The role of vascularization in nerve regeneration of nerve graft
2020-03-07TiamSaffariMeiwandBedarCarolineHundepoolAllenBishopAlexanderShin
Tiam M. Saffari , Meiwand Bedar , Caroline A. Hundepool, Allen T. Bishop Alexander Y. Shin
1 Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN, USA
2 Department of Plastic, Reconstructive and Hand Surgery, Erasmus Medical Center, Rotterdam, the Netherlands
Abstract Vascularization is an important factor in nerve graft survival and function. The specif ic molecular regulations and patterns of angiogenesis following peripheral nerve injury are in a broad complex of pathways. This review aims to summarize current knowledge on the role of vascularization in nerve regeneration, including the key regulation molecules, and mechanisms and patterns of revascularization after nerve injury. Angiogenesis, the maturation of pre-existing vessels into new areas, is stimulated through angiogenic factors such as vascular endothelial growth factor and precedes the repair of damaged nerves. Vascular endothelial growth factor administration to nerves has demonstrated to increase revascularization after injury in basic science research. In the clinical setting, vascularized nerve grafts could be used in the reconstruction of large segmental peripheral nerve injuries. Vascularized nerve grafts are postulated to accelerate revascularization and enhance nerve regeneration by providing an optimal nutritional environment, especially in scarred beds, and decrease fibroblast infiltration. This could improve functional recovery after nerve grafting, however, conclusive evidence of the superiority of vascularized nerve grafts is lacking in human studies. A well-designed randomized controlled trial comparing vascularized nerve grafts to non-vascularized nerve grafts involving patients with similar injuries, nerve graft repair and follow-up times is necessary to demonstrate the efficacy of vascularized nerve grafts. Due to technical challenges, composite transfer of a nerve graft along with its adipose tissue has been proposed to provide a healthy tissue bed. Basic science research has shown that a vascularized fascial f lap containing adipose tissue and a vascular bundle improves revascularization through excreted angiogenic factors, provided by the stem cells in the adipose tissue as well as by the blood supply and environmental support. While it was previously believed that revascularization occurred from both nerve ends, recent studies propose that revascularization occurs primarily from the proximal nerve coaptation. Fascial f laps or vascularized nerve grafts have limited applicability and future directions could lead towards off-the-shelf alternatives to autografting, such as biodegradable nerve scaffolds which include capillary-like networks to enable vascularization and avoid graft necrosis and ischemia.
Key Words: angiogenesis; fascial f lap; nerve graft; nerve injury; nerve regeneration; peripheral nerve; vascular endothelial growth factor; vascularization; vascularized nerve graft
Introduction
Vascularization is one of the great challenges faced in nerve reconstruction, especially in nerve autograft substitutes such as nerve allografts or conduits that do not contain living cells (Muangsanit et al., 2018). In these substitutes, vascularization needs to be achieved to ensure cellular survival and avoid central necrosis, which is observed in nerve grafts with large diameters and long defects (Auger et al., 2013; Muangsanit et al., 2018). The diffusion limit of oxygen to meet the demands of cellular metabolism is around 100-200 μm, which is an essential to recognize when evaluating the role of vascularity in neural regeneration (Jain et al., 2005; Rouwkema et al., 2010). There is an increased understanding of nerve regeneration and cell biology with compelling evidence for the association of arterial blood vessels and nerves, in particular the alignment of peripheral nerves with blood vessels. The nervous system forms intricate branching networks reaching every organ in the body and relies on the vascular tree to supply oxygen and nutrients to meet substantial metabolic demands supporting organ development (James and Mukouyama, 2011).
It has been suggested that vascular endothelial cells guide the regeneration of peripheral nerve axons by producing vascular endothelial growth factor (VEGF) to induce angiogenesis preceding the repair of damaged nerves (Cattin et al., 2015; Figure 1). VEGF is a potent multifunctional cytokine that stimulates the outgrowth of Schwann cells and blood vessels, while enhancing axonal outgrowth from the dorsal root ganglia (Sondell and Lundborg, 1999; Wongtrakul et al., 2002; Dumpich and Theiss, 2015; Lee et al., 2016). As a result, VEGF has become the focus of numerous investigations with regards to its effects on the peripheral nerves (Hobson et al., 2000; Storkebaum and Carmeliet, 2004; Li et al., 2008; Mohammadi et al., 2013; Lee et al., 2016). Clinical attempts to augment vascularization and improve nerve graft outcomes using VEGF have led to conf licting results (Best and Mackinnon, 1994; D’Arpa, 2016), suggesting that the specif ic molecular regulations and patterns of angiogenesis following peripheral nerve injury are broader and more complex.

Figure 1 Schematic illustration of a peripheral nerve.
In this review, the role of vascularization in nerve regeneration will be discussed, including the key regulation molecules in vascularization, the vascular system of the nerve, the mechanisms of vascularization and the application of vascularized nerve grafts (VNG) in peripheral nerve reconstruction.
Search Strategy and Selection Criteria
Literature research was performed using PubMed, Medline, Cochrane, Web of Science and Google Scholar databases using the following combination of keywords: “angiogenesis” OR “vascularization” OR “blood perfusion” AND “nerve regeneration” OR “nerve graft” OR “nerve transplantation” until August 2019. The results were further screened by title and abstract to only present studies in peripheral nerve injuries in both animals and humans. Other inclusion criteria were articles (i) written in English, (ii) published in the last 35 years and (iii) that had available abstracts. Articles describing nerve studies in spinal cord surgery, facial and eye surgery were excluded. Six exceptionally valuable articles published prior to 1985 were included based on snowballing.
Mechanisms of Vascularization
Formation of a vascular network of peripheral nerves
The processes underlying the formation of blood vessels are vasculogenesis and angiogenesis (Akhavani et al., 2008). Vasculogenesis describes the formation of the primitive vasculature in the embryo. After development of this primary vascular system, remodeling of pre-existing vessels into new areas forms mature vasculature as a result of angiogenesis. Central to the control of angiogenesis is VEGF, which is also critical to the maturation and stabilization of vessels in this process (Akhavani et al., 2008). VEGF-induced effects are mediated through receptor tyrosine kinases, predominantly expressed on endothelial cells (Neufeld et al., 1999).
A cadaver study identif ied f ive different patterns of blood supply to the nerves varying from no dominant arterial pedicle to multiple dominant arterial pedicles forming a continuous artery that accompanies the nerve (el-Barrany et al., 1999). While identifying vascular supply to peripheral nerve contributes to our knowledge, this does not clarify the mechanisms of peripheral nerve revascularization.
Revascularization of the nerve
The vascular tree and the nervous system branching networks are often patterned similarly in peripheral tissues and share several anatomical and functional characteristics (James and Mukouyama, 2011). This parallel pattern has raised questions regarding their interactions to explain whether both networks are independent of one another. In the embryonic limb skin, endothelial cells migrate toward and align with peripheral nerves in response to nerve and Schwann cell-derived VEGF-A; this is called angiogenesis (James and Mukouyama, 2011). Subsequently, the pattern of axons provides a spatial template for the pattern of arterial vessel branching resulting in congruence of these two networks.
Transplanted nerves have no vascular supply and need to undergo revascularization in the recipient bed (Bassilios Habre et al., 2018). Experimental models have investigated the pattern of endoneurial perfusion. These models provided evidence that the primary mechanism of revascularization in a conventional autograft is longitudinal inosculation (host vessels grow into the graft from both ends and anastomose with donor vessels) (Lind and Wood, 1986; Penkert et al., 1988; Best et al., 1999). The evidence of peripheral neovascularization or dependence on the graft bed as a source of revascularization had not evolved until later when centripetal revascularization, growth from the surrounding tissue bed, was described (Tarlov, 1945). Inosculation results in endothelial-lined formed vessels at the nerve graft coaptation site. When the nerve graft metabolic requirements exceed the ability of the inosculated vessels to provide adequate blood flow, centripetal revascularization occurs from the surrounding bed (Terzis et al., 1995). Previously, it was believed that inosculation occurs equally from both nerve graft ends, but recent research using novel micro CT visualization techniques has shown that inosculation occurs primarily from proximal to distal (Chalfoun et al., 2003; Saffari et al., 2019). This supports the fact that success of the grafted nerve is partly affected by the length of the graft, as a longer graft is subject to higher risk of necrosis in the mid-section (Terzis et al., 1995; Saffari et al., 2019). Prolonged denervation time leads to intraneural f ibrosis and core necrosis negatively affecting the nerve regeneration process (Bassilios Habre et al., 2018). Moreover, it was realized that multiple thin diameter cable grafts instead of single large diameter grafts could overcome central necrosis secondary to faster revascularization in smaller diameter cables (Breidenbach and Terzis, 1987).
To appreciate the potential benef its and limitations of our understanding of vascularization, it is important to comprehend the basic physiology of its effect on nerve regeneration in basic science and clinical applications as discussed below.
Effect of Vascularization on Nerve Regeneration
Basic science applications
Since 1976, studies have suggested the superiority of VNGs. The major reason for the lack of evidence in peripheral nerve injuries is the fact that studies include the use of different animal species, reconstructive strategies, follow-up times and outcome measures to assess nerve regeneration. These methodological differences make translation from animal models to humans challenging and nearly impossible. An overview of the available studies is provided.
It is postulated that vascularity improves results of nerve grafts by increasing the number of Schwann cells, while minimizing intraneural f ibrosis and enhancing axonal regeneration (Oudega et al., 2001). Thus, vascularity would accelerate revascularization compared to non-vascularized nerve grafts (NVNG) (Bertelli et al., 1996; Donzelli et al., 2016). A well vascularized bed does not only decrease fibroblast infiltration, but also provides an optimal nutritional environment (Donzelli et al., 2016). In a scarred, non-vascularized wound bed model, increase in myelination has been found when providing vascularization (Koshima and Harii, 1985; Ozcan et al., 1993). However, no differences were found in nerve conduction velocity (Mani et al., 1992). When intraneural blood supply is limited, the f ibroblasts replace the Schwann cells, causing a f ibrotic distal nerve and scarred endoneurial tube (Breidenbach and Terzis, 1987). In normal, vascularized beds, reported results have been conflicting. Some studies found no differences between VNGs and NVNGs (McCullough et al., 1984; Pho et al., 1985; Seckel et al., 1986) in rat models, while in rabbit models increased remyelination and enhanced contraction force have been reported in VNGs (Shibata et al., 1988; Kanaya et al., 1992). These results may be explained by the length of the gap and regeneration process in these animal models. The rat model could be used as a bioassay to evaluate treatments but translation to larger animals may be complicated as differences may occur due to the small gap (< 11 mm) and superlative regeneration rate in rats.
Other studies have focused on VEGF administration to the nerve site to enhance vessel formation and thus nerve regeneration and subsequently motor recovery (Wongtrakul et al., 2002; Lee et al., 2016). It has been demonstrated that VEGF increases revascularization (Wongtrakul et al., 2002; Lee et al., 2016), however, the application of VEGF did not improve functional motor nerve recovery in the long term (Lee et al., 2016). It is assumed that producing a supportive microenvironment after nerve injury including stable blood supply may have more effect than the application of VEGF alone. A vascularized fascial flap containing adipose tissue and a vascular bundle has been suggested to improve revascularization through the excreted angiogenic factors, provided by the stem cells in the adipose tissue as well as the blood supply and environmental support. The application of the superf icial inferior epigastric artery fascial f lap to provide vascularization to the nerve graft site in an experimental model has been described (Saffari et al., 2020) and found to increase revascularization of transplanted nerve allografts (Saffari et al., 2019) (Figure 2). Further animal model studies are required to determine the role of fascial f laps in nerve reconstruction.
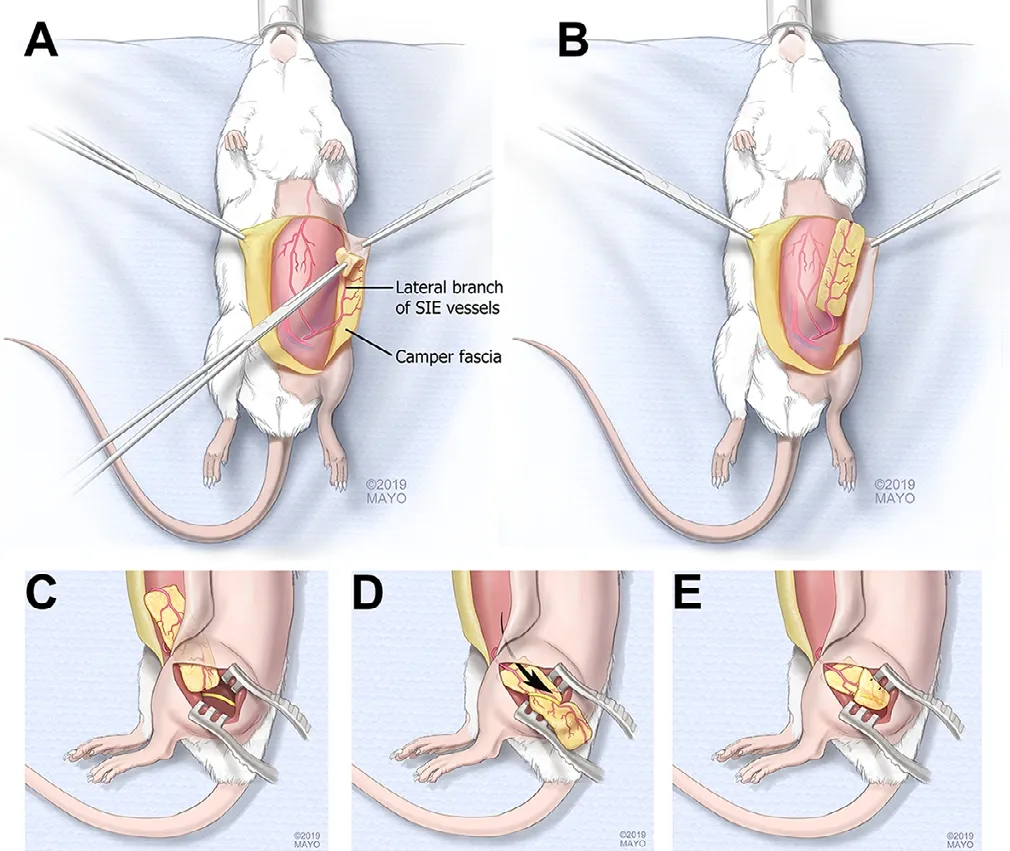
Figure 2 Schematic drawing of the superf icial inferior epigastric fascia (SIEF) f lap harvest.
Clinical applications
In 1870, Phillipeaux and Vulpian performed the first successful nerve autograft (Dellon and Dellon, 1993; Saffari et al., 2019) in cases in which direct tensionless nerve repair could not be achieved. In the years after, the increased application of cable grafting instead of trunk grafts, improved clinical outcomes (Tarlov, 1945; Seddon, 1947; Dellon and Dellon, 1993). To further overcome the problem of ischemia in conventional nerve grafts and optimize nutrient delivery, Strange (1947) introduced the first pedicled VNG in 1945 by using a pedicled ulnar nerve graft for the reconstruction of the median nerve. It was not until 1976, when the use of a free VNG was described; a 24 cm superf icial radial nerve graft based on the radial artery to reconstruct a median nerve in a 26-year-old woman (Taylor and Ham, 1976). Postoperative angiography at six weeks conf irmed successful transfer of the radial artery and positive Tinel’s sign progressed to the distal border of the flexor retinaculum at six months, which indicated that at least a portion of the axons had recovered (Taylor and Ham, 1976). Although this result was encouraging, technical difficulties were recognized and the procedure was suggested to be only performed in young patients, based on a case report and opinion of the authors.
In the 1980’s, several experimental and clinical studies investigated the effectiveness of adding vascularization to a nerve graft (Daly and Wood, 1985; Koshima and Harii, 1985; Pho et al., 1985; Restrepo et al., 1985; Doi et al., 1987; Shibata et al., 1988; Rose et al., 1989). Some of these studies demonstrated superior results of VNGs compared to NVNGs, while others suggested that the sensory- and motor functional recovery after VNGs were not significantly enhanced. The risk of thrombosis of the anastomosis in a VNG with subsequent necrosis of the nerve graft was considered by some authors as concerning and they advised against use of VNGs (Merle and Dautel, 1991). Conclusive findings on the superiority of VNGs in a clinical setting remain lacking due to multiple confounders, different duration of outcomes and the studies being mostly case reports or small case series (Farzan et al., 2005; del Piñal et al., 2007; Bertelli and Ghizoni, 2009; Terzis and Kostopoulos, 2009, 2010a, b). There is no clear consensus on the clinical indications for VNGs. The application of VNGs should be considered in the following cases: large length of nerve gap of more than 6-7 cm, large diameter of the injured nerve, scarred recipient bed that could not support a NVNG and substantial pre-operative delay of more than 24 months (Koshima and Harii, 1985; Restrepo et al., 1985; Shibata et al., 1988; Kallio and Vastamäki, 1993; Terzis and Kostopoulos, 2010a; D’Arpa, 2016). It is imperative to understand that this recommendation is based on case reports, small case series and the anecdote of expert opinions.
Doi et al. (1992) compared 27 cases of free vascularized sural nerve grafts in the upper extremity to 22 non-vascularized sural nerve grafts. These grafts were used to repair axillary, median, ulnar, radial and digital nerves, with a mean nerve gap of 6.0 cm in the VNG group versus 4.7 cm in the NVNGs. Two years postoperatively, the VNGs performed better in terms of rate of axonal regeneration, rate of electromyographic return and motor- and sensory outcome (Doi et al., 1992). Significant changes were found evaluating (i) the abductor pollicis brevis muscle (M2.5, S3 and M1, S2.3) for the median nerve, (ii) the abductor digiti minimi muscle (M3.3, S3 and M2, S2) for the ulnar nerve and (iii) extensor digiti communis muscle (M3.5 and M1) for the radial nerve, comparing the successful VNGs to conventional grafts using the Medical Research Council scale, respectively. The authors concluded that VNGs are technically difficult and equally good to conventional grafts. The variability in use e.g., different motor nerves, mixed motor and sensory nerves, or sensory nerves, and difficulties in consistent measures of outcome, combined with the small numbers, make a def inite conclusion difficult. Nevertheless, others have endeavored describing techniques to repair defects of larger than 12 cm (Koshima et al., 2003; Hasegawa et al., 2004).
Similarly, Terzis and Kostopoulos (2010a) found good to excellent sensory return, depending on the injury, after VNG reconstruction of 21 cases with upper extremity nerve injuries in which NVNGs had failed. In their lower extremity cases with injuries at the level of the knee or thigh, these same techniques were applied to regain muscle strength when denervation time was less than six months in patients reconstructed with large nerve grafts and regained remarkable muscle strength of at least M4, mostly in traction avulsion injuries (Terzis and Kostopoulos, 2010b). Unfortunately, these results have yet to be duplicated.
VNGs are also often used in the reconstruction of proximal nerve lesions, such as traumatic brachial plexus injuries (BPI) (D’Arpa, 2016). In the largest BPI case series to date, 151 reconstructions for posttraumatic BPI were described (Terzis and Kostopoulos, 2009). Free and pedicled vascularized ulnar grafts were used for reconstruction and concluded that patients with long denervation times (> 12 months) yielded inferior results compared with those that were operated earlier (< 6 months) (Terzis and Kostopoulos, 2009). A similar study found unsatisfying recovery of elbow flexion and wrist extension after BPI when repaired around 4.6 months after trauma (Bertelli and Ghizoni, 2009). While there was no direct comparison to NVNGs in these studies, the importance of denervation time was well-stated and described as less receptivity of the neuromuscular junction when nerve repair is delayed for a long period of time (Wu et al., 2014). It is accepted that outcomes are correlated with both the time course and the degree of denervation, however, an exact cut off point has not been def ined, which may be worthwhile to evaluate. With no direct comparison to NVNGs, conclusions on the superiority of VNGs is only speculated and not proven.
To summarize, the above mentioned studies have used VNGs to repair large nerve defects to improve outcomes in complex and unique cases. Ideally, clinical studies comparing VNGs to NVNGs should be randomized and involve patients with similar injuries, nerve graft repair and follow-up times. Due to diversity in cases, critical analysis has not been feasible to date, recognizing a still existing clinical problem. Moreover, reconstructions are evaluated mostly by investigating axonal regeneration via Tinel’s sign, electromyographic return and functional motor recovery. While these outcome measurements are clinically relevant, they are not a direct ref lection of the applied vascularization. Very recent research investigated the use of ultrasound to describe intraneural hypervascularization proximal to the site of nerve injury (Arányi et al., 2018). Although the increased blood flow does not directly implicate for patient management, it may reflect neovascularization and provide insight into pathophysiological processes after nerve injury treatments or serve as a potential prognostic tool, as it is known that the number of endoneurial capillaries significantly increases after nerve injury (Nukada, 1988). Implementing ultrasound as prognostic tool in future clinical trials would allow for direct measurement of the vascularization.
Technical challenges
The most frequently used VNGs are the saphenous and sural nerve graft, due to their dominant arterial pedicles and the acceptable donor site morbidity (el-Barrany et al., 1999). Several other VNGs, as described above, are associated with higher donor site morbidity or are mainly used in free f lap reconstructions and not solely as nerve grafts. In very specific patients with brachial plexus avulsions of C8 and T1, the ulnar nerve can be used as a VNG based on the superior collateral artery (Hattori et al., 2005; Figure 3).
Vascularization of a long nerve segment can be achieved by using a VNG alone, placed together or enveloped with a NVNG, or a vascularized adipose f lap can be placed around a NVNG to induce angiogenesis (Terzis and Kostopoulos, 2010a). When using a sural nerve graft, blood supply will be based on the sural artery or the saphenous vein can be arterialized (Breidenbach and Terzis, 1984). In cases of a larger nerve defect, grafts up to 60 cm could be harvested from the saphenous nerve, depending on the height of the patient (Terzis et al., 1995). Other options are the superf icial peroneal nerve with its vascular pedicle, to reconstruct multiple nerves ranging from 14 to 30 cm (Breidenbach and Terzis, 1984), or the superficial radial nerve which could be harvested with the radial artery (Shaf i et al., 2010). To use this VNG, however, it is advocated that the radial artery should be reconstructed with a vein graft, in order to minimize donor site morbidity.
Although VNGs have the potential to improve nerve reconstruction after injury, the surgeries are demanding and require microvascular experience. Technical challenges in matching diameter by cabling, while preserving blood supply, and finding recipient vessels if performed as a free tissue transfer may prevent widespread adoption. In addition, considerations in the clinical application include location of obtaining sufficient peripheral nervous tissue and the associated donor site morbidity. Not only is the choice of donor site of importance, the injury site limits graft options as well, especially when the injury is situated near joint creases. In these cases, the environment of the nerve graft could be optimized by using a vascularized adipose-nerve composite transfer. Foo and colleagues described a novel technique of transferring a posterior interosseous nerve graft along with vascularized synovial and adipose tissue based on a branch of the posterior interosseous artery for neuromas of digital nerves, to provide a healthy tissue bed (Foo et al., 2019). In large nerve defects with concomitant vascular trauma, immediate revascularization of a damaged artery has priority, limiting options for VNG harvest. In these cases, vascularized allografts could be applied (Bain, 2000), potentially vascularized with the use of a f lap.
Future Directions
Although several studies have discussed the surgical techniques of VNGs (el-Barrany et al., 1999; Shafi et al., 2010; Mozaffarian et al., 2018), the clinical use has been sparse. Limited availability of VNGs in patients with nerve injuries stimulates the need for alternative methods for introducing vascularity to nerve reconstructions. The number of biomedical companies involved in regenerative medicine and tissue engineering has been steadily increasing, representing future interest in off-the-shelf suitable alternatives to autografting, such as biodegradable scaffolds which maintain mechanical properties and ultrastructure. Both biomaterials and decellularized nerve grafts have been combined with growth factors or stem cells to provide options to reconstruct nerve defects (Patel et al., 2018). Moreover, exosomes, extracellular vesicles carrying microRNA, derived from Schwann cells, macrophages or stem cells provide exciting prospects for future treatment as the exosomes overcome the obstacles associated with cell therapy (Ching and Kingham, 2015; Qing et al., 2018). Other promising techniques include the generation of capillary-like networks in scaffolds to support the growth and viability of tissue substitutes that require blood supply, mainly when nutrient demand cannot be covered by diffusion processes in the center of these three-dimensional constructs (Laschke and Menger, 2012; Auger et al., 2013).
Future three-dimensional printing may be able to precisely recreate the structure of the scaffold to mimic a native nerve. Apart from the design and ultrastructure of the scaffold, several different factors are important and currently studied, such as biodegradation. The slow pace of nerve regeneration necessitates the scaffold to not be a fast degrading polymer, especially when reconstructing large defects. Moreover, the acetic environment created by the degradation of biocompatible polymers needs to be addressed in order to maintain a healthy bed for nerve regeneration (Sung et al., 2004). Other considerations may be the thickness of the outer scaffold, which should not exceed 100-200 μm, defined as the diffusion limit of oxygen, aforementioned (Jain et al., 2005). The application of three-dimensional printed nerves with or without vessels will evolve over the next decade and may be implemented and improve outcomes in peripheral nerve injuries, especially to successfully repair large segmental defects.
Conclusions
Peripheral nerves are living dynamic tissues that thrive on nutritive blood supply. The interaction of vessels and nerves after a nerve injury is complex. Although the relationship between angiogenesis and neuroregeneration was originally thought to be related to nutritional factors, recently more evidence advocates for the Schwann cells to guide neuronal precursors via a complex pathway of factor secretion, among which VEGF, to enhance nerve regeneration. Revascularization of nerve grafts after injury relies mostly on environment and primarily on inosculation from host vessels. Well-designed clinical studies comparing VNGs to NVNGs remain lacking and existing studies are inconclusive. Future studies should be randomized and involve patients with similar injuries, nerve graft repair and follow-up times to be able to critically elucidate differences between VNGs and NVNGs. The technical obstacles could be overcome by provision of vascularized fascial f laps that enhance revascularization. The field of tissue engineering is evolving and predicted to take a larger part in treatment options for peripheral nerve injuries using biodegradable scaffolds.
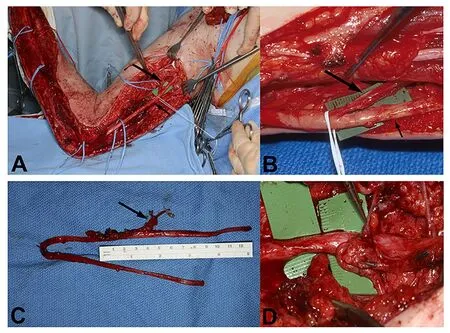
Figure 3 Vascularized ulnar nerve graft (VUNG) harvest.
Acknowledgments:The authors would like to thank Jim Postier (Rochester, MN) for the artwork of Figures 1 and 2.
Author contributions:TMS, MB, CAH, ATB and AYS wrote and critically reviewed the manuscript. All authors approved the final manuscript.Conf licts of interest:The authors declare no conf licts of interest.
Financial support:This work was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number RO1 NS102360 (to AYS).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Konstantin D. Bergmeister, BG Unfallklinik Ludwigshafen, Germany; Xin Zhao, Huashan Hospital Affiliated to Fudan University, Shanghai, China.
Additional file:Open peer review reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of an injured ascending reticular activating system with recovery from a minimally conscious state to normal consciousness in a stroke patient: a diffusion tensor tractography study
- New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target?
- Advanced diffusion magnetic resonance imaging in patients with Alzheimer’s and Parkinson’s diseases
- Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury
- Decoding epigenetic codes: new frontiers in exploring recovery from spinal cord injury
- Insights into platinum-induced peripheral neuropathy-current perspective
