Comparing silicone oil-induced ocular hypertension with other inducible glaucoma models in mice
2020-03-07JieZhang,YangHu
Glaucoma, “a silent thief of sight” and the leading cause of irreversible blindness, is characterized by retinal ganglion cell (RGC) death and optic nerve degeneration. Elevation of intraocular pressure (IOP), also called ocular hypertension, is the most recognized risk factor for glaucoma (Jonas et al., 2017). Current therapies only target reduction of IOP, but irreversible RGC death continues even after IOP is normalized (Wormald et al., 2020), indicating the critical clinical need to prevent degeneration and/or replace lost RGCs. Deciphering the key molecular mechanisms of glaucomatous degeneration and identifying or testing novel therapeutic targets for neuroprotection and regeneration, requires reliable, reproducible, and reversible ocular hypertension/glaucoma models in experimental animals. Due to the similarity of its anatomical structure and aqueous humor dynamics to those of the human eye, its strong reproductive ability, genetic plasticity, well developed transgenic techniques, and low price, the mouse has been used extensively to model glaucoma. While genetic mouse models are valuable to understand the roles of a specif ic gene in IOP elevation and/or glaucomatous neurodegeneration, the pathologic effects of a mutation may not manifest for months or even years (Libby et al., 2005; Chi et al., 2010). Inducible ocular hypertension that develops more quickly and is more severe is preferable for testing experimental manipulations and studying neurodegeneration mechanism associated with IOP elevation.
IOP rises or falls in response to two processes: aqueous inf low to the anterior chamber through the pupil from the ciliary body in the posterior chamber and aqueous outflow through the trabecular meshwork (TM) at the angle of the anterior chamber. After traversing the TM, the aqueous is collected into the limbal venous plexus via collector channels and f inally f lows into the episcleral veins to enter the blood circulation. When aqueous inflow and outflow reach a steady state, normal IOP is maintained; when aqueous inf low exceeds outf low, IOP rises; when aqueous outflow exceeds inflow, IOP falls. Almost all of the previous inducible glaucoma models increase IOP by decreasing aqueous outf low, either through occluding the angle of the anterior chamber or by damaging the TM. These models are excellently summarized in a recent comprehensive review (Pang and Clark, 2019). We recently developed an inducible ocular hypertension model in mice by preventing aqueous inf low into the anterior chamber (Zhang et al., 2019a, b). Here we compare our new model to several common inducible mouse models of ocular hypertension, and brief ly summarize the unique features, major drawbacks and potential applications of these models in basic and preclinical research.
Ocular hypertension and glaucomatous neurodegeneration induced by intracameral injection of silicone oil (SO): SO is used as a tamponade in retinal detachment repair surgery because it is buoyant with high surface tension. However, SO is lighter than the aqueous and vitreous f luids and an excess can physically occlude the pupil, which in turn prevents aqueous f low into the anterior chamber. This obstruction leads to a post-operative complication with increased aqueous pressure in the posterior chamber and anterior displacement of the iris, which causes angle-closure, blockage of aqueous outf low through TM, and a further increase in IOP (Ichhpujani et al., 2009). We recently faithfully replicated this well-documented, human secondary glaucoma in mouse eyes by intracameral injection of SO (Zhang et al., 2019a, b). SO injected into anterior chamber effectively blocks the pupil, which thwarts aqueous inflow. Aqueous humor, which is constantly produced by the ciliary body, accumulates in the posterior chamber and consequently increases the IOP of the posterior segment. When the iris is pushed forward and the iris root touches the posterior corneal surface, the anterior chamber angle closes. The angle closure further impedes the outf low of aqueous humor through TM and also contributes to IOP elevation. Thus, by mimicking this human secondary glaucoma, we create a novel ocular hypertension model in mice with conf ined aqueous accumulation in the posterior chamber and elevated IOP of the posterior segment.
In contrast to the SO model, intracameral injection of polystyrene microbeads physically obstruct aqueous outflow by accumulating and occluding the TM and Schlemm’s canal (Sappington et al., 2010). Refinements of this basic technique include adding viscoelastic material or replacing polystyrene microbeads with magnetic microbeads (Pang and Clark, 2019). Despite mixed success between labs in reproducing the microbead model, the microbeads model has become popular and contributed to many significant novel findings in glaucoma research. Compared with the acute IOP elevation in the SO model, microbeads injection increases IOP more slowly and mildly, which may more closely resemble chronic glaucoma in the clinic. A caveat is the relatively modest neurodegeneration in this mouse model, which leaves a narrow window for preclinical testing of neuroprotective therapies. Another important caveat to its use, however, is that it is challenging to retain microbeads at the angle of the anterior chamber and to control the degree of aqueous outf low blockade. In order to maintain a long duration of elevated IOP at the necessary level to cause RGC loss, reinjection is normally required, which increases complications such as corneal opacity, neovascularization, and ulceration, as well as endophthalmitis.
Another common method to decrease aqueous outflow is to use a diode laser to photocoagulate the outflow structures, the TM, limbal plexus or episcleral veins; damage of these structures closes intertrabecular spaces and major outf low channels and thus increases the resistance of the outflow pathway (Pang and Clark, 2019). This method needs expensive ophthalmic laser equipment and highly specialized skills, as well as multiple repeats of the procedures to increase the chance of IOP elevation. It is also more successful in large animals, such as non-human primate, because it is difficult to apply to the small mouse eye. Laser photocoagulation in mouse eye is normally associated with relatively severe complications, including permanent and extensive sclera damage, outer retina injuries, intraocular inf lammation, and retinal and choroidal ischemia. Compared to this model, the SO model is much simpler, more stable, and, importantly, undoubtedly much less damaging to the eye. Since SO causes ocular hypertension in both human patients and mice, it is reasonable to hypothesize that the same procedure may be adapted to different animal species with minimal confounding factors. The anatomy of the non-human primate visual system closely resembles that of humans and includes a macula and lamina cribrosa not present in mouse. An non-human primate SO glaucoma model is the most likely to predict human responses to ocular hypertension and therapies.
Similar to the SO model, which mimics clinical secondary glaucoma in human, topical ocular administration of the glucocorticoid, dexamethasone, in mice also replicates human secondary glaucoma and induces ocular hypertension and glaucomatous neurodegeneration (Zode et al., 2014). Glucocorticoids are commonly used to treat ocular inf lammation. However, for reasons that are not well understood, prolonged treatment with glucocorticoids causes IOP elevation and secondary open angle glaucoma in about 30% of patients (Fini et al., 2017). Although there is evidence to support the role of glucocorticoids in TM dysfunction and IOP elevation in mice (Pang and Clark, 2019), the multiple systemic functions of glucocorticoids complicate interpreting the pathogenesis of glucocorticoid-induced glaucomatous neurodegeneration.
Compared to these commonly used ocular hypertension and glaucoma mouse models, the SO model has some advantages that we summarize in Table 1: 1) A single SO injection into anterior chamber allows a reliable, sufficient, and stable IOP elevation without the multiple treatments required by other models. 2) The size of the SO droplet stays stable for a long period and also correlates well with IOP elevation; unresponsive animals can be predicted by the size of the SO droplet very soon after injection and excluded promptly. 3) RGC and optic nerve degeneration is consistent and progressive, and the degeneration correlates well with imaging and visual function deficits similar to those observed in patients. 4) Because the unique pathogenesis of the pupillary block spares the ocular elements in the anterior part of the eye from damage by high IOP, the clear cornea, lens and transparent SO allow light to the retina for in vivo assessment of visual function and morphology. 5) Because SO injection does not permanently damage ocular tissues and the SO can be removed easily to lower IOP quickly to normal, another unique feature of this model is that the ocular hypertension is reversible. Thus, it can mimic the clinical setting and test whether lowering IOP together with neuroprotection, regeneration, or cell replacement therapies provides additional benef its for visual function recovery.
Like every other model, the SO model also has limitations and complications. An important disadvantage is that the increased IOP can only be detected after dilation removes the pupillary block and aqueous inflow is restored. The SO droplet touching the surface of the iris in combination with the large mouse lens forms a rigid barrier that seals the pupil and essentially disconnects anterior chamber from posterior segment. This seal acts as a dam that keeps IOP low in the anterior segment and high in the posterior segment where the aqueous accumulates. Unfortunately, posterior IOP cannot be measured directly, at least in mouse eyes. Only when the mouse pupil is sufficiently dilated that it is no longer covered by the SO droplet are the anterior and posterior chambers reconnected and the aqueous permitted to quickly f lood into the anterior chamber. This f low exceeds outf low from the TM allowing increased IOP in the anterior chamber to be detected. This may also be the reason for another disadvantage of the SO model, which is that the IOP elevation is acute instead of chronic. Thus, this model replicates secondary acute glaucoma in the clinic but not the majority of chronic glaucoma patients. However, the SO model can be modif ied to be milder and more chronic by more frequent pupil dilation because every dilation releases the aqueous accumulated at the back of the eye and serves as treatment to lower IOP. Although very rare, corneal opacity caused by band-shaped degeneration or neovascularization can occur, which may be associated with the interaction of excessive SO with the endothelial cells of the cornea.
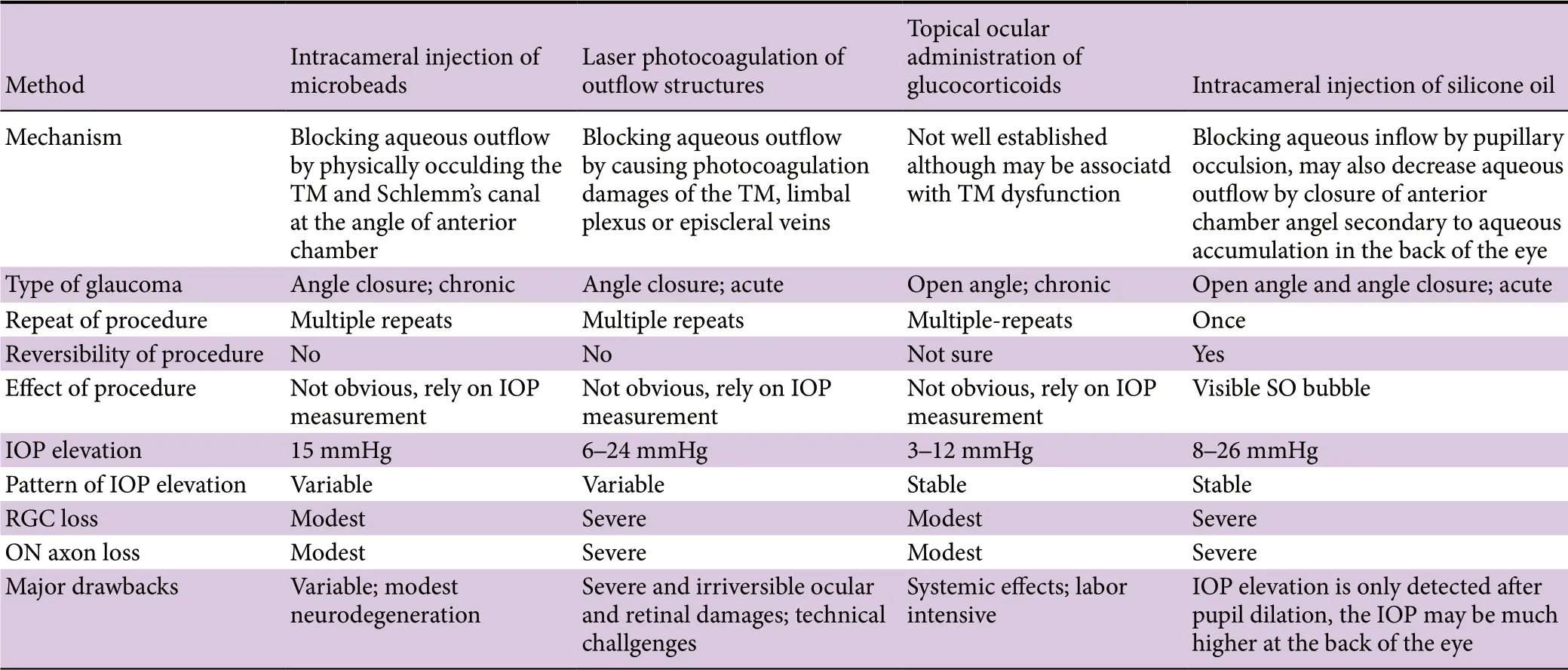
Table 1 Comparison of inducible ocular hypertension mouse models
In summary, a simple but effective ocular hypertension model in mice is important for investigating glaucomatous neurodegeneration and testing treatments for neuroprotection. Thus, we resolved to develop a straightforward mouse ocular hypertension model that closely mimics SO-induced secondary glaucoma observed in human patients. The procedure is simple, the IOP elevation is stable, and the neurodegeneration associated with ocular hypertension is severe. Importantly, the ocular hypertension is reversible because the SO can be easily removed. No special equipment or repeated procedures is required. We predict it will expedite selection of neuroprotectants and facilitate establishing the pathogenesis of acute ocular hypertension-induced glaucoma. But the value and shortcomings of this model will become clear only after it is analyzed by more investigators.
We thank Dr. Alan Tessler for critically reading the manuscript.
YH was supported by NIH grants EY024932, EY023295 and EY028106 and grants from BrightFocus Foundation and Glaucoma Research Foundation.
Jie Zhang, Yang Hu*
Department of Ophthalmology, Stanford University School of Medicine, Palo Alto, CA, USA (Zhang J, Hu Y)Department of Ophthalmology, Union Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan, Hubei Province, China (Zhang J)
*Correspondence to:Yang Hu, MD, PhD, huyang@stanford.edu.
orcid:0000-0002-7980-1649 (Yang Hu)
Received:November 20, 2019
Peer review started:November 28, 2019
Accepted:January 2, 2020
Published online:February 28, 2020
doi: 10.4103/1673-5374.276330
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of an injured ascending reticular activating system with recovery from a minimally conscious state to normal consciousness in a stroke patient: a diffusion tensor tractography study
- The role of vascularization in nerve regeneration of nerve graft
- New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target?
- Advanced diffusion magnetic resonance imaging in patients with Alzheimer’s and Parkinson’s diseases
- Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury
- Decoding epigenetic codes: new frontiers in exploring recovery from spinal cord injury
