Concentrations of SerumIL-4 and IL-6
Following the protocol provided by the manufacturer and described previously (Blanchetot et al., 2016), commercial ELISA kits 69-57625 and 69-63251 (MSKBIO,Wuhan, China) were used to assay the concentrations of IL-4 and IL-6 in fish serum. In brief, 10 μL serum was mixed with 40 μL diluent provided in the kits and incubated at 37℃ for 30 min. Then 50 μL enzyme-labeling reagent was added to the serum. After washing, 50 μL of chromogenic reagents A and B were added sequentially to the labeled serum and incubated in dark for 15 min. The colorification was terminated with 50 μL terminator. Absorption values at 450 nm were read on a microplate reader(Thermo Multiskan Go, United States) and the concentrations of serum IL-4 and IL-6 were subsequently determined by referring to corresponding standard curves.
2.7 Expression Analyses of Key Immune-Relating Genes
Six individuals were randomly selected from each experimental group, anaesthetized and dissected on ice. The spleen tissue of each individual was carefully removed and immediately frozen in liquid nitrogen. Total RNA was extracted using EASY Spin Plus Tissues/Cells Rapid RNA Extraction Kit (Aidlab, RN2802) following the method described by Peng (2016) and Su (2019). The quality and concentration of RNA were checked by gel electrophoresis and NanoDrop 1000 UV/visible spectrophotometer(Thermo Scientific), respectively. High-quality RNA was then reverse transcribed into the first strand cDNA using an M-MLV First Strand Kit (Invitrogen, C28025-032) following the manufacturer’s protocols. Quantitative PCR was performed on a CFX 96TM Real-Time System (Bio-Rad) with a total volume of 10 μL consisting of 5 μL 2×Super Mix (Bio-Rad, 172-5201AP), 3 μL double-distilled water, 0.5 μL forward and reverse primer (10 μmol L-1,each), and 1 μL cDNA template. The following cycle condition was used: 95℃ for 5 min, followed by 40 cycles of 95℃ for 20 s, 61℃ for 20 s, 72℃ for 20 s. A melting curve analysis (MCA) was used to confirm the specificity of the PCR products. In total, the expressions of four immune-relating genes encoding NF-κB inhibitor alpha (IkBα),tumor necrosis factor α (TNFα), cyclooxygenase-2 (COX-2), and proto-oncogene protein c-fos (FOS) were analyzed. The 18S rRNA gene was used as an internal reference. All primers used were synthesized by Tsingke Biotech (Hangzhou, China) and the sequence information is shown in Table 2.
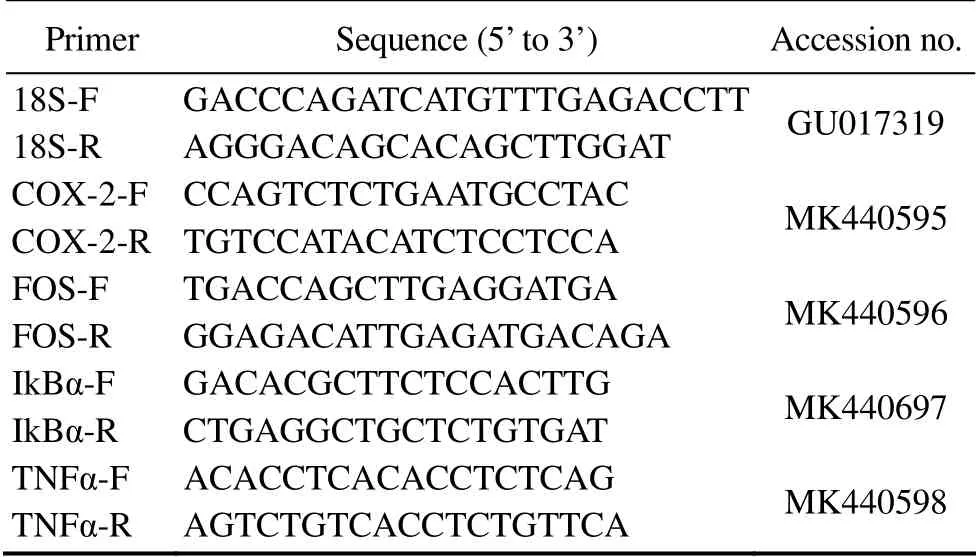
Table 2 Sequence information of the immune-relating genes investigated and the 18S rRNA gene
2.8 Statistical Analysis
One-way ANOVAs followed by Tukey’s post hoc tests were performed to compare the differences in haemocyte counts, lysozyme content, and activities of lysozyme, antiprotease and myeloperoxidase among groups. Gene expressions were compared using the Duncan multiple range test (Tallarida and Murray, 1987). All the statistical analyses were performed using OriginPro 8.0. Data were presented as mean ± SE (standard error) and a P-value less than 0.05 was identified as a statistical significance.
3 Results
3.1 The Impact of the Dietary Intake of Triterpene-Enriched OE on Haemocyte Counts
The RBC counts of healthy black sea bream fed with diet containing 0.5 g kg-1triterpene-enriched OE were significantly higher (about 1.24 times, Fig.1A, F1,17= 8.19, P= 0.01) than that of the control (individuals without Cd exposure as well as OE intake). Upon exposure to 0.5 mg L-1Cd2+without dietary intake of OE for 10 ds (the negative control), RBC counts significantly declined to about 57.37% of the control (Fig.1B, F1,17= 65.38, P = 4.82E-7).However, dietary intake of OE at the concentrations tested exerted an evident rescue effect on the RBC counts of Cdtreated sea bream (Fig.1B, F2,26= 1.63, P = 0.22).
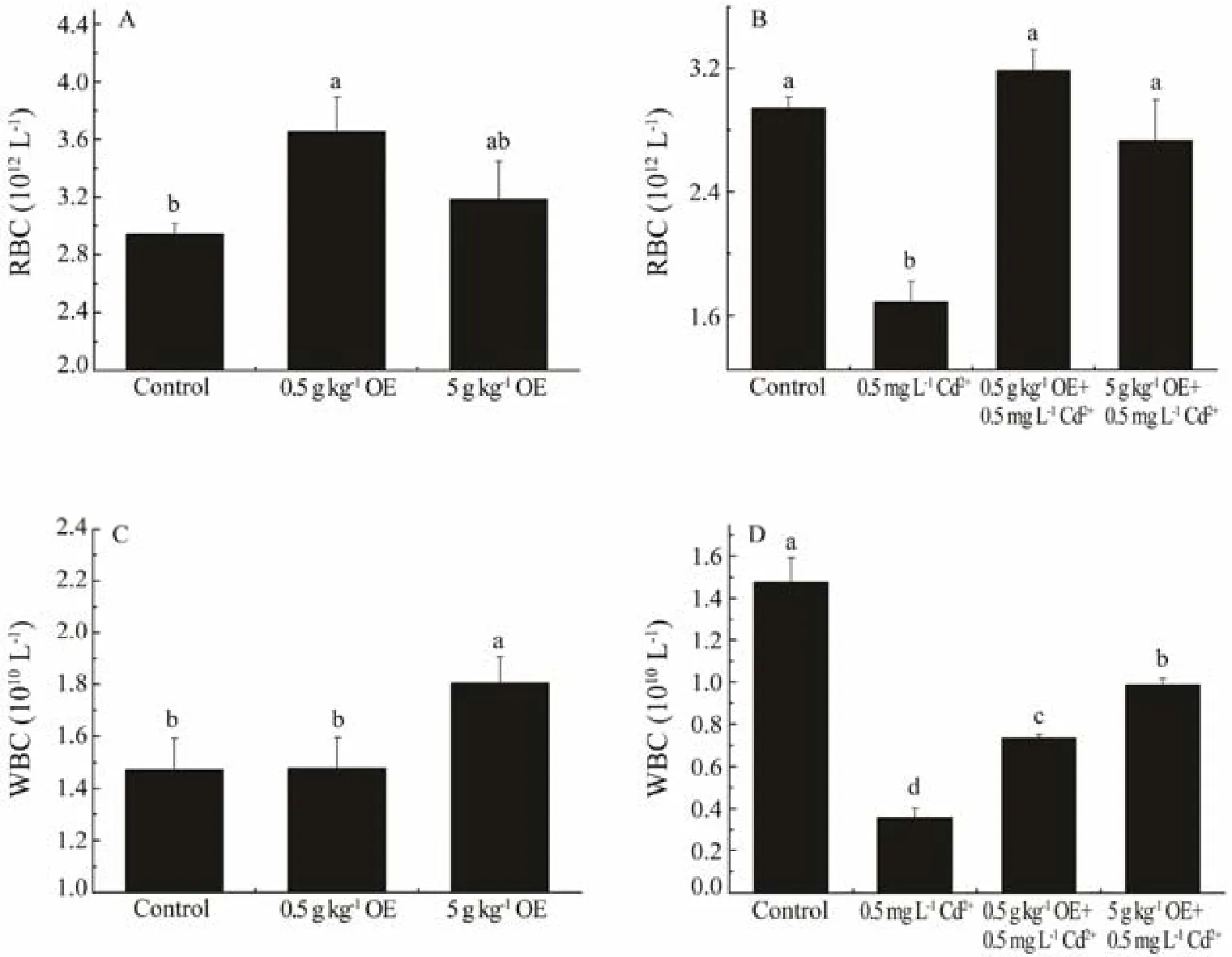
Fig.1 The RBC and WBC counts of black sea bream after 10 d of treatment with or without triterpene-enriched olive extract (OE). Mean values that do not share the same superscript are significantly different at P < 0.05.
Similarly, compared to that of the control, the WBC count of healthy black sea bream fed with diet containing 5 g kg-1OE was significantly increased to about 1.23 times of that of control (Fig.1C, F1,17= 4.57, P = 0.04). The WBC of sea bream exposed to 0.5 mg L-1Cd2+-spiked seawater without OE intake was significantly reduced to about 24.24% of that of control (Fig.1D, F1,17= 77.23, P = 1.61E-7). When dietary OE was administrated during Cd exposure, the WBC counts of black sea bream were significantly recovered to approximately 2.05 and 2.76 times of that of the negative control for groups supplied with 0.5 and 5 g kg-1OE, respectively (Fig.1D, F2,26= 90.20, P =6.86E-12).
3.2 The Impact of Dietary Intake of Triterpene-Enriched OE on the Concentration and Activity of Lysozyme
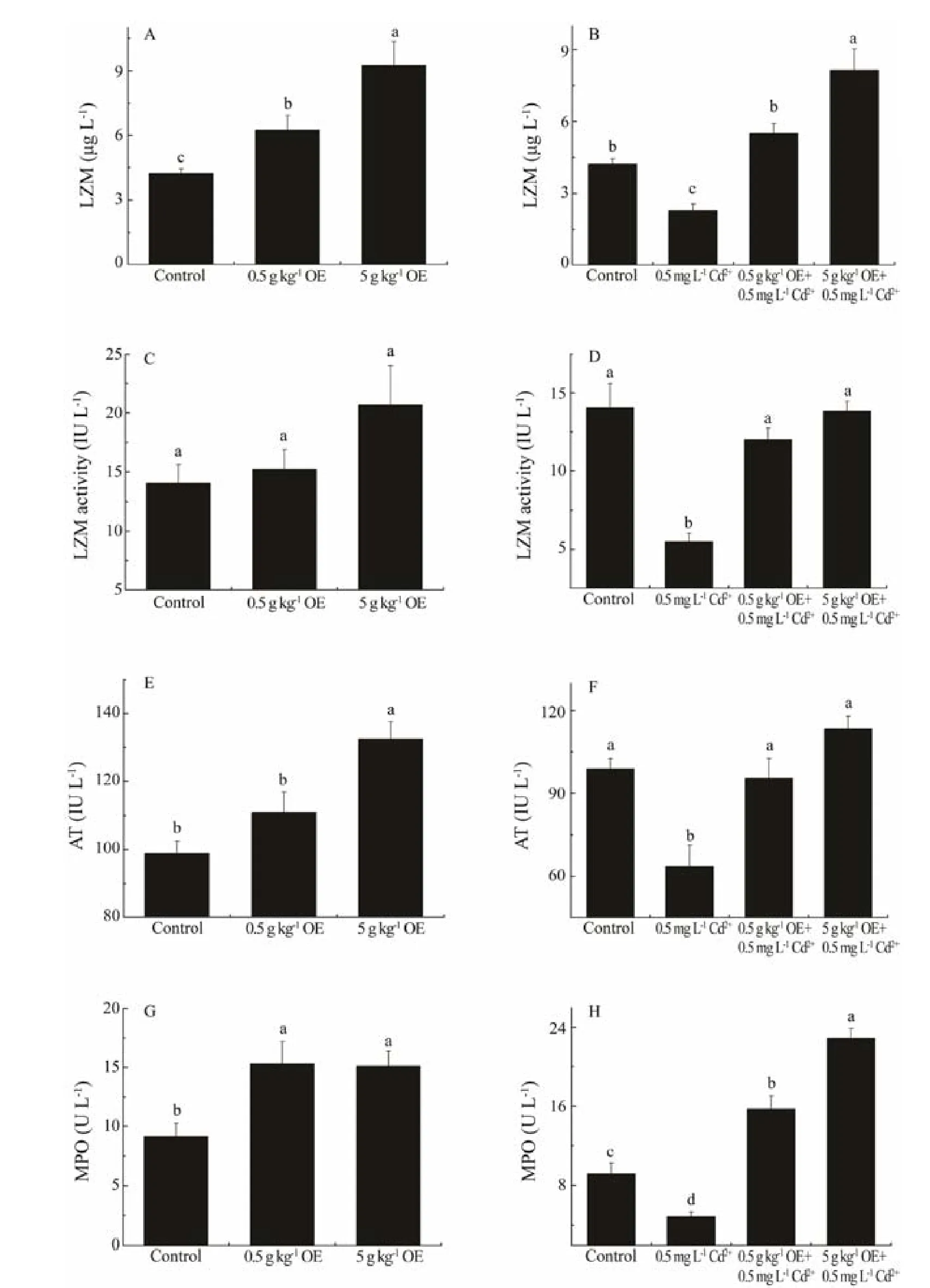
Fig.2 The concentration and activityof lysozyme, the activities of antiprotease and myeloperoxidase in the serum of black sea bream after 10 d of treatment with or without triterpene-enriched OE. Mean values that do not share the same superscript are significantly different at P < 0.05.
The in vivo concentration of lysozyme was significantly up-regulated after 10-days administration of OE, being increased to about 1.48 and 2.20 times of that of control,respectively, when black sea bream were fed with 0.5 and 5 g kg-1OE (Fig.2A, F2,26= 4.72, P = 0.02). Exposure of sea bream to 0.5 mg L-1waterborne Cd2+resulted in a significant decline in the in vivo content of lysozyme, which was only about 54.12% of that of control (Fig.2B, F1,17=29.85, P = 5.20E-5). In addition, upon being supplemented with dietary OE and exposure to Cd2+simultaneously, the in vivo content of lysozyme was significantly higher than that of negative control (Fig.2B, F2,26= 20.35, P = 6.78E-6), which was similar or even higher than that of individuals without being exposure to Cd2+.
Although the activity of lysozyme was not significantly induced by the dietary intake of OE for black sea bream without Cd2+treatment (Fig.2C, F2,26= 2.30, P = 0.12), the adverse impact of Cd2+exposure on lysozyme activity was significantly mitigated by the administration of OE (Fig.2D,F2,26= 46.99, P = 5.01E-9) as was observed in recovered levels comparable to that of fish without Cd2+treatment(Fig.2D, F2,26= 1.15, P = 0.33).
3.3 The Impacts of Dietary Intake of Triterpene-Enriched OE on the Activities of Antiprotease and Myeloperoxidase
Black sea bream fed with diet containing 5 g kg-1OE yielded a significantly higher (approximate 1.34 times) antiprotease activity than that of control (Fig.2E, F1,17= 28.79,P = 6.31E-5). While the antiprotease activity was significantly suppressed by Cd2+exposure, the adverse impact was significantly mitigated by the simultaneous intake of dietary OE as was reflected by antiprotease activity recovering to that of black sea bream without Cd2+treatment (Fig.2F, F2,26= 3.17, P = 0.06).
The myeloperoxidase activity significantly increased by about 66.85% and 64.66% in black sea bream fed with diets containing 0.5 and 5 g kg-1OE, respectively (Fig.2G,F2,26= 5.82, P = 0.01). Similar to other immune parameters investigated, the myeloperoxidase activity declined dramatically to 52.76% of that of control after 10-day exposure to 0.5 mg L-1Cd2+(Fig.2H, F1,17= 13.93, P = 1.81E-3).When OE was administrated and Cd2+exposure was done simultaneously, the activity of myeloperoxidase of groups supplied with 0.5 and 5 g kg-1OE, respectively, was significantly up-regulated to about 1.71 and 2.49 times of that of negative control (Cd2+exposure group), respectively(Fig.2H, F2,26= 88.96, P = 7.95E-12).
3.4 The Impacts of Dietary Intake of Triterpene-Enriched OE on the Concentrations of IL-4 and IL-6
When the fish was feed with diet containing 5 g kg-1OE but not exposure to Cd2+, the content of serum IL-4 of black sea bream was about 1.77 times higher than that of control (Fig.3A, F1,17= 37.54, P = 1.46E-5). Though the exposure of sea bream to 0.5 mg L-1Cd2+for 10 days led to a significant decline (approximately 29.15%) (Fig.3B,F1,17= 9.88, P = 6.29E-3), the serum IL-4 content of black sea breams fed with 0.5 and 5 g kg-1OE was significantly increased to as high as 2.23 and 1.79 times of that of the negative control, respectively (Fig.3B, F2,26= 69.54, P =1.03E-10).
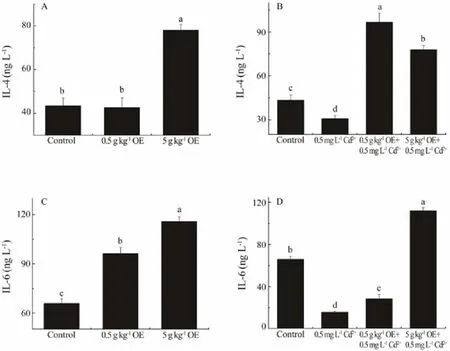
Fig.3 The concentrations of IL-4 and IL-6 in the serum of black sea bream after 10 d of treatment with or without triterpene-enriched OE. Mean values that do not share the same superscript are significantly different at P < 0.05.
The content of serum IL-6 of black sea bream was significantly increased by dietary intake of triterpene-enriched OE (Fig.3C, F2,26= 68.28, P = 1.24E-10). About 1.46 and 1.75 times of serum IL-6 content of the control was detected when black sea bream were feed with diets containing 0.5 and 5 g kg-1OE, respectively (Fig.3C). Though the IL-6 content of fish exposing Cd2+for 10 days was significantly reduced to only 38.98% of that of control (Fig.3D,F1,17= 324.83, P = 4.73E-10), this adverse impact was significantly mitigated by OE administration (Fig.3D, F2,26=322.09, P = 0.00).
3.5 The Impacts of Dietary Intake of Triterpene-Enriched OE on the Expressions of Immune-Relating Genes
Except for COX-2, the expressions of genes under investigation were all significantly up-regulated by the administration of OE (Fig.4, P < 0.05). Compared to that of control, the expressions of IkBα significantly ascended by 58.51% and 61.88% in fish feeding with diets containing 0.5 and 5 g kg-1OE, respectively. Similarly, the expression of TNFα of fish individuals fed with 0.5 and 5 g kg-1OE, respectively, increased to 1.96 and 2.08 times of that of control. There is about 76.92% and 82.00% increase in the expression of FOS in black sea breams fed with 0.5 and 5 g kg-1OE, respectively. The expression of COX-2 was up-regulated to 5.44 times of that of control by administration of 5 g kg-1OE.
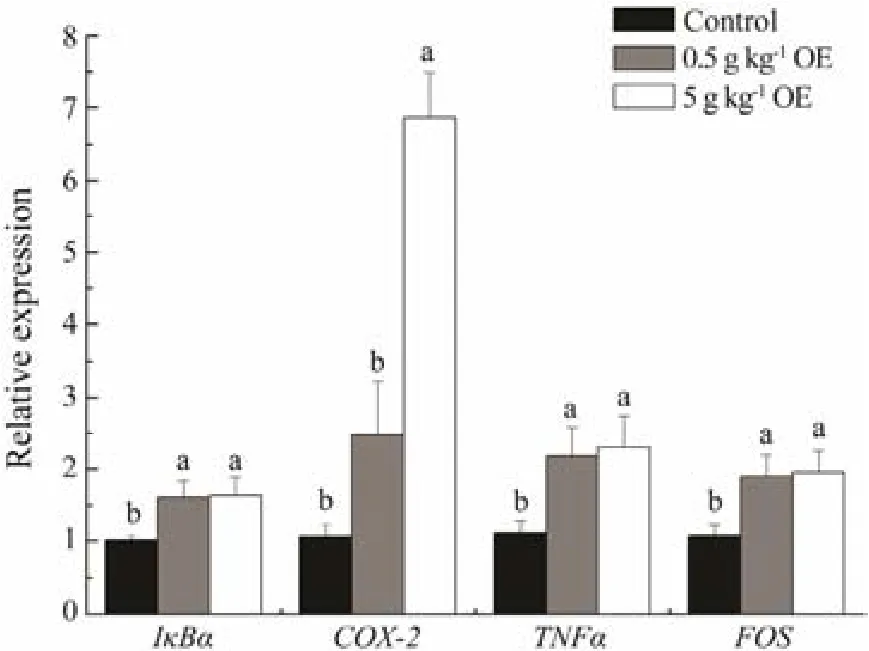
Fig.4 The relative expressions of genes encoding IκBα,COX-2, TNFα, and FOS in black sea bream after 10 days of treatment with or without triterpene-enriched OE. Mean values that do not share the same superscript are significantly different at P < 0.05.
4 Discussion
It has been suggested that plant extracts, such as those from Astragalus radix, Ganoderma lucidum, Achyranthes aspera, Trigonella foenum graecum and Muscari comosum, can be used in aquaculture as immunopotentiators to increase the immunity of cultured fish species (Rao et al.,2006; Yin et al., 2008; Baba et al., 2014; Awad et al., 2015;Bahi et al., 2017). The results of this study showed that triterpene-enriched OE has similar immune enhancement function and therefore can be applied as an immunopotentiator for commercial teleost. In addition, according to the data obtained, dietary intake of triterpene-enriched OE may exert beneficial immunomodulatory effects through increasing haemocyte counts and activating immune-relating biomolecules and signaling pathways (Fig.5).
Since blood cells play crucial roles in the immune response, haematological parameters such as blood cell counts are widely used as indicators of the immune status in fish (Agrawal and Mahajan, 1980). In the present study,intake of dietary OE exerted significant mitigation effects on blood cell counts when black sea bream were exposed to Cd2+. This finding indicated an immune enhancement function of triterpene-enriched OE without provoking undesired collateral effects (anemia), which is comparable to the effects of other herbal immunopotentiators reported in other fish species. For example, similar findings have also been documented in Labeo rohita fed with Ocimum sanctum extract and Oncorhynchus mykiss fed with Spirulina platensis (Das et al., 2015; Yeganeh et al.,2015). In addition, the induction impact of dietary intake of triterpene-enriched OE on blood cell counts may be due to the up-regulation of IL-6 synthesis. In addition to playing an important role in immune response, IL-6 has been proven to be a hematopoietic regulatory factor that can promote the production of leucocyte and erythrocyte(Ulich et al., 1989; Patchen et al., 1991). In this study, the in vivo content of IL-6 was found to be significantly increased by dietary OE intake, which may, therefore, lead to an increase in haemocyte counts due to its hematopoietic regulatory function.
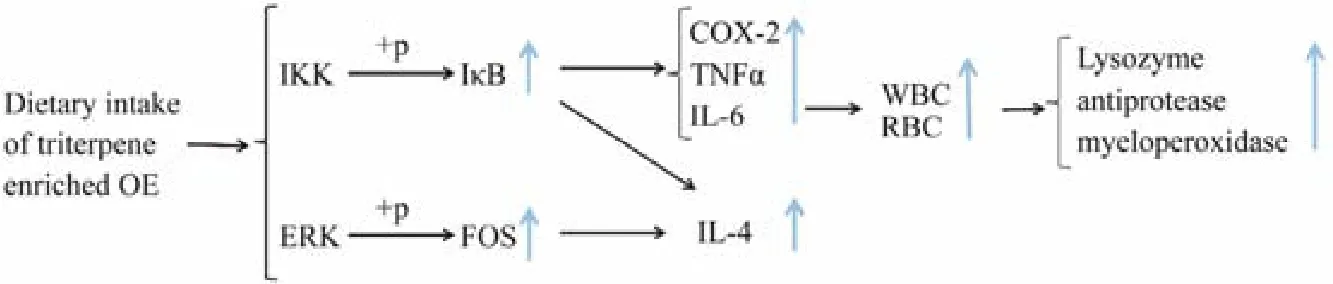
Fig.5 Potential affecting pathways underpinning the immune enhancement effect of triterpene-enriched OE in black sea bream.
It is well known that lysozyme, antiproteases, and myeloperoxidase play crucial roles in the nonspecific immune response in both mammals and teleost (Workenhe et al.,2010). However, the impacts of triterpenes extracted from olive on these parameters are not clear to date. The results obtained in the present study suggested that the up-regulation of lysozyme, antiproteases, and myeloperoxidase may be a mechanism through which the dietary intake of triterpene-enriched OE exerts immune enhancement in black sea bream.
While the lysozyme activity was not significantly improved by the dietary intake of OE, the in vivo content of lysozyme was significantly increased through OE administration as was found in this study. In addition, the adverse impact on lysozyme induced by Cd2+exposure was found to be significantly mitigated by OE intake. Since lysozyme is an important defense molecule counteracting against pathogens through dissolving the peptidoglycan structure of pathogen (Fearon and Locksley, 1996; Medzhitov and Janeway, 1998), our data indicated that OE administration can improve the immune response of black sea bream through activating lysozyme, which is similar to the findings in other species (Rao et al., 2006; Zheng et al., 2015). For instance, comparable impacts on lysozyme have been revealed in Schizothorax prenanti fed with oxidized konjac glucomannan and L. rohita fed with Achyranthus (Rao et al., 2006; Zheng et al., 2015). In addition, since lysozyme is mainly produced by neutrophils, a leucocyte subtype (Fearon and Locksley 1996; Medzhitov and Janeway 1998), the increase in lysozyme content may attribute to the increase in leucocyte induced by OE through IL-6 as mentioned above.
The up-regulation of myeloperoxidase activity upon OE administration was similar to those reported in Asian catfish, Clarias batrachus, and rainbow trout, O. mykiss,that are fed with diets supplemented with other dietary immunostimulants (Kumari and Sahoo, 2006; Awad et al.,2013). Myeloperoxidase, one of the most important antimicrobial enzymes, is mostly released by the neutrophils during the oxidative respiratory burst (Dalmo et al., 1997;Palic et al., 2005). Therefore, it is possible that the increase of the activity of myeloperoxidase is caused by OE induced leucocyte production via IL-6 as well.
In agreement with the results in Oreochromis mossambicus fed with Eclipta alba leaf aqueous extract (Christybapita et al., 2007) and O. mykiss fed with Nigella sativa and Quercetin extracts (Awad et al., 2013), the activity of antiprotease in black sea bream was found to be significantly induced by dietary intake of 5 g kg-1triterpene-enriched OE in the present study. In addition to fighting against pathogen infection, antiprotease is also known to protect cells from damages incurred by protease (McKerrow et al., 1999; Ellis, 2001; Sharifuzzaman and Austin,2009). In immune response, when mature neutrophils are activated, elastase-based proteases will be released and subsequently they will reinforce the inflammatory response.In this circumstance, the activation of antiprotease, which inhibits the activity of elastase, will help to maintain homeostasis (McKerrow et al., 1999; Ellis, 2001; Sharifuzzaman and Austin, 2009). Therefore, the significant increase in the activity of antiprotease detected upon OE administration indicated an enhanced immune response, which may be provoked by the increased release of elastase by neutrophils.
It has been well demonstrated that several immunogenic pathways such as toll-like receptor (TLR) pathway,NF-κB signaling pathway, and ERK signaling pathway will be activated to ensure effective protection against pathogen infection (Seth et al., 2006; Zha et al., 2018,2019). Challenged by foreign particles such as those from pathogenic organisms, individual TLR specifically recognizes different pathogen-associated molecular patterns(PAMPs) and subsequently activates myeloid differentiation factor 88 (MyD88)-dependent downstream pathways(Kaisho and Akira, 2006; Kawasaki and Kawai, 2014).MyD88 recruits members of the IL-1 receptor-associated kinase (IRAK) through death domain-death domain interactions. This process subsequently activates the NF-κB signaling pathway through molecules such as tumor necrosis factor receptor-associated factor (TRAF), TAK1-binding protein 1 (TAB1), TAK1-binding protein 2 (TAB2),and TGF-β-activated kinase-1 (TAK1) (Li and Qin, 2005;Sato et al., 2005; Kaisho and Akira, 2006). The activation of TAK1 then breaks down the IKK complex, phosphorylates NF-κB inhibitor alpha (IkBα), and eventually activates the immune effector NF-κB (Oeckinghaus et al.,2011). The activation of NF-κB will then modulate immune-related cytokines such as TNFα, IL-6, and COX-2(Barton and Medzhitov, 2003; Kaisho and Akira, 2006;Seth et al., 2006; Workenhe et al., 2010). In addition, during the specific immune response, antigen peptide, the polypeptide hydrolyzed by protease, and major histocompatibility complex-II (MHC-II) will form antigen peptide-MHC-II molecule complex on the surface of antigen-presenting cells (APC), which can subsequently be recognized by corresponding CD4+ T cells (Kolls, 2013). In addition to provoking the IKK pathway, this process also activates the extracellular regulated protein kinases (ERK),which phosphorylates the proto-oncogene protein c-fos(FOS) and thereafter promotes the production of cytokines such as IL-4 (Zhang et al., 2004; Ríos, 2010). In the present study, it was found that the expressions of IkBα,TNFα, COX-2, and FOS genes were significantly up-regulated by dietary intake of triterpene-enriched OE, indicating OE probably exerts immuneenhancement effect through activating both NF-κB and ERK signaling pathways (Fig.5).On one hand, OE administration may up-regulate the phosphorylation of IkBα and therefore lead to the induction of TNFα, COX-2, and IL-6. On the other hand, dietary intake of triterpene-enriched OE probably up-regulates the phosphorylation of FOS through the ERK pathway and therefore results in the increase of IL-4 contents. In addition, the present study demonstrated that the expression of IkBα was induced upon OE administration. With an NFκB binding site within its promoter, the expression of IkBα is regulated by NF-κB (Lee et al., 2003). Therefore,the induction of IkBα may be a feed- back response to the upregulation of NF-κB.
In conclusion, evident immune enhancement effect of dietary intake of triterpene-enriched OE in black sea bream was revealed in the present study, which was probably due to its comprehensive beneficial impacts on blood cells and key immune-relating molecules and pathways. In addition, the present study indicated that triterpene is latently one of the key functional biomolecules of OE exerting immune enhancement impact.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2018YFD0900603), the National Natural Science Foundation of China (No. 31672634) and the research funding from Innovation Division, Lucta (Guangzhou) Flavours Co., Ltd.
杂志排行
Journal of Ocean University of China的其它文章
- Abyssal Circulation in the Philippine Sea
- Numerical Study of Storm Surge Inundation in the Southwestern Hangzhou Bay Region During Typhoon Chan-Hom in 2015
- Interannual Variability and Scenarios Projection of Sea Ice in Bohai Sea Part I: Variation Characteristics and Interannual Hindcast
- Probability Distribution of the Hull Motion and Mooring Line Tension of Two Floating Systems
- Suppression of Vortex-Induced Vibration by Fairings on Marine Risers
- The Characteristics of Storm Wave Behavior and Its Effect on Cage Culture Using the ADCIRC+SWAN Model in Houshui Bay, China
