Microinjection of a growth factor cocktail affects activated microglia in the neocortex of adult rats
2020-03-07RuoXuLiuJieMaNingGuoShaoJunLiu
Ruo-Xu Liu, Jie Ma, Ning Guo, Shao-Jun Liu
State Key Laboratory of Proteomics and Department of Neurobiology, Institute of Military Cognition and Brain Sciences, Beijing, China
Abstract Microglia, as the resident immune cells in the central nervous system, play important roles in regulating neuronal processes, such as neural excitability, synaptic activity, and apoptotic cell clearance. Growth factors can activate multiple signaling pathways in central nervous system microglia and can regulate their immune effects, but whether growth factors can affect the morphological characteristics and ultrastructure of microglia has not been reported. After microinjecting 300 nL of a growth factor cocktail, including 10 μg/mL epidermal growth factor, 10 μg/mL basic f ibroblast growth factor, 10 μg/mL hepatocyte growth factor and 10 μg/mL insulin-like growth factor into adult rat cortex, we found that the number of IBA1-positive microglia around the injection area increased significantly, indicating local activation of microglia. All CD68-positive labeling co-localized with IBA1 in microglia. Cell bodies and protrusions of CD68-positive cells were strongly attached to or were engulf ing neurons. Characteristic huge phagosomes were observed in activated phagocytes by electron microscopy. The phagosomes generally included non-degraded neuronal protrusions and mitochondria, yet they contained no myelin membrane or remnants, which might indicate selective phagocytosis by the phagocytes. The remnant myelin sheath after phagocytosis still had regenerative ability and formed “myelin-like” structures around phagocytes. These results show that microinjection of a growth factor cocktail into the cerebral cortex of rodents can locally activate microglia and induce selective phagocytosis of neural structures by phagocytes. The study was approved by the Institute of Laboratory Animal Science, Beijing Institute of Basic Medical Sciences (approval No. IACUC-AMMS-2014-501) on June 30, 2014.
Key Words: adult neocortex; CD68; IBA1; microinjection; phagocyte; selective phagocytosis; ultrastructure
Introduction
Microglia are a type of glial cell located throughout the central nervous system. Microglia are derived from monocytes that originate from the yolk sac and spread throughout the central nervous system by invading the primitive brain tissue in early development. During prenatal development, microglia rapidly proliferate and ultimately become the resident immune cells in the brain (Alliot et al., 1999; Ginhoux and Prinz, 2015; Lenz and McCarthy, 2015; Wolf et al., 2017; Gomes-Leal, 2019) with a stable population throughout life (Ajami et al., 2007; Ginhoux and Prinz, 2015). In the postnatal brain of mammals, microglia transform into a highly ramif ied phenotype and constantly screen their environment (Wolf et al., 2017). They also play an active part in many basic processes in healthy brain physiology, including cell proliferation and synaptic connectivity (Lenz and McCarthy, 2015).
Microglia can be activated by any type of pathological event or change in brain homeostasis (Wolf et al., 2017). This activation process is highly diverse and depends on the context and type of stress or pathology, such as brain trauma, spinal cord injury or hypoxic/ischemic damage (Perez-Dominguez et al., 2019). Once stimulated, microglia rapidly undergo a morphological transformation and retract their highly ramif ied, branched processes to take on an amoeboid form (Lenz and McCarthy, 2015; Ransohoff and El Khoury, 2015). Some bioactive substances, including inf lammatory cytokines, bioactive factors, lipopolysaccharides, and chemokines (Kreutzberg, 1996), also strongly inf luence the pathological outcome or immune response of microglia (Wolf et al., 2017). Accumulating evidence also shows that microglia are involved in the regulation of neuronal excitability, synaptic activity, neurogenesis, and clearance of apoptotic cells in the healthy adult brain. Although reviews mention the inf luence of growth factors on signal transduction in microglia (ElAli and Rivest, 2016; Kaminska et al., 2016), no report has described the activation of microglia by growth factors. This study explored the protective effect of nerve growth factors on the nervous system by local injection of a high concentration growth factor cocktail into normal rats.
Materials and Methods
Animals
Sixteen adult male Sprague-Dawley rats weighing 250 g were purchased from SPF (Beijing) Biotechnology Co., Ltd. [license No. SCXK (Jing) 2019-0010]. All rats were maintained on a 12-hour day/night cycle with lights on at 06:00 at 24°C and 30-50% relative humidity. This study was approved by the Institute of Laboratory Animal Science, Beijing Institute of Basic Medical Sciences (approval No. IACUC-AMMS-2014-501) on June 30, 2014.
Microinjection of growth factor cocktail
Under sodium pentobarbital anesthesia (50 mg/kg body weight, P3761, Sigma), the rats were randomly divided into control and experimental groups (n = 8) and received saline and growth factor cocktail microinjections, respectively. During surgery, animals were fixed on a stereo positioner (RWD, Shenzhen, China) and the skull was exposed through a vertical skin incision. After determining the precise coordinates (1.8 mm from bregma and 2 mm from the midline), small holes with a diameter of 1.5 mm were drilled on both sides of the skull. A glass needle f illed with growth factors or saline was inserted into the prefrontal cortex to a depth of 2 mm. A total of 300 nL (15 nL/pump × 20 times) cocktail or saline was injected into each side of the cortex within 15 minutes. The skin on the skull was sutured after the foramen was sealed with bone wax. The growth/neurotrophic factor cocktail was composed of epidermal growth factor (Cat# E4127 Lot: SLBJ4118V; Sigma, St. Louis, MO, USA), basic fibroblast growth factor (Cat# PMG0033, Lot 489821E; Invitrogen, Waltham, MA, USA), hepatocyte growth factor (Cat# 375228-5UG, Lot: D00165582; Millipore, Darmstadt, Germany), and insulin-like growth factor (Cat# GF306, Lot: 2576396; Millipore). The final concentration of each factor was 10 μg/mL.
Immunof luorescence staining
Four days after microinjection, rats in the control and experimental groups (n = 5) were anesthetized with sodium pentobarbital (50 mg/kg body weight) and then perfused with saline solution followed by 4% formaldehyde in icecold phosphate buffer for 100 minutes. The brain cortex was removed, post fixed for 4 hours in 4% formaldehyde, and then incubated in 20% sucrose solution overnight at 4°C. Frozen 20 μm sections were cut and incubated in phosphate-buffered saline containing 4% donkey serum, 0.3% bovine serum albumin, and 0.3% Triton for 2 hours at room temperature, and then incubated with different primary antibodies [IBA1 (ionized calcium binding adapter molecule 1), a macrophage/microglia-specific protein that is upregulated upon cell activation; CD68, a macrophage-specific marker that is associated with lysosomes; NeuN: a neuronal marker] at 4°C for 24 hours. The incubated sections were then washed in phosphate-buffered saline for 10 minutes, three times, and then incubated with appropriate Alexa f luor-conjugated goat-anti mouse/rabbit secondary antibodies (1:500 dilution) for 2 hours at room temperature. After three washes in phosphate-buffered saline, sections were counterstained with Hoechst 33258 (Lot: B28838, 1:1000; Sigma-Aldrich) or DAPI (Lot: D9642, 1:1000; Sigma-Aldrich) for 10 minutes, and mounted using F4680 FluoromountTMaqueous mounting medium (Lot# SLBQ2436V; Sigma) to prevent f luorescence quenching. Antibodies used were: anti-NeuN (Cat# ab104224, Lot: GR138829-25, mouse, 1:500, or Cat# ab177487, Lot: GR249899-5, rabbit, 1:500; Abcam, Cambridge, MA, USA), anti-IBA1 (Cat# 019-19741, rabbit, 1:1000; Wako, Osaka, Japan), anti-CD68 (Cat# ab955, mouse, 1:1000; Abcam). Fluorescein-conjugated secondary antibodies corresponded to the primary antibodies. Images were captured using a laser confocal microscope (Zeiss, Oberkochen, Germany) and data from sections at the same anatomical level were compared and analyzed.
Electron microscopy
Four days after microinjection, rats receiving cocktail (n = 3) or saline (n = 3) were perfused with saline, and then with a solution containing 1.25% glutaraldehyde and 1% paraformaldehyde for 100 minutes. The brain was then removed and immersed in the same solution for 2 hours. Vibratome slices of appropriate brain regions were cut and some of the slices were prepared for electron microscopy (Hitachi, Tokyo, Japan). Selected slices were incubated in 2% osmium tetroxide in 0.1 M phosphate buffer for 1 hour. After dehydration in ascending concentrations of ethanol, slices were embedded in EPON 812. Polymerization was performed at 40°C for 6 hours and at 45°C for 24 hours. Semi-thin sections were stained with toluidine blue and observed under an electron microscope. Ultrathin sections were counterstained with lead citrate and observed using electron microscopy. The images were analyzed using Zeiss analysis software (Version 12.0.1.22; Zeiss, Oberkochen, Germany) or Volocity analysis software (PerkinElmer, Hopkinton, MA, USA).
Results
Cortical injection of growth factor cocktail activates local phagocytes
In the control group, IBA1-positive microglia were evenly distributed among NeuN-positive neurons. They were small cells (between 6 and 15 μm in diameter) and had a triangular or circular shape. Variable numbers of cell protrusions extended from cell bodies and branched further to form even f iner protrusions that meandered evenly between cortical neurons. No protrusions or deformed cell bodies were directly attached to NeuN-positive neurons (Figure 1A). The number of IBA1-positive cells around the saline injection site was 10,110/mm3with no significant change in cell morphology. No CD68-positive cells were found in the control group (Figure 1B).
Around the growth factor cocktail injection site, the density of IBA1-positive microglia was 21,565/mm3, which was higher than the density in the cortex of the control group. The density of IBA1-positive cells decreased and their morphology changed significantly as the slice plane gradually moved away from the needle track. In the core region of the injection site, the diameter of IBA1 cells increased to 20-50 μm. These cells presented an irregular shape and hypertrophic protrusions that had no obvious boundary with the cell body. The IBA1 cells were distributed among NeuN-positive cells (Figure 2A). Some IBA1 cell bodies and protrusions were located around or even had direct contact with neurons.
A considerable number of CD68-positive cells was also present in the bottom two thirds of the cylindrical area of 1 mm in diameter around the needle trace and below the injection site. The density of CD68-positive cell was 12,625/mm3. There was no significant cell number reduction in this area; however, CD68-positive cells were almost never seen outside the tissue cylinder. CD68-positive cells were basically round with a diameter between 20-50 μm. Few processes originated from the bodies of CD68-positive cells. Most CD68-positive cells were in direct contact with NeuN-positive neurons, or even formed local crescent-shaped structures around neuronal cell bodies. Crescent notches were commonly formed on the neuronal surface in direct contact with CD68-positive cells, which indicates that neurons have been invaded by microglia (Figure 2B). In the IBA1 and CD68 double-immunostained sections, all CD68-staining was co-localized within IBA1-positive cells (Figure 2C).
Ultrastructure of activated microglia
Overview of the ultrastructure of activated microglia
Activated primary microglia around the injection site were observed by electron microscopy. The primary activated microglia had an irregular or a triangular shape and were significantly increased in size, usually having a diameter of 25-50 μm or more. A large number of pseudopods were observed to emanate from the activated microglia. Membrane fusion was occasionally spotted between two activated microglia, which indicated that two cells were merging together. Microglial nuclei were small with high electron density heterochromatin plaques under the nuclear membrane. The nuclear heterochromatin either gathered around the perinuclear membrane or aggregated to form irregular masses “drifting” in the euchromatin-aggregated nucleoplasm. The cell cytoplasm had the characteristic ultrastructure of activated microglia, which had high-contrast electron negative staining that appeared dark and contained a large amount of rough endoplasmic reticulum. Complete or incompletely engulfed nerve tissue, mainly including dispersed and phagocytized dystrophic neurites, was common in the region peripheral to the activated microglia/phagocytes (Figure 3A).
Compared with primary cells, secondary activated microglia became larger and displayed a similar f ine structure in spite of a slightly brighter electron staining with more engulfed nerve tissues and more pseudopods around its periphery than primary cells (Figure 3B).
Huge phagosomes in phagocytes
Huge phagosomes (16 μm × 12 μm) of the pocket type were found in phagocytes (Figure 3C). The pocket-shaped phagosomes were observed to open outside phagocytes through a thin neck at the upper tip (Figure 3C-c). These huge phagosomes were encapsulated by bilayer membranes and were isolated from the phagocyte cytoplasm. The two membranes layers faced the cytoplasm and the phagosome respectively, with a gap between them (Figure 3C-d). In addition to the remnants of neuronal protrusions and secondary lysosomes enclosed by the membrane structures, a large number of bulky mitochondria with clear structures existed in phagosomes (Figure 3C-d and -e). Although dystrophic neurites were engulfed by phagocytes, no “myelin-like” structures or remnants thereof were observed in the huge phagosomes or in the cytoplasm of phagocytes. Apart from one or two huge phagosomes, other phagosomes were not observed in the cytoplasm of phagocytes.
“Myelin-like membrane structure” outside phagocytes
A layer of myelin-like membrane structure was wrapped around the phagocyte. They osculated either directly or indirectly by leaving a gap (Figure 3C). The structure of this “myelin-like” multilayer membrane was identical to the myelin as seen by high magnification electron microscopy. The membrane structure outside the phagocytes was further traced, and when a phagocyte swallowed a myelinated nerve fiber, only the internal neurites were selectively phagocytosed, while the outer layer of the “myelin-like” structure was left aside (Figure 3C), so that the residual myelin-like structure was stacked outside the phagocyte.
Further observations revealed that the folded myelin structure remnant could be partially stretched around the phagocytes (Figure 3D) and wrapped around the outer surface of the phagocytes to form a “myelin-like” membrane structure (Figure 3D). Umbrella-shaped membrane structures were also observed to sprout from the remnant myelin sheath, which indicated that myelin sheath regeneration originates from the remnant myelin sheath (Figure 3D).
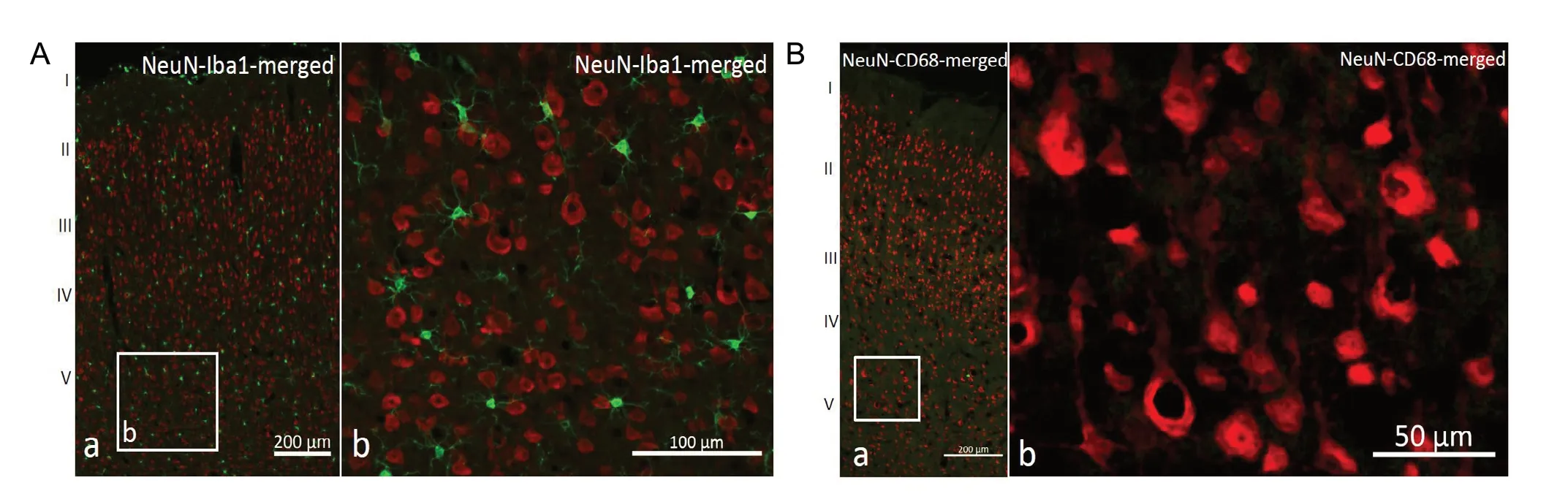
Figure 1 IBA1 and CD68 distribution in the rat cortex around the injection site after saline microinjection.
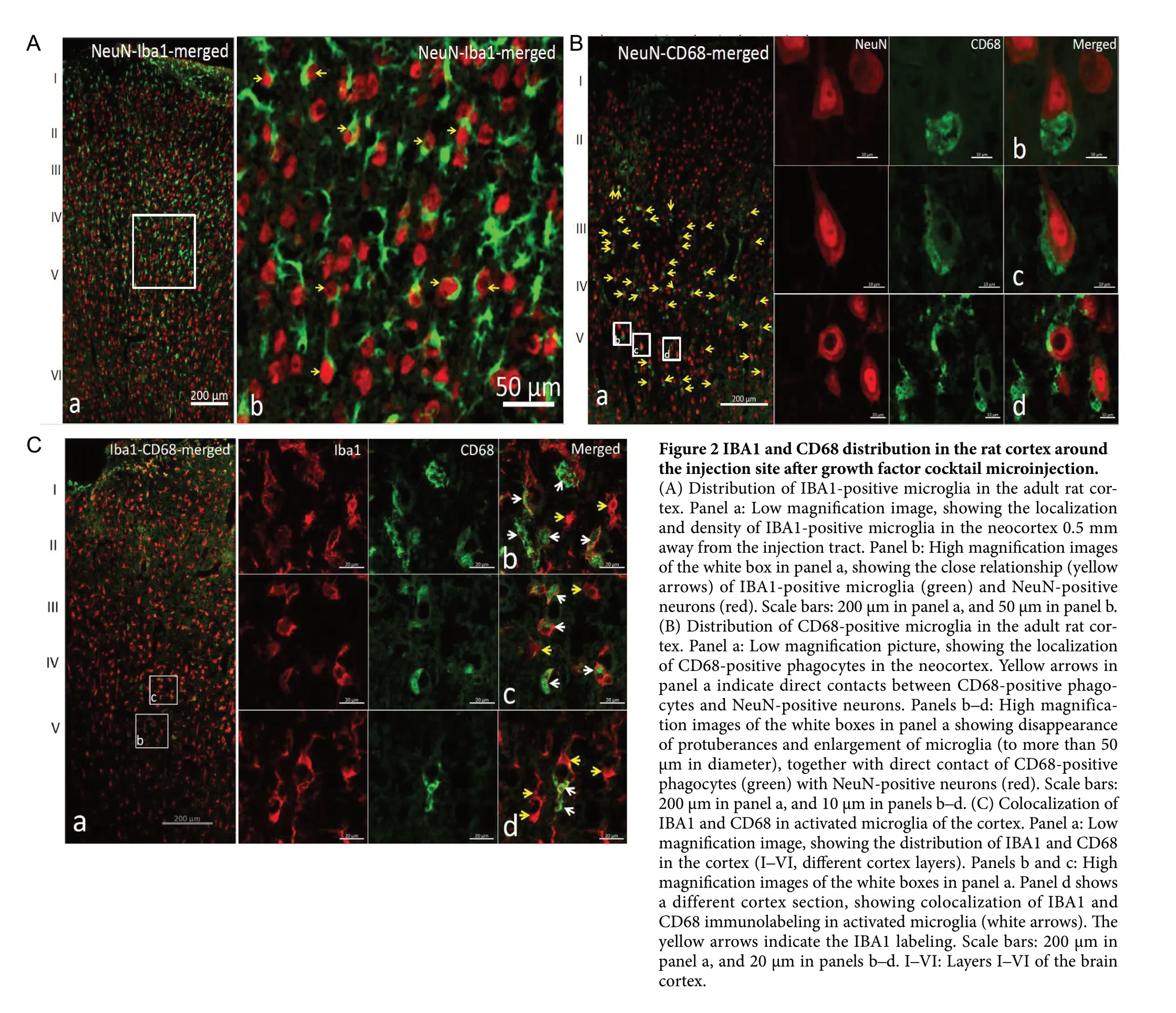
Figure 2 IBA1 and CD68 distribution in the rat cortex around the injection site after growth factor cocktail microinjection.
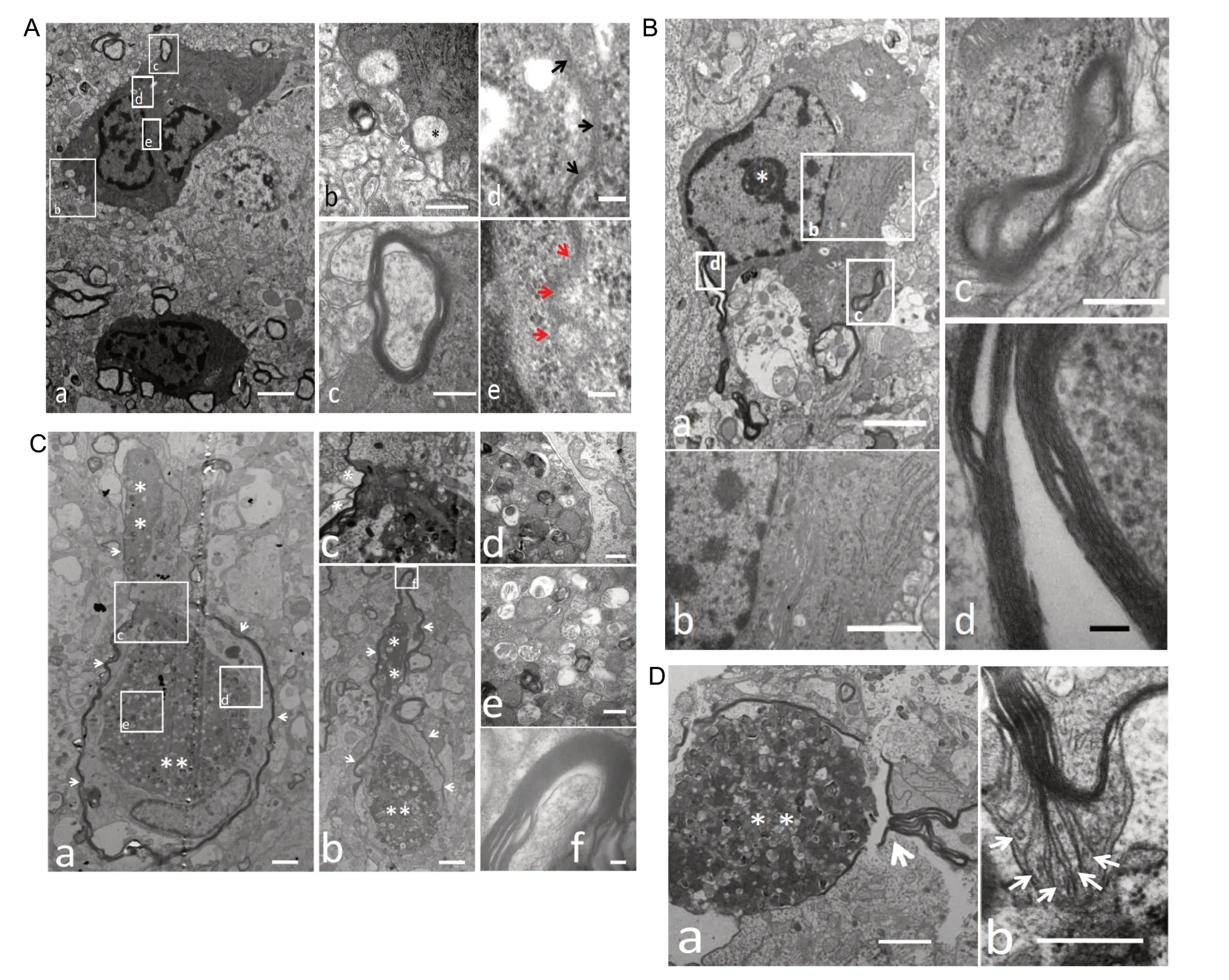
Figure 3 Ultrastructure of activated microglia.
Discussion
Microinjection of growth factors activates microglia in the adult neocortex
IBA1 is a 17 kDa molecule that is expressed at high levels in microglia of the cerebral cortex. It can act as a marker to distinguish the former from inf iltrated monocytes (Hendrickx et al., 2017; Konishi et al., 2017; Xu et al., 2017). Around the cocktail-injected region, both the number and volume of IBA1-positive cells were significantly increased. The characteristic ultrastructure of primary and secondary activated microglia indicates microglial activation around the injection site (Tremblay et al., 2013; Kaur et al., 2017).
CD68 is a receptor protein with a molecular weight of 110 kDa, and is expressed in macrophages derived from monocytes as well as in phagocytes and activated microglia. CD68 has been used as a specif ic marker of differentiated mature microglia and exogenous macrophages (Ramprasad et al., 1996; Ginhoux and Prinz, 2015; Minett et al., 2016; Xu et al., 2017; McGill et al., 2018). All CD68-positive labeling co-localized with IBA1, demonstrating that CD68-positive cells are activated microglia rather than macrophages recruited from blood mononuclear cells.
For microinjection, we used a very fine glass needle of 200 μm diameter. The injection volume was 300 nL, which minimizes volume damage. Moreover, no significant enrichment of IBA1-positive cells was observed around the needle tract in the control group, indicating that neither microneedle penetration nor volume injury was sufficient to induce microglial activation. In the experimental group, enriched IBA1- and CD68-positive cells were observed at the injection area, strongly supporting the activation of microglia and phagocytes caused by the injection of the growth factor cocktail.
Although many factors can cause microglial activation in the brain, the direct introduction of growth factors leading to the activation of local microglia and phagocytes has not previously been reported. There are a considerable number of growth factor receptors on the membranes of microglia and phagocytes (Liu et al., 1998; Hermann et al., 2005; Moransard et al., 2010; Ogundele et al., 2017), and the injected growth factor cocktail may cause activation of microglia through these receptors. Alternatively, the growth factors can act on their receptors in neurons (Bondy and Lee, 1993; Nadarajah et al., 1998; Wright, 1998; Powell et al., 2001; Puehringer et al., 2013), resulting in physiological and biochemical changes that secondarily activate microglia. Despite these possible explanations, the precise mechanism by which growth factors provoke local microglia and phagocytes is not completely understood.
Many diseases that seriously affect quality of life are strongly associated with the cortex. For example, brain aging and senile dementia, cerebral palsy caused by hypoxia at childbirth, brain trauma, stroke, and cerebral infarction can involve different degrees of cerebral cortex damage. Extensive experimental and preclinical studies using stem cell transplantation for the treatment of brain diseases have been carried out. Stem cells that survive in the transplantation area can locally produce varying concentrations of numerous growth factors, such as hepatocyte growth factor, epidermal growth factor, f ibroblast growth factor and insulin-like growth factor. Growth factor has a strong neuroprotective effect and can be used as a protective drug against brain damage. However, our study suggests that local, high concentration growth factors may cause further damage by activating microglia.
Huge phagosomes in phagocytes
Electron microscopy showed a complete bilayer cell membrane and a gap between the two layers around the huge phagosome in phagocytes, which indicates that the giant phagosome may be formed by endocytosis. It is possible that the phagocytes continue to phagocytose extracellular nerve tissue that gradually fuse into giant phagosomes in the cell body. Although some myelinated nerves were observed to be partially engulfed in the periphery of phagocytes, no myelin structure or its remnants were observed in either huge phagosomes or in the cytoplasm of phagocytes. This may result from the specif ic phagocytosis of axons within the myelinated nerve fibers by activated microglia. Secondary lysosomes and a large number of intact mitochondria remained in the phagosomes. The presence of these mitochondria may provide energy for the degradation of phagosomes, either directly or indirectly.
Structure and origin of the extracellular myelin-like membrane surrounding phagocytes
Myelin-like membrane structures were observed to encapsulate phagocytes or were folded near them. The connectivity between these two kinds of membranes suggests that the extracellularly wrapped myelin sheath originates from the nearby folded myelin structure. The formation of this morphology may be caused by the mutual movement of phagocytes and myelin sheath. This is the first report of such a myelin-like membrane structure surrounding phagocytes. This study also shows that some protuberances with membranes grow from the remnant myelin sheath. Together, these findings show that the remnant myelin sheath structure still has the ability to regenerate and move.
Scientific significance
The present study found that exogenous growth factors can activate microglia/phagocytes in the cerebral cortex and trigger the phagocytic effect of microglia. Observing large independent phagosomes in phagocytes will help us to understand the phagocytic ability of activated microglia/phagocytes. Myeloid-like membrane structures were observed to locate outside phagocytes. This phenomenon can provide insight into the specif ic phagocytosis of nerve fibers by microglia. Further study is warranted to elucidate the specif ic mechanism of selective phagocytosis.
Acknowledgments:We thank Xin Xu (National Center of Biomedical Analysis, China) and Kai Wang (National Center of Biomedical Analysis, China) for the assistance in image captures with laser scanning confocal microscope and Sa Zhang (National Center of Biomedical Analysis, China) for electron microscope experiment.
Author contributions:Study concept: RXL, NG and SJL; methodology: JM, RXL; investigation, validation, formal analysis, data collection, manuscript writing, reviewing and editing: RXL and SJL; fundraising: SJL. All authors approved the final version of the paper.
Conf licts of interest:The authors declare that they have no competing interests.
Financial support:This work was supported by a grant from State Key Laboratory of Proteomics of China, No. SKLP-K201401 (to SJL); the National Key Project of Basic Research of China, No. 2009CB918301 (to SJL); the National Natural Science Foundation of China, No. 30430310, 30140001, 30370460 (to SJL). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement:This study was approved by the Institute of Laboratory Animal Science, Beijing Institute of Basic Medical Sciences (approval No. IACUC-AMMS-2014-501) on June 30, 2014. All experimental procedures were performed in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Com-mercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Yun-Qing Li, Air Force Medical University, China.Additional file:Open peer review report 1.
CORRIGENDUM
Corrigendum: Resveratrol reduces brain injury after subarachnoid hemorrhage by inhibiting oxidative stress and endoplasmic reticulum stress
doi:10.4103/1673-5374.276363
In the article titled “Resveratrol reduces brain injury after subarachnoid hemorrhage by inhibiting oxidative stress and endoplasmic reticulum stress”, published on pages 1734-1742, Issue 10, Volume 14 of Neural Regeneration Research (Xie et al., 2019), the author list is written incorrectly as “Yun-Kai Xie1,#, Xin Zhou1,2,#, Hong-Tao Yuan1, Jie Qiu1, Dan-Qing Xin1, Xi-Li Chu1, Da-Chuan Wang1,*, Zhen Wang2,*” instead of “Yun-Kai Xie1,#, Xin Zhou1,2,#, Hong-Tao Yuan1, Jie Qiu1, Dan-Qing Xin1, Xi-Li Chu1, Da-Chuan Wang2,*, Zhen Wang1,*”.
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of an injured ascending reticular activating system with recovery from a minimally conscious state to normal consciousness in a stroke patient: a diffusion tensor tractography study
- The role of vascularization in nerve regeneration of nerve graft
- New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target?
- Advanced diffusion magnetic resonance imaging in patients with Alzheimer’s and Parkinson’s diseases
- Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury
- Decoding epigenetic codes: new frontiers in exploring recovery from spinal cord injury
