Regional volume changes of the brain in migraine chronif ication
2020-03-07XiaoYanChenZhiYeChenZhaoDongMengQiLiuShengYuanYu
Xiao-Yan Chen , Zhi-Ye Chen , Zhao Dong Meng-Qi Liu Sheng-Yuan Yu
1 Department of Neurology, First Medical Center of Chinese PLA General Hospital, Beijing, China
2 Department of Radiology, First Medical Center of Chinese PLA General Hospital, Beijing, China
3 Department of Radiology, Hainan Hospital of First Medical Center of Chinese PLA General Hospital, Sanya, Hainan Province, China
Abstract The pathophysiology of migraine is complex. Neuroimaging studies reveal functional and structur al changes in the brains of migraine patients. We sought to explore regional volume differences in intracranial structures in patients with episodic and chronic migraine. Sixteen episodic migraine patients, 16 chronic migraine patients, and 24 normal controls were recruited and underwent 3.0 T MRI scanning. The volumes of 142 brain regions were calculated by an automatic volumetric algorithm and compared with clinical variables. Results demonstrated that the volumes of specif ic regions in the frontal and occipital lobes, and the right putamen, were increased and the volume of the fourth ventricle was decreased in the episodic migraine patients compared with controls. The volumes of the left basal forebrain, optic chiasm, and, the fourth ventricle were decreased in the chronic migraine patients, while the occipital cortex and the right putamen were larger. Compared to episodic migraine patiants, chronic migraine patients displayed larger left thalamus and smaller frontal regions. Correlation analysis showed that headache frequency was negatively correlated with the volume of the right frontal pole, right lateral orbital gyrus, and medial frontal lobes and positively correlated with the volume of the left thalamus. The sleep disturbance score was negatively correlated with the volume of the left basal forebrain. This suggests that migraine patients have structural changes in regions associated with pain processing and modulation, affective and cognitive processing, and visual perception. The remodeling of selective intracranial structures may be involved in migraine attacks. This study was approved by the Ethics Committee of Chinese PLA General Hospital (approval No. S2018-027-02) on May 31, 2018.
Key Words: brain volume; chronic migraine; frontal lobe; magnetic resonance imaging; migraine; remodeling; thalamus; visual processing system
Introduction
Migraine is a common neurological disorder which can cause significant disability (GBD 2016 Neurology Collaborators, 2019). Episodic migraine (EM) refers to fewer than 15 headache days per month, while chronic migraine (CM) implies headache occurring ≥ 15 days per month for at least 3 months with migraine features on ≥ 8 days every month (No authors listed, 2018). About 3% of patients may transform from EM to CM within a year (Lipton et al., 2015; Scher et al., 2017; Borkum, 2018). The prevalence of CM was reported to be 0.6-1.7% in Asia-Pacif ic region (Stark et al., 2013) and 1-2% in the global population (Burch et al., 2019). A number of risk factors, such as increased headache frequency, acute medication overuse, and depression are related to the transition from EM to CM, termed “chronification” (Buse et al., 2019). CM associated with medication overuse, also called “medication overuse headache” (Goudarzi et al., 2016), returns to episodic form after medication withdrawal, and may not actually be CM (No authors listed, 2018).
The pathogenesis of migraine chronif ication has been only partially revealed. Neuroimaging studies of CM have found functional and microstructural alterations in the brainstem, hypothalamus, basal ganglia, and cortex, sites that are involved in pain processing. Some of the changes correlate with headache frequency and/or duration, while others may be associated with mood and emotion, cognitive dysfunction and insomnia (Coppola et al., 2017; Neeb et al., 2017; Schulte et al., 2017; Androulakis et al., 2018; Domínguezet al., 2019; Lee et al., 2019; Woldeamanuel et al., 2019). These functional and structural alterations ref lect central plasticity of CM, but whether the changes were the etiology or the effects of migraine chronification has yet to be revealed. Neuroimaging findings were variable across different studies, probably due to the diversities of patients’ background, clinical aspects, imaging processing methods, and statistical power. Previous structural imaging studies have investigated alterations in gray matter volume or thickness (Coppola et al., 2017; Neeb et al., 2017; Woldeamanuel et al., 2019), iron deposition (Domínguez et al., 2019), white matter lesions (Zheng et al., 2014; Neeb et al., 2015) and textural features of gray matter (Chen et al., 2017). Among those morphometric studies, volumetric analysis was most widely performed, and may well ref lect brain remodeling in migraine. We intended to assess for the intracranial volume changes in EM and CM by calculating the volume of 142 brain regions according to a commercial template (Neuromorphometrics, Somerville, MA, USA) using an automatic morphometric algorithm over the whole brain. Compared to previous volumetric studies that usually investigated the cortical voxelwise microstructural changes (Coppola et al., 2017; Neeb et al., 2017; Palm-Meinders et al., 2017), this study directly calculated the volume differences not only in the cortex, but also in the white matter and the ventricles in a more macroscopic view using the automatic morphometric algorithm.
This study investigated the global and regional brain volume changes in EM and CM patients compared with normal controls (NCs), the global and regional brain volume differences between EM and CM patients, and the correlation of altered brain-region volumes with headache duration, frequency, and intensity and the scores for anxiety, depression, cognition, and sleep quality in all migraine patients.
Subjects and Methods
Fifty-six subjects were included in this prospective cross-sectional study, comprising 16 EM patients, 16 CM patients and 24 NCs. Migraine patients were consecutively recruited from the Outpatient Headache Clinic of the Department of Neurology of the First Medical Center of Chinese PLA General Hospital. Diagnosis of EM and CM fulf illed the criteria of ICHD-3 (No authors listed, 2018) and only migraine patients without aura were included, aura being relatively uncommon in the Chinese population. Inclusion criteria were age 18-60 years old, right-handed, willing to engage in the study, and not taking prophylactic medication or acute headache medications for more than 10 days per month during the last 3 months. Patients with chronic somatic or psychiatric disorders such as hypertension, diabetes mellitus, hypercholesterolemia, cardiovascular diseases, brain trauma, neoplasm, infection, connective tissue diseases, other subtypes of headache, chronic somatic pain, severe anxiety or depression before the onset of headache, or substance addiction were also excluded Inclusion and exclusion criteria of NCs were similar to those of the patients, except for the diagnosis of migraine.
Demographic information and general headache data were collected. All the patients completed a Visual Analogue Scale (VAS) (Aicher et al., 2012) for pain and a Migraine Disability Assessment (MIDAS) questionnaire (Hung et al., 2006) for assessing the impact of headache. The ten-point VAS was structured with a score greater than 6 corresponding to severe pain, a score of 4-6 indicating moderate pain, and a score lower than 4 indicating mild pain. The MIDAS score is derived from f ive questions about missed time from work, household work, and missed days of nonwork activities. The higher the MIDAS score, the more severe the disability caused by migraine. Both patients and NCs underwent Hamilton Anxiety Scale (HAMA) (Matza et al., 2010, Nair et al., 2015), Hamilton Depression Scale (HAMD) (Zimmerman et al., 2013), Montreal Cognitive Assessment (MoCA) Beijing Version (www.mocatest.org), and General Sleep Disturbance Scale (GSDS) (Lee, 2007) assessment for evaluating anxiety, depression, cognition and sleep quality, respectively. A HAMA score 8-14 indicates mild anxiety; 15-23 indicates moderate anxiety; and ≥ 24 indicates severe anxiety (Matza et al., 2010). A HAMD score of 8-16 indicates mild depression; 17-23 indicates moderate depression; and ≥ 24 indicates severe depression (Zimmerman et al., 2013). The total score of MoCA is 30 and when the score falls below 26, cognitive impairment is present. The lower the MoCA score is, the worse the cognitive function. GSDS is a self-rated, 8-point scale arranged from 0 (never) to 7 (every day); and higher GSDS scores indicate more severely disturbed sleep (Lee, 2007). The above scales were evaluated by trained doctors. This study was approved by the Ethics Committee of Chinese PLA General Hospital (approval No. S2018-027-02) on May 31, 2018 (Additional file 1). All the participants signed informed consents (Additional file 2) which complied with the Declaration of Helsinki before participating in the study. This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (Additional file 3).MRI acquisition
Nicotine, alcohol, and caffeine were prohibited at least 12 hours prior to MRI scanning. For EM patients, MRI examinations were performed at least 3 days after the last migraine attack. Headache status was recorded for CM patients at MRI examination. All the MRI examinations were carried out on a GE 3.0 Tesla MRI scanner (DISCOVERY MR750, GE Healthcare, Milwaukee, WI, USA) with a conventional 8-channel quadrature head coil. High-resolution structural images were collected by a three-dimensional T1-weighted fast spoiled gradient recalled echo sequence with the parameters as follows: repetition time = 6.3 ms, echo time = 2.8 ms, f lip angle = 15 degrees, field of view (Bilgiç, Kocaman et al., 2016) = 25.6 × 25.6 cm2, matrix size = 256 × 256, number of acquisition = 1180 contiguous axial slices, slice thickness = 1 mm. Subjects with obvious structural abnormalities and lesions in the brain, as assessed by a radiologist and neurologist, unless they were a few white matter lesions which may be related to migraine, were excluded from the study.
Data processing
All the MRI structural images were processed using computational Anatomy plugins (CAT, http://www.neuro.uni-jena.de/cat/) implemented within MATLAB 7.6 (The Mathworks, Natick, MA, USA) based on Statistical Parametric Mapping 12 (SPM 12, http://www.f il.ion.ucl.ac.uk/spm/). The volumes of 142 brain regions were automatically extracted from gray matter, white matter and cerebrospinal f luid according to the software as in our previous methods (Chen et al., 2018) composed of several procedures: spatial registration of individual brain images to an International Consortium for Brain Mapping (ICBM) space template (East Asian brains) (Mazziotta et al., 2001) to obtain normalized images, segmentation of normalized tissue into gray matter, white matter and cerebrospinal fluid, and volume extraction of 142 brain regions according to the Neuromorphometrics template.
Statistical analysis
The sample size was calculated by PASS 11 power analysis software (NCSS, Kaysville, UT, USA) based on our preliminary data with type 1 error rate of 0.5 and power of 90% in the one-way analysis of variance power analysis. Sample sizes of 14, 14, and 21 were obtained from the three groups whose means were to be compared. The total sample of 49 had 91% power to f ind means to be different among the groups using an F test with significance level of 0.05. The common standard deviation was assumed to be 0.21 in the means and 0.40 within a group.
Statistical analysis was performed with IBM SPSS 22.0 (IBM Corp., Armonk, NY, USA). The continuous data in demographics and clinical profiles were presented as the mean ± standard deviation. The differences in age, HAMA, HAMD, MoCA, sleep disturbance scale, total volume of gray matter, white matter, cerebrospinal f luid, and total intracranial volume were compared among the three groups using a one-way analysis of variance test followed by pairwise comparison using the Bonferroni method. Sex ratios of CM and EM were compared with the chi-squared test. Headache duration, headache frequency, VAS, number of doses of each medication per month, and MIDAS were compared between the EM and CM groups with an independent samples t-test. The differences in brain-region volume among EM, CM and NCs were calculated with a general linear model using age, sex and total intracranial volume as covariates followed by pairwise comparison corrected by the Bonferroni method. Partial correlation analyses were performed between the volumes of altered brain regions and the clinical parameters in EM and CM patients compared with controls for age, sex, and total intracranial volume. A value of P < 0.05 was considered statistically significant.
Results
Demographics and clinical data of EM, CM, and NCs
A total of 56 participants (16 EM patients, 16 CM patients and 24 NCs) were enrolled in this study. Only two patients in the CM group were headache-free during MRI scanning. Table 1 shows the demographics and clinical data. There were no statistical differences in age and sex among the three groups. Years with headache, headache intensity, and number of acute analgesics taken per month were not significantly different between EM and CM groups. Both EM and CM patients were significantly more anxious, depressed, and had worse sleep disturbance than NCs (P < 0.001). CM patients were more depressed and had more severe sleep disturbance than EM patients (P < 0.05). Cognitive function as measured by MoCA was much lower in CM patients than in EM patients and NCs (P = 0.001). CM patients endured a much higher burden of migraine disability than EM patients (P < 0.001).
Volume differences in intracranial structures among EM patients, CM patients and NCs
The total intracranial volume and total volume of gray matter, white matter, and cerebrospinal f luid yielded no statistical differences among EM patients, CM patients, and NCs (P > 0.05; Table 2). The results did not change after adjusted by age and using the Bonferroni correction for multiple group comparison.
Region-volume analyses with multiple comparisons found significantly larger volumes of several regions in the frontal lobe, occipital lobe, right putamen and lower volume of the 4thventricle of EM patients compared with NCs (P < 0.05; Table 3 and Figure 1).
Compared with NCs, greater volume was detected in the left lingual gyrus, left fusiform gyrus, and right putamen, while the optic chiasm, the 4thventricle, and basal forebrain in CM patients (Table 3 and Figure 2) were lower in volume compared with NCs.
Compared with EM patients, the frontal lobe volume was smaller and the left thalamus volume was larger in CM patients (Table 3 and Figure 3).
Correlation of clinical parameters and the volume of different brain regions
Headache frequency (headache days per month) was negatively correlated with the volume of the right frontal pole (r = -0.571, P = 0.001), right lateral orbital gyrus (r = -0.395, P = 0.034), and left and right medial frontal lobes (r = -0.501, P = 0.006; r = -0.493, P = 0.007, respectively). Headache frequency was positively correlated with the volume of the left thalamus (r = 0.583, P = 0.001). The sleep disturbance scale was negatively correlated with the volume of the left basal forebrain (r = -0.410, P = 0.024; Figure 4). No significant correlation was found between other clinical parameters and the volume of brain regions.
Discussion
This structural MRI study analyzed volume alterations of regions in the gray matter, the white matter, and the ventricles in migraine patients. In this study, the altered volume in several regions of prefrontal cortex, occipital lobe, right putamen, the 4thventricle, the optic chiasm, and thalamus of migraine patients may indicate a central plasticity in migraine pathogenesis and chronif ication.
The prefrontal cortex has a key role in cognitive processing and pain modulation by wide connections with other brain areas, including the parietal cortex, insula, hippocampus, amygdala, thalamus, basal nuclei and periaqueductal gray matter (Ong et al., 2019). Previous studies have reported impaired prefrontal cortical function in migraine patients as shown by neuropsychological testing (Lev et al., 2010, 2013). Voxel-based morphometry studies generally demonstrated volume or density reduction of the frontal cortex in migraine patients (Kim et al., 2008; Schmitz et al., 2008; Valfrè et al., 2008; Jin et al., 2013; Coppola et al., 2017). Different from most voxel-based morphometry studies, EM patients in our study showed volume expansion of the frontal and occipital lobes. Those selected areas have been reported in previous studies to demonstrate volume alteration in migraine patients (Kim et al., 2008; Schmitz et al., 2008; Valfrè et al., 2008; Jin et al., 2013; Bilgiç et al., 2016; Yu et al., 2016; Coppola et al., 2017; Palm-Meinders et al., 2017). Therefore, we speculated that those regions indeed had alteration in EM patients.
Similar to our results, one study using whole-brain vertex-by-vertex analysis also found thickened prefrontal cortex in migraine patients without aura (Kim et al., 2014). More surface-based morphometry studies reported increased thickness in the somatosensory cortex (DaSilva et al., 2007) and motion-processing visual areas (Granziera et al., 2006) in EM patients. However, other surface-based morphometry studies reported thinner cortex in the frontal lobe, occipital lobe (Yu et al., 2016) and basal ganglia (Yuan et al., 2013) in migraine patients. The results from structural MRI studies are inf luenced by several factors, such as the patients’ ethnicity, migraine duration and/or frequency (Maleki et al., 2012), MRI parameters, morphometric methods, statistical efficacy, and ictal or interictal state (Coppola et al., 2015). In our study, those enlarged frontal areas in EM patients were no more larger in CM patients compared with NCs and the volume of some frontal regions was negatively correlated with headache frequency. The increased prefrontal cortex in EM patients may indicate adaptation of the central nervous system, which would enhance descending pain modulation at the stage of migraine attack. The volume decrease in prefrontal cortex in patients with higher headache frequency may ref lect maladaptive volume reduction (Liu et al., 2017) and dysfunction of pain inhibition, which would facilitate migraine attacks and promote migraine chronif ication (Krummenacher et al., 2010; Bräscher et al., 2016). The mechanism of remodeling of migraine brain is unclear, but is considered to be related to the number and size of neuronal or glial cells and their synapses, and altered interstitial fluids or blood f low (May, 2011) probably resulting from neural excitability, neuroinflammation, vascular constriction or dilation, and neural degeneration.
Interestingly, our study detected decreased basal forebrain size in CM patients compared with NCs, which was not reported in previous structural MRI studies. The basal forebrain contains at least three distinct populations of neurons (cholinergic, glutamatergic, and GABAergic), which have extensive projections to cortical and subcortical areas to regulate arousal, motivation, and other behaviors (Agostinelli et al., 2019). An animal study found increased pain sensitivity after destruction of basal forebrain cholinergic neurons in rats, revealing that cholinergic transmission of basal forebrain may be involved in pain processing (Vierck et al., 2016). A quantitative electroencephalogram study found that migraine patients suffered from highly asymmetric values in anterior δ power and posterior α and θ waves, suggesting altered activity patterns in the cholinergic brainstem or basal forebrain nuclei and thalamocortical connections prior to migraine attack (Bjørk and Sand, 2008). Further, the basal forebrain is considered to play a role in regulating the sleep—wake cycle in animals (Hermanstyne et al., 2013). In our study, the volume of basal forebrain was negatively correlated with the sleep disturbance scale, which further supported that altered basal forebrain may be associated with poorer sleep in migraine patients.
The visual processing pathway is strongly associated with migraine pathophysiology and clinical manifestations (Marzoli and Criscuoli, 2017). Light stimulation is a possible trigger of migraine attack. Visual aura is the most frequent form of migraine aura. Photophobia is a common accompanying symptom of migraine. Light hypersensitivity in migraine has been considered to be associated with light transmission from the retina to the trigeminovascular thalamocortical pathway (Noseda et al., 2010). Several studies reported a thinner retinal nerve fiber layer (Reggio et al., 2017; Tak et al., 2018) and/or retinal ganglion cell layer (Reggio et al., 2017; Gunes et al., 2018) in migraine patients, and the thickness may be negatively correlated with headache frequency (Reggio et al., 2017). Our study demonstrated volume reduction of the optic chiasm in CM patients, which further supports the structural alteration of the visual processing pathway. Similar to a previous surface-based morphometry study which reported thickened motion-processing visual regions (MTt and V3A) in migraine patients (Granziera et al., 2006), our study found thickened occipital regions in EM and CM patients, indicating the remodeling of central visual system in migraine patients.
The deep gray matter basal ganglia are important in integrating sensory, motor, motivation, cognitive and pro-
cedural learning functions (Kreitzer and Malenka, 2008). Previous studies have found volume alteration, functional connectivity changes, and iron deposits (Kruit et al., 2009; Yuan et al., 2013; Rocca et al., 2014) in the basal ganglia of migraine patients. Similar to previous studies (Rocca et al., 2014; Neeb et al., 2017), our study demonstrated an enlarged right putamen in both EM and CM patients, supporting the role of the basal ganglia in migraine patients. Another essential deep gray matter structure in migraine is the thalamus, which is responsible for pain processing, sleep—wake cycle regulation, awareness, cognitive behaviors, and the modulation of visual information in migraine (Younis et al., 2019). The volume reduction of thalamic nuclei (Magon et al., 2015) or thalamic microstructural alteration (Granziera et al., 2014) in migraine has reported in previous studies. Our previous study found that the thalamic volume was increased in medication-overuse headache patients compared to NCs (Chen et al., 2017). In this study, neither EM nor CM patients showed volume difference from NCs in the thalamus, which was consistent with a previous study (Granziera et al., 2014). However, compared with EM patients, CM patients presented with thalamic enlargement on the left side (P = 0.004), and the volume was positively correlated with headache days per month, indicating the plasticity of the thalamus in migraine chronif ication.
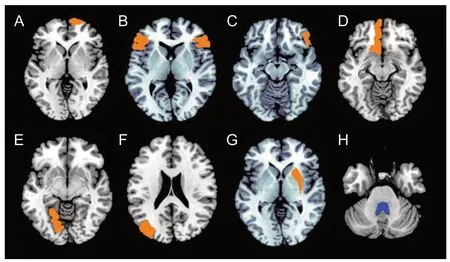
Figure 1 Brain regions positive for volume alteration in episodic migraine compared with normal controls (P < 0.05, Bonferroni corrected).
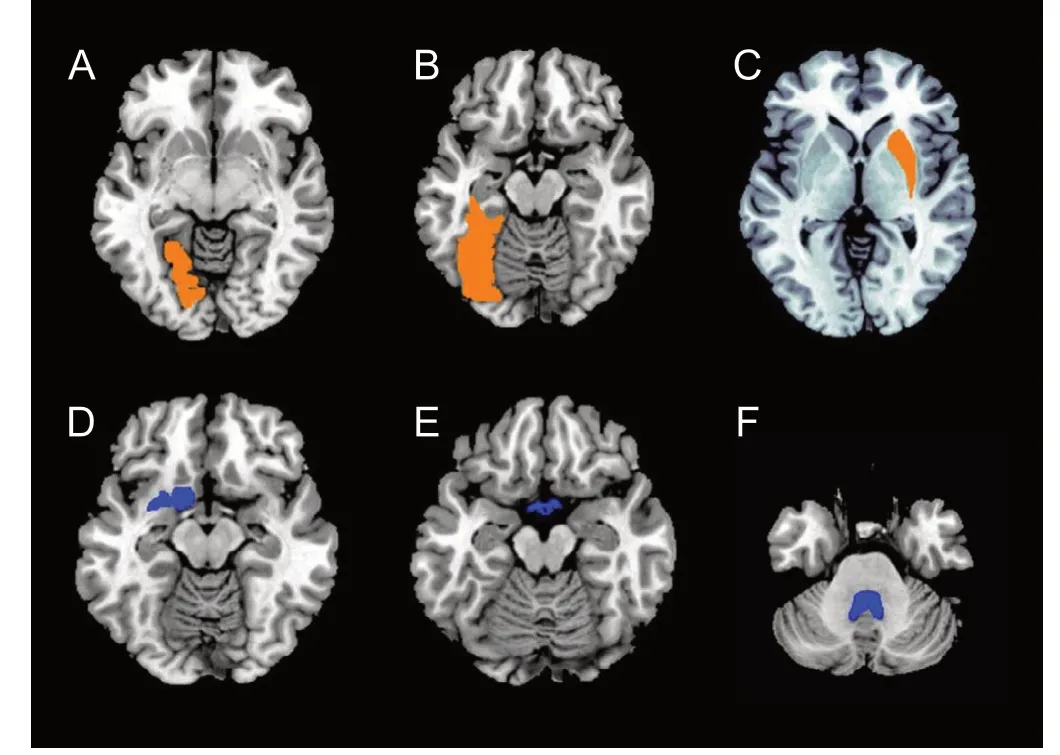
Figure 2 Brain regions with volume alteration in chronic migraine compared with normal controls (P < 0.05, Bonferroni corrected).

(A-C) Orange color represents larger regions in the right frontal pole (P = 0.021), right lateral orbital gyrus (P = 0.035), and bilateral medial frontal cerebrum (P = 0.002). (D) Blue color represents smaller brain regions in the left thalamus (P = 0.009).
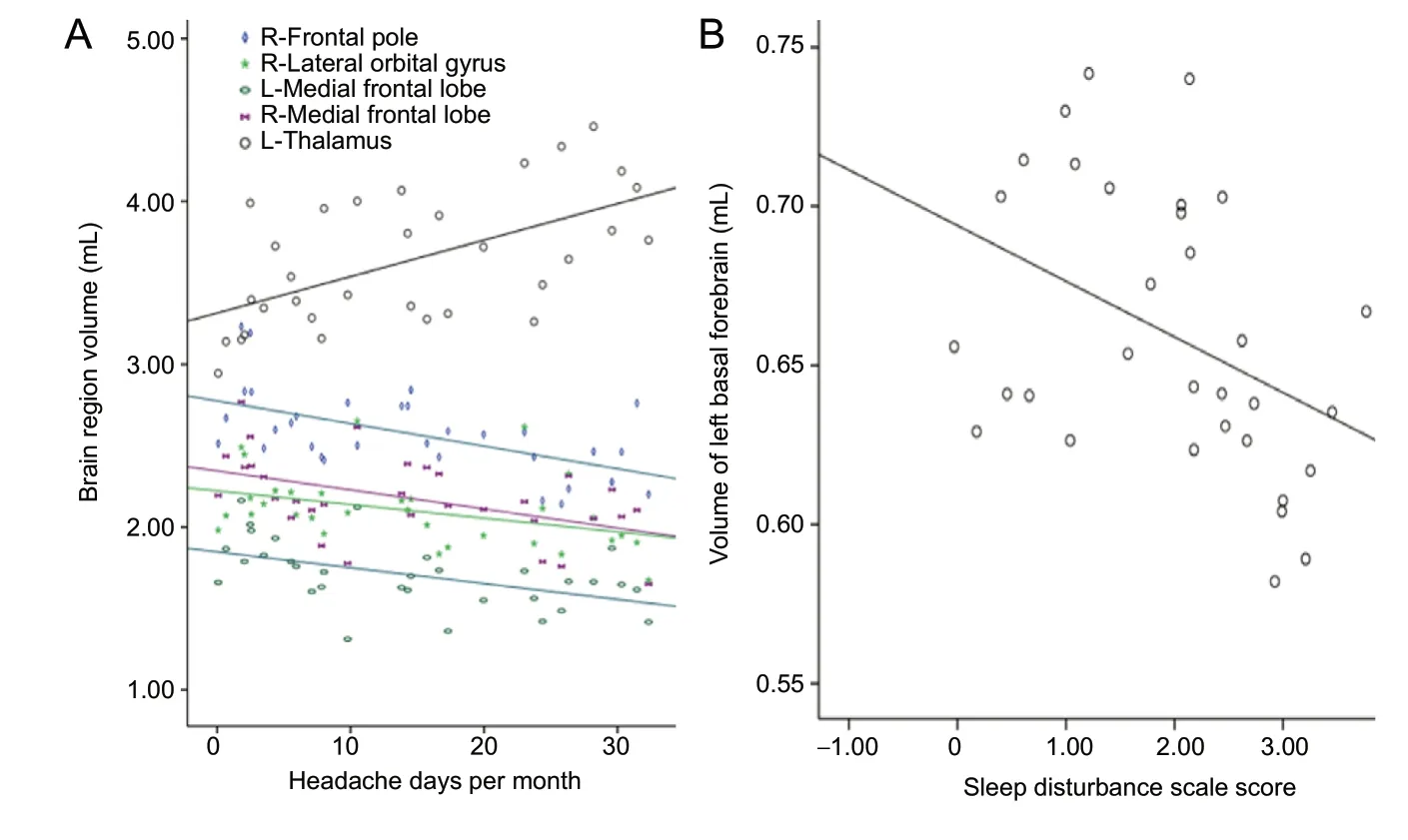
Figure 4 Correlation analysis of clinical prof iles and the volume of positive brain regions.
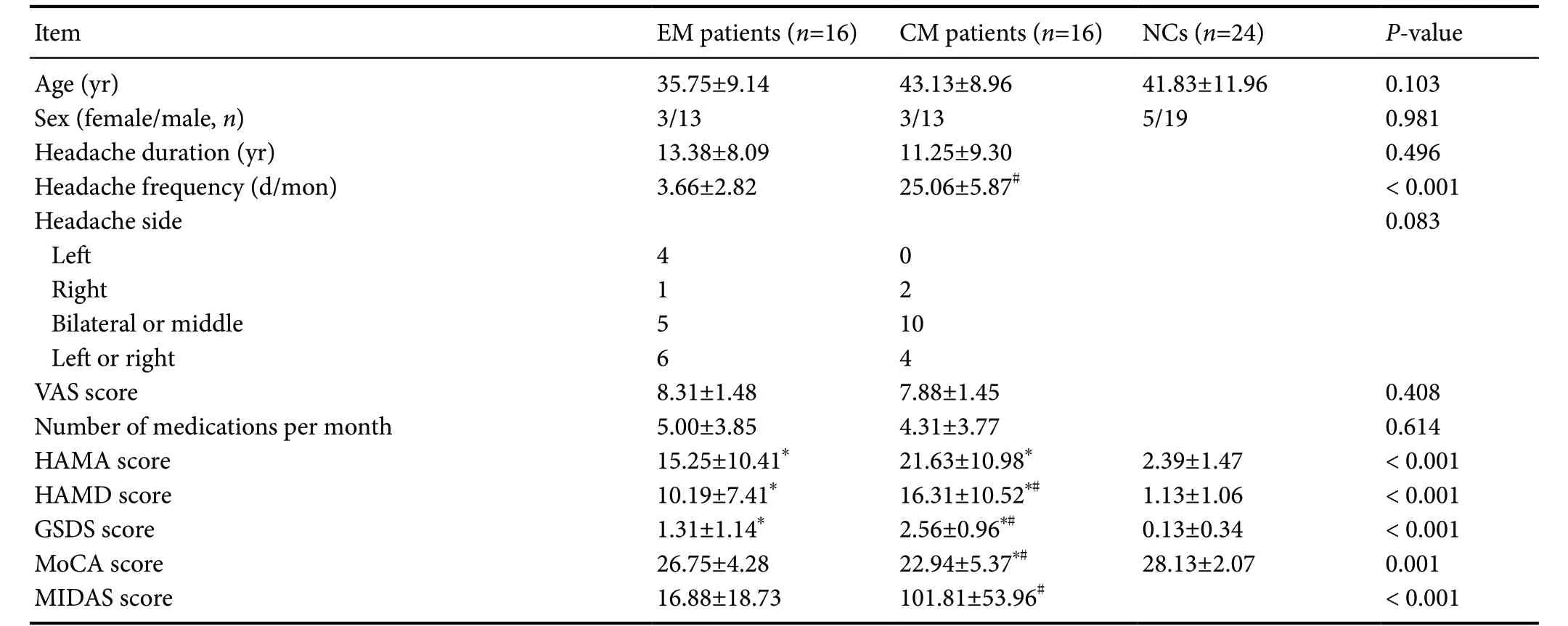
Table 1 Demographic and clinical information of EM patients, CM patients and NCs
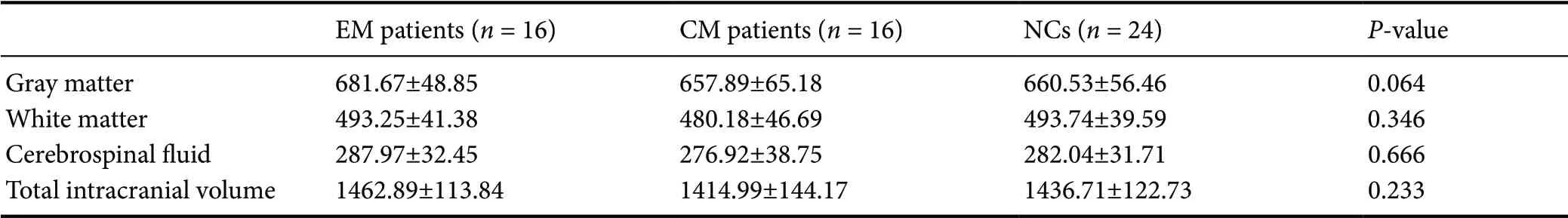
Table 2 Total intracranial volume (mL) of intracranial structure in EM patients, CM patients and NCs
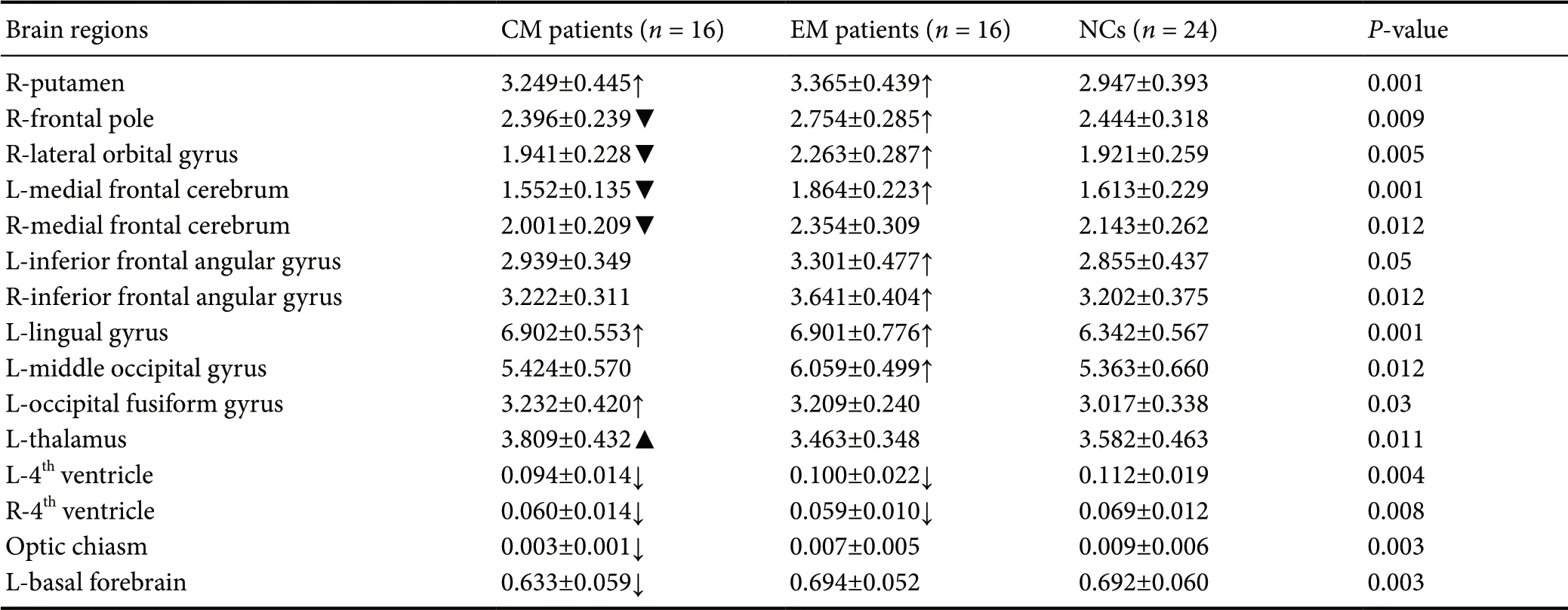
Table 3 Regional volume differences of brain regions among EM patients, CM patients and NCs
The decreased volume of the 4thventricle in EM and CM patients may reflect enlargement of the brainstem or cerebellum, although we did not find statistical differences in the size of those structures. The brainstem contains the ascending trigeminal pathway and descending pain modulatory system. The volume of brainstem conf licted with data from previous studies. Some studies showed that gray matter volume of the brainstem decreased in migraine patients (Marciszewski et al., 2018) and CM patients (Bilgiç et al., 2016). Others demonstrated larger brainstem and subnuclei in migraine with aura (Petrusic et al., 2019) and in medication-overuse headache (Chen et al., 2018). The volume of the cerebellum in migraine patients was generally decreased in several studies (Bilgiç et al., 2016; Demir et al., 2016; Messina et al., 2017).
Our study has some limitations. First, because most of the CM patients had headache on MRI scanning, it is not possible to exclude that the non-edematous volumetric alterations observed in CM patients might be inf luenced by the presence of headache (Coppola et al., 2015). Nevertheless, it is impossible to perform subgroup analysis according to patients with or without headache when taking MRI examinations, because the sample was too small to allow this. The sample was small since CM without medication overuse was less prevalent. Second, we only recruited migraine patients without aura. Whether the volume alteration in migraine with aura occurs in the same or different brain regions is unknown. Third, this was a cross-sectional study, and we could not prove that chronification caused volume alteration or that volume alteration caused chronif ication.
In conclusion, this study revealed regional volume changes in the brains of CM and EM patients. The volume alterations were mainly located in the frontal lobe, visual processing system, basal ganglia, and thalami, which are involved in pain modulation, affective/cognitive processing, visual processing and pain processing. The volume of some brain regions may dynamically change with different migraine frequencies. However, the underlying mechanism of the volume alteration needs further investigation.
Acknowledgments:We are very grateful to the colleagues in the headache center of Chinese PLA General Hospital for collecting patients’ data and the radiology department for MRI scanning.
Author contributions:Study concept and design: SYY; data acquisition: XYC, ZYC, MQL, and ZD; data analysis and interpretation: XYC and ZYC; article drafting: XYC and ZYC; revision for intellectual content: SYY. All authors read and approved the final manuscript.
Conf licts of interest:The authors declare that they have no conf licts of interest.
Financial support:This work was supported by the Natural Science Foundation of Hainan Province of China, No. 818MS153 (to ZYC); the National Natural Science Foundation of China, No. 81771200 (to ZD); the National Key Research and Development Projects of Beijing Science and Technology Plan of China, No. Z161100002616013 (to SYY); the Special Financial Grant from the China Postdoctoral Science Foundation, No. 2014T70960 (to ZYC); the Nursery Technology Innovation Fund of Chinese PLA General Hospital, No. 12KMM39 (to XYC). The funder had no role in the study design, data collection, anagement, analysis, and interpretation; paper writing; or decision to submit the manuscript for publication.
Institutional review board statement:The study was performed in strict accordance with the Declaration of Helsinki and relevant ethical requirement of the Ethics Committee of Chinese PLA General Hospital (approval No. S2018-027-02) on May 31, 2018.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms from the conscious participants. In the forms, the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understood that their names and initials will not be published.
Reporting statement:This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of the medical school of Chinese PLA.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed in the current study will be available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional f iles:
Additional file 1:Hospital Ethics Approval.
Additional file 2:Informed Consent Form.
Additional file 3:STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist.
CORRIGENDUM
Corrigendum: Resveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: a molecular docking study exploring possible neuroprotection
doi:10.4103/1673-5374.278561
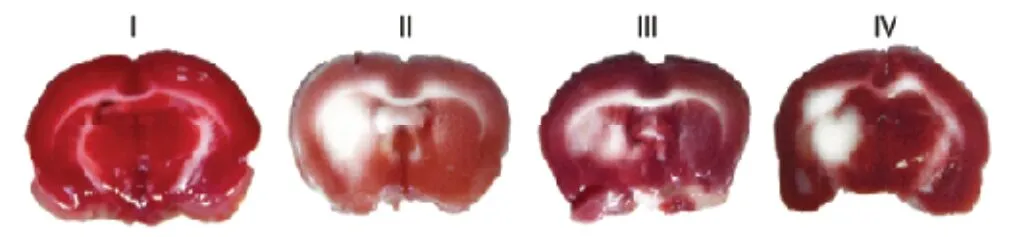
In the article titled “Resveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: a molecular docking study exploring possible neuroprotection”, published on pages 568—575, Issue 4, Volume 10 of Neural Regeneration Research (Pandey et al., 2015), Figure 1B images were provided incorrectly. The correct images of Figure 1B are shown below:
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of an injured ascending reticular activating system with recovery from a minimally conscious state to normal consciousness in a stroke patient: a diffusion tensor tractography study
- The role of vascularization in nerve regeneration of nerve graft
- New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target?
- Advanced diffusion magnetic resonance imaging in patients with Alzheimer’s and Parkinson’s diseases
- Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury
- Decoding epigenetic codes: new frontiers in exploring recovery from spinal cord injury
