Insights into platinum-induced peripheral neuropathy-current perspective
2020-03-07AndrijanaLaziJelenaPopoviTatjanaPauneskuGayleWoloschakMilenaStevanovi
Andrijana Lazić, Jelena Popović, Tatjana Paunesku, Gayle E. Woloschak, Milena Stevanović,
1 Institute of Molecular Genetics and Genetic Engineering, University of Belgrade, Belgrade, Serbia
2 Feinberg School of Medicine, Department of Radiation Oncology, Northwestern University, Chicago, IL, USA
3 Faculty of Biology, University of Belgrade, Belgrade, Serbia
4 Serbian Academy of Sciences and Arts, Belgrade, Serbia
Abstract Cancer is a global health problem that is often successfully addressed by therapy, with cancer survivors increasing in numbers and living longer world around. Although new cancer treatment options are continuously explored, platinum based chemotherapy agents remain in use due to their efficiency and availability. Unfortunately, all cancer therapies affect normal tissues as well as cancer, and more than 40 specif ic side effects of platinum based drugs documented so far decrease the quality of life of cancer survivors. Chemotherapy-induced peripheral neuropathy is a frequent side effects of platinum-based chemotherapy agents. This cluster of complications is often so debilitating that patients occasionally have to discontinue the therapy. Sensory neurons of dorsal root ganglia are at the core of chemotherapy-induced peripheral neuropathy symptoms. In these postmitotic cells, DNA damage caused by platinum chemotherapy interferes with normal functioning. Accumulation of DNA-platinum adducts correlates with neurotoxic severity and development of sensation of pain. While biochemistry of DNA-platinum adducts is the same in all cell types, molecular mechanisms affected by DNA-platinum adducts are different in cancer cells and non-dividing cells. This review aims to raise awareness about platinum associated chemotherapy-induced peripheral neuropathy as a medical problem that has remained unexplained for decades. We emphasize the complexity of this condition both from clinical and mechanistical point of view and focus on recent findings about chemotherapy-induced peripheral neuropathy in in vitro and in vivo model systems. Finally, we summarize current perspectives about clinical approaches for chemotherapy-induced peripheral neuropathy treatment.
Key Words: chemotherapy-induced peripheral neuropathy; CIPN; DNA-Pt adducts; dorsal root ganglia; DRG; model systems; molecular mechanisms; neurotoxic; platinum based chemotherapy; Pt; sensory neurons treatment; side effects
Introduction
Side effects of anticancer therapies of varying severity often persist over many years. Many excellent reviews have focused of complications that follow chemotherapy or radiation therapy. Most focus on complications following a specific type of cancer or on specif ic groups of complications. For example, a recent review by Stone and DeAngelis (2016) discusses syndromes affecting the central and peripheral nervous system (PNS) in response to more than two dozen chemotherapy treatments and radiotherapies, regardless of the primary disease. PNS complications have been noted for as many as 21 different chemotherapy agents including cisplatin, carboplatin and oxaliplatin (Stone and DeAngelis, 2016). It is important to note that autonomic, motor and sensory complications associated with these therapies are different and drug-specif ic. It is therefore unlikely that the same underlying mechanisms can be associated with all of these varied treatments. Consequently, approaches to mitigate these complications are unlikely to be universally applicable. Thus, attempts to explain all cancer therapy complications by a single wide-ranging concept, such as oxidative stress (Areti et al., 2014), may not be the complete explanation for a particular therapeutic complication. For example, the newly emerging concept of the microbiome gut-brain axis was recently proposed as an explanation for complications caused by cancer therapies (Bajic et al., 2018), and it is conceivable that different parts of microbiome are affected differently by specif ic chemotherapeutic drugs. Consequently, same drugs rarely have the same effects in all cancer patients for reasons that are not only drug specif ic, but also patient specif ic.
Chemotherapy-induced peripheral neuropathy (CIPN) is a subset of PNS complications often associated with platinum agents according to the Multinational Association of Supportive Care in Cancer neurological complications working group (Chan et al., 2019). Platinum (Pt) based chemotherapy drugs are the main theme of this review which separates purported molecular targets for these groups of chemotherapy agents into gene and protein regulation effects, subcellular organelle effects involving endoplasmic reticulum and mitochondria, and effects on specif ic categories of biomolecules such as microtubules, ion channels and nuclear DNA. It is important to recognize that the same molecular targets critical for success of anti-cancer treatments and the same biochemical mechanisms of action that make chemotherapy drugs effective against cancer are also the ones that cause normal tissue toxicities. Neuronal cells of the PNS are exposed to chemotherapy drugs in the course of treatment and accumulate the same molecular damage as do cancer cells, for example damage of the genomic DNA (Chan et al., 2019). However, while mutation accumulation and genomic instability primarily decrease success of mitosis of cancer cells, their effects on transcription and translation are more important for non-dividing cells. Other “less important” effects of chemotherapy drugs, e.g., endoplasmic reticulum stress also have different effects in rapidly dividing versus irreversibly differentiated cells. In a population of cycling cancer cells some of the daughter cells may manage to evade cellular stress by sheer uncontrolled increase of newly produced cell mass. On the other hand, functional endoplasmic reticulum is overwhelmingly important for neuronal cells with carefully orchestrated protein translation necessary for lifelong maintenance.
This review emphasizes the complexity of CIPN from clinical and mechanistic point of view. Even with contemporary resources and advancements in the medical science the problem of CIPN remains unsolved. CIPN pain is mostly managed with opioids, which underscores the fact that the molecular effects of Pt-based drugs on non-dividing cells such as dorsal root ganglia (DRG) neurons are insufficiently studied. We focus on recent findings regarding CIPN target cells and tissues and update information about in vitro and in vivo model systems for CIPN as crucial elements to understand Pt associated CIPN complexity.
Search Strategy and Selection Criteria
We conducted a literature search using NCBI/PubMed database with following terms: cisplatin; oxaliplatin; carboplatin: CIPN models, CIPN mechanisms in period between April 2019 and August 2019. We have also reviewed additional papers that were referenced in the obtained publications and some of them included in this review.
Undesired Side Effects of Platinum-Based Chemotherapy Drugs
Pt based chemotherapy agents are some of the best known anticancer therapies. They are used to treat cancers of most organ systems — urogenital such as testicular, prostate and bladder cancer; female cancers such as ovarian, breast and cervical cancers; head and neck cancers, esophageal and stomach cancer, small and non-small cell lung carcinomas, melanoma, neuroblastoma, mesothelioma as well as Hodgkin’s and non-Hodgkin’s lymphomas, sarcomas and multiple myeloma. As with most small molecule therapies, Pt chemotherapy is not tumor specific and always affects normal tissue as well. Literature suggests that as many as 40 side effects may be ascribed to these drugs. These therapy associated complications include nephrotoxicity, ototoxicity, neurotoxicity, cardiotoxicity, hematological toxicity, hepatotoxicity, and gastrointestinal toxicity (Crona et al., 2017; Fung et al., 2018; Oun et al., 2018). The effects differ between patients as well as for different doses and drug delivery routes, but they are most specif ically associated with the type of the Pt drug itself. Three drugs are widely in use clinically (Chen et al., 2013): cisplatin, carboplatin and oxaliplatin which have different effects on central and peripheral nervous systems (Stone and DeAngelis, 2016). Many other are under development (Sarkar, 2018), in hope that adverse side effects can be reduced with other Pt formulations.
In brains of cisplatin treated patients, concentration of this metal ranges between 0.33 and 2.9 μg/g (Nakagawa et al., 1996). At these concentrations, cisplatin causes impairment or death of cells in different areas such as hippocampus suggesting a mechanistic basis for chemotherapy associated cognitive impairment (Andres et al., 2014). Neurotoxic effects on peripheral nerves leading to CIPN often results in discontinuation or reduction of therapy. This, in turn, affects efficacy of treatment and quality of life (Miltenburg and Boogerd, 2014; Boyette-Davis et al., 2015; Carozzi et al., 2015; Chiorazzi et al., 2015; Kanat et al., 2017; Kerckhove et al., 2017; Paice et al., 2017; Starobova and Vetter, 2017; Ma et al., 2018). There are currently no effective strategies for either prevention or treatment of CIPN.
Overview of Chemotherapy-Induced Peripheral Neuropathy Caused by Exposure to Platinum-Based Chemotherapy
While sensation of pain is associated with disturbance of sensory function associated with damage of large myelinated sensory fibers, CIPN also affects motor and autonomic functions caused by general damage of PNS. Causes of damage include oxidative stress, change of calcium homeostasis, axon degeneration and membrane remodeling, all of it caused by neuroinf lammation and triggering of apoptotic pathways as reviewed in Chiorazzi et al. (2015). As many as 68% of patients receiving chemotherapy develop CIPN within the first month of treatment. The severity and persistence of CIPN often depend on pre-existing nerve damage associated with other diseases such as diabetes (Seretny et al., 2014).
Three most important Pt based chemotherapy drugs differ by their neurotoxic potential, with cisplatin and oxaliplatin more damaging than carboplatin. In each case, toxicity of a specific Pt drug reflects its reactivity — how labile its leaving groups are as they bind different biomolecules. In consequence, the most toxic drugs are those that have most labile group(s) (Oun et al., 2018). Positive charge of metal ions with vacant d-orbitals allows them to bind electronegative sites in proteins and nucleic acids (Ali et al., 2013). DNA damage is generally considered to be the main cause of apoptosis observed in sensory neurons (Gill and Windebank, 1998).
Different techniques have been used to assess the quantity of Pt association with specif ic proteins or trace elements in biological samples (Palm-Espling et al., 2013; Galvez et al., 2018). In vitro experiments with rat sensory neurons showed that increased concentrations of cisplatin (10-50 μM), oxaliplatin or carboplatin (100-500 μM) corresponded to increased cell death and apoptosis (Kelley et al., 2014). At the same time, carboplatin or oxaliplatin versus cisplatin concentrations causing a similar degree of cytotoxicity had a ratio of 10 to 1. Nevertheless, cytotoxicity in all three cases corresponded to production of reactive oxygen species and accumulation of 8-oxoguanine DNA damage (Kelley et al., 2014). The level of cisplatin accumulation in patients’ brain, between 0.33 and 2.9 μg/g (Nakagawa et al., 1996) corresponds to that found in cells incubated with 5 μM cisplatin in cell culture (Hermann et al., 2013). Therefore, study with rat sensory neurons started with only two fold higher cisplatin concentration than that can be expected in patients.
The main target of Pt drugs in vivo are believed to be bodies of sensory neurons located in DRG (Ta et al., 2006). Exposure to Pt-based compounds causes morphological changes that are especially obvious in nucleoli whose appearance changes with decreased production of ribosomal RNA. Stalled transcription is explained by formation of DNA-Pt adducts; in large neuronal cells, dependent on active transcription and translation, this leads to apoptosis (McWhinney et al., 2009). Particular sensitivity of DRG neurons to Pt-based compounds is ascribed to their dense fenestrated vascularization and lack of a blood-brain barrier (Jimenez-Andrade et al., 2008; Carozzi et al., 2015). The absence of lymphatic drainage in DRG further exacerbates the situation (Karlsson et al., 2017). There are still doubts regarding the mechanisms of cellular uptake and efflux of Pt compounds. While early studies suggested that Pt enters the cell mainly by passive diffusion (Wang and Lippard, 2005), more recent findings suggest that active uptake may also be involved in the uptake of Pt based drugs into DRG neurons. Proteins present on neuronal cell membrane: organic cation transporter-2, copper transporter-1, and other copper associated molecules such as Atox1 were all implicated in Pt uptake (Palm-Espling et al., 2013; Sprowl et al., 2013; Cavaletti et al., 2014). Changes within DRG and sensory neurons affect distal extremities starting from cold sensitivity which transforms into long-lasting burning pain, numbness, and tingling. In addition to sensory neurons, other neural components involved in CIPN include satellite cells, Schwann cells and neuronal and glial cells in the spinal cord (Han and Smith, 2013). Pt adducts may also cause axonal changes in addition to neuronal damage (Kanat et al., 2017).
Biological Mechanisms Underlying Platinum Associated Chemotherapy-Induced Peripheral Neuropathy
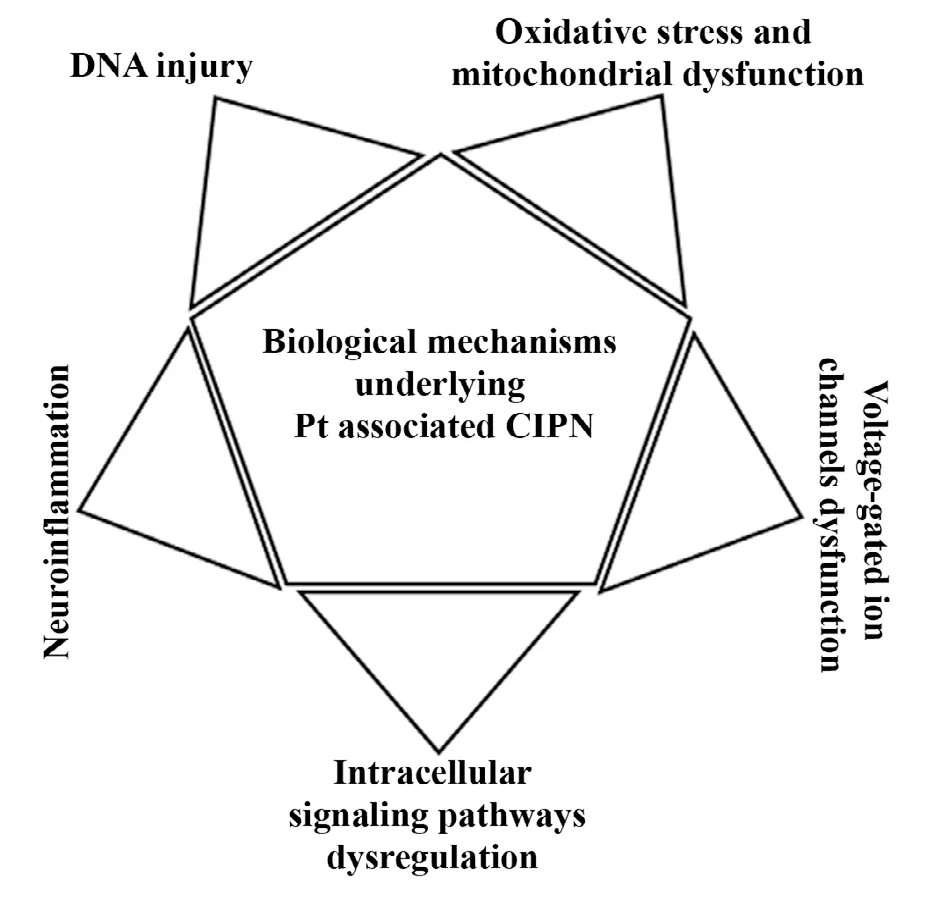
Figure 1 Summary of biological mechanisms underlying peripheral neuropathy induced by Pt based chemotherapy drugs.
Many different mechanisms of action were proposed for CIPN induction by Pt based chemotherapy drugs (Figure 1). For example, a recent review focused on Pt induced deregulation of ion channels and compromised mitochondrial DNA transcription as causes of altered excitability of peripheral neurons (Zajaczkowska et al., 2019). This work also emphasizes activation of microglia and astrocytes as initial stages in neuroinf lammation, while Colvin et al. (2019) recognize role of cytokines in this process as well. In general, it seems that chemotherapeutic antitumor mechanisms are also responsible for their neurotoxic effect (Zajaczkowska et al., 2019). Chemotherapeutic agents deregulate activity of cell membrane proteins such as receptors and ion channels and affect mitochondrial and nucleolar morphology. This disbalances intracellular homeostasis, signaling and neurotransmission, and the final outcomes include production of reactive oxygen species (ROS), neuroinflammation, DNA damage, demyelination, cytoskeleton damage and microtubule disruption, axonal degeneration and cell death (Carozzi et al., 2015; Malacrida et al., 2019; Zajaczkowska et al., 2019).
DNA injury
Binding between Pt and DNA produces intra-strand and inter-strand DNA and DNA-protein cross-links called DNA adducts (Wang and Lippard, 2005). These complex DNA lesions inhibit the DNA base excision repair (Kelley et al., 2014) as well as transcription (Yan et al., 2015). While these changes in cellular activity are important for Pt-drugs anticancer activity, they also injure non-mitotic cells such as DRG neurons because of their requirements to sustain high metabolism and protein and organelle production necessary for maintenance of long axons. Thus, in sensory neurons of DRG-induced DNA damage leads to neuronal atrophy. Most important for resolution of Pt caused DNA damage is the base excision DNA repair pathway. Of all the proteins involved in base excision DNA repair apurinic/apyrimidinic endonuclease/redox factor-1 is especially important for resolution of DNA damage caused by cisplatin and oxaliplatin. In fact, enhancing the activity of apurinic/apyrimidinic endonuclease/redox factor-1 protected cisplatin-treated cultured neurons from neurotoxicity (Areti et al., 2014; Kelley et al., 2014, 2016).
Oxidative stress, mitochondrial dysfunction
Oxidative stress and mitochondrial dysfunction are also indicated as critical for development of CIPN (Podratz et al., 2011). Oxidative stress causing neurodegeneration can be a result of reduction of antioxidant defenses and bioenergetic failure (Podratz et al., 2011; Giordano et al., 2014). Mammalian nerves with their long axons are especially vulnerable to ROS due to their high content of phospholipids and relatively poor cellular antioxidant defenses. At the same time, axoplasm is f illed with mitochondria and numerous studies suggest that axonal mitotoxicity contributes to CIPN (Canta et al., 2015). Pt compounds directly bind mitochondrial DNA (mDNA) just as they bind to nuclear DNA. Unlike nuclear DNA which can be repaired by base excision DNA repair, repair of Pt-mDNA adducts is not efficient (Prakash and Doublie, 2015). Presence of Pt adducts inhibits mDNA transcription and replication and causes morphological changes in the mitochondria. For example, mitochondria of peripheral nerves in chemotherapy treated animals were found to be swollen and vacuolated (Xiao and Bennett, 2012). Electron transport chain and ATP generation are easily disturbed in damaged mitochondria resulting in energy failure and overproduction of ROS. All of these events cause redox imbalance, opening of mitochondrial permeability transition pores, depolarization of mitochondrial membrane and release of cytochrome c, deregulation of calcium homeostasis and initiation of apoptosis. All of these events — decrease in cellular metabolism and increase in production of ROS ultimately cause structural damage of peripheral nerves (Zheng et al., 2011; Waseem et al., 2018).
Intracellular signaling dysregulation
Pt induces unregulated proteolysis through an array of intracellular signaling pathways. In its role of the second messenger, intracellular Ca2+plays a very important role in cellular homeostasis and its disturbance is also involved in CIPN (Leo et al., 2017a, b). Since Ca2+is stored in mitochondria and endoplasmic reticulum, the integrity of these organelles is of great importance for Ca2+homeostasis. Alternations in Ca2+concentration may interfere with membrane excitability, neurotransmitter release and gene expression (Carozzi et al., 2015). Paclitaxel induces rapid mitochondria depolarization and Ca2+release by activation of the mitochondrial permeability transition pore (Tsubaki et al., 2015). Axonal degeneration may be triggered by activation of protein kinases and caspases. Mitogen-activated protein kinase has been the most studied in CIPN (Tsubaki et al., 2015). Cisplatin and oxaliplatin induce apoptosis of DRG neurons via mitogen-activated protein kinase mechanism. In mitogen-activated protein kinase inhibitor experiments Pt induced DRG damage in vitro was prevented (Scuteri et al., 2009).
Voltage-gated ion channels dysfunction
Development of CIPN has also been associated with malfunction of ion channels such as sodium and potassium channels and transient receptor potential (TRP) channels. Voltage-gated channels initiate and propagate action potentials in neural cells, and disturbance in their function changes peripheral nerve excitability. TRP channels TRPA1, TRPV1 and TRPM8 detect thermal, mechanical and chemical signals in DRG neurons. While all of these signals misf ire in CIPN, not all of them do so in response to Pt drugs. For example, reduction in potassium channels and the spontaneous activity of nociceptors were observed in DRG in a paclitaxel-induced CIPN model (Deuis et al., 2014). Interestingly, while oxaliplatin alters sodium and potassium channels functions in vivo and in vitro (Deuis et al., 2014; Poupon et al., 2018), effect of cisplatin on these ion channels was not noted in neurons (Kanat et al., 2017). Preclinical studies on nonselective TRP cation channels have shown that they play a crucial role in cold and mechanical sensitivity induced by oxaliplatin and cisplatin (Kanat et al., 2017; Zajaczkowska et al., 2019).
Inf lammatory cytokines and chemokines
Neuroinflammation as a critical mechanism contributing CIPN complexity is an emerging concept. This process is characterized by inf iltration and activation of immune cells, peripheral glial (satellite and Schwann cells) and central glial cells (astrocytes and microglia) and their production of cytokines and chemokines. Association between pain hypersensitivity and an acute pro-inflammatory immune response has been demonstrated in rodent models with different chemotherapeutics. For example, drugs that suppress activation of immune cell alleviate pain hypersensitivity (Lees et al., 2017). Preclinical studies have also implicated cytokine signaling in CIPN. In the DRG and spinal cord CIPN is associated with an increase of pro-inflammatory cytokines (tumor necrosis factor-α, interleukin (IL)-1β and IL-6) and a decrease of anti-inf lammatory cytokines (IL-10 and IL-4) (Lees et al., 2017). Chemokines can also act as pro-nociceptive mediators following nervous system injury and disease. In rodents, chemokines CCL2/CCR2 have a major role in chronic pain (White and Miller, 2010). Cytokine IL-10 on the other hand suppresses numbness and pain, while CCL2/CX3CL1 increase infiltration of macrophages and support production of ROS (Colvin, 2019).
Clinical Attempts to Treat Mechanisms of Chemotherapy-Induced Peripheral Neuropathy
Current CIPN therapeutic strategies depend on pain symptom relief and therapies used to treat neuropathic pain do not prevent CIPN. Pharmacotherapy includes analgesics, anticonvulsants (pregabalin, gabapentin), antidepressants, opioids and serotonin-noradrenalin reuptake inhibitors (duloxetine and venlafaxine). Cancer and noncancer-related neuropathic pain have distinct molecular signatures. Trials assessing neuropathic pain drugs in CIPN often include patients with non-pain neuropathy symptoms, such as sensory loss. Generally, cancer pain includes mixed mechanisms and rarely presents as a purely neuropathic pain, but rather as a complex with inflammatory and/or ischemic components. Accordingly, a distinction between cancer and noncancer-related neuropathic pain is almost impossible. Various nonpharmacologic strategies including vitamins, minerals and herbs have also been studied in CIPN with limited success (Majithia et al., 2016). It can be hoped that future findings revolving around the etiology of CIPN will enable the development of a mechanism-based therapy, aimed to treat underlying causes of this condition rather than its fully developed signs- and symptoms.
The effects of cisplatin on cells are generally associated with DNA adducts and oxidative and nitro-oxidative stress affecting both nuclear and mitochondrial genomes (Carozzi et al., 2010; Han and Smith, 2013; Areti et al., 2014; Starobova and Vetter, 2017; Wang et al., 2018). While experimental strategies targeting ROS gave positive results in preclinical studies, the results from clinical studies were less satisfying. Many antioxidants have been tested including vitamin B6, vitamin E, omega-3 fatty acids, glutamine, gluthatione, acetyl-L-carnitine and α-lipoic acid with controversial results (Albers et al., 2007; Beijers et al., 2012; Schloss et al., 2013; Piccolo and Kolesar, 2014). For example, α-lipoic acid demonstrated promise in preclinical studies (Melli et al., 2008), but did not have protective effect in patients receiving chemotherapy (Melli et al., 2008; Guo et al., 2014). Stimulation of endogenous antioxidants has been suggested as an alternative to external antioxidant supplementation with the transcription factor peroxisome proliferator-activated receptor alpha emerging as a candidate in animal CIPN studies (Donvito et al., 2016). Early stage clinical studies have focused on palmitoylethanolamide as its modulator (Okine et al., 2019).
A recent systematic review describes a total of 26 different treatment options for CIPN, including pharmacological, light, scrambler, magnetic field, acupuncture, dietary and long-wave diathermy therapy (Hou et al., 2018). However, evidence for clinical efficacy of new pharmaceutic and non-pharmaceutic treatments has not been positive and today Duloxetine is the only one drug recommended by the American Society of Clinical Oncology for the treatment of CIPN. This drug is an antidepressant and nerve pain medication for diabetes. Photobiomodulation or low-level laser therapy, is also occasionally used for treatment of CIPN. While this treatment is also believed to show moderate benef its in CIPN patients, a lengthy list of other treatments showed no appreciable value to CIPN sufferers. List of unsuccessful CIPN treatments includes topical KA (4% ketamine and 2% amitriptyline), tricyclic antidepressants, andamitriptyline, and nortriptyline (Hou et al., 2018).
Thiol WR-2721, also known as Ethyol®or Amifostine was also clinically tested for prevention of CIPN. Active metabolite of this thiol, another thiol known under label WR1065 protected differentiated neurons from cisplatin induced damage (Popovic et al., 2019). This thiol accumulates in normal tissues more efficiently than in tumors and protects the normal tissues from side effects of radiation and chemotherapy without decreasing the therapeutic efficacy of these anti-cancer treatments (Senzer, 2002; Lorusso et al., 2003; Small, 2003; Wasserman et al., 2005). Amifostine was tested against CIPN in several clinical trials (Beijers et al., 2012; Schloss et al., 2013; Albers et al., 2014; Piccolo and Kolesar, 2014). This thiol was also tested in translational animal models (Treskes and van der Vijgh, 1993; Yalcin et al., 2003) with varied success. For that reason, further evaluation of molecular mechanisms that may be responsible for success of in vitro use of this drug should be considered. In human neurons co-treated in vitro with cisplatin and WR1065, WR1065 has decreased ROS and preserved cell viability and neurite outgrowth (Popovic et al., 2019). X-ray f luorescence microscopy (XFM) of neurons treated by cisplatin in the presence of WR1065 has shown fewer cells with Pt accumulation and lower Pt concentration compared to XFM of cells treated with cisplatin alone. Based on Pt LIII near edge X-ray absorption spectroscopy, the addition of WR1065 to DNA or protein mixtures with cisplatin alters chemical bonds formed by Pt atoms. This finding argues that WR1065 modulates chemical interactions between cisplatin Pt (NH3)2Cl2 and DNA. It is possible that further research delving more deeply into Pt and thiol chemistry may provide a route to use Amifostine as a treatment for CIPN symptoms (Popovic et al., 2019). We agree with the conclusion of (Hou et al., 2018) that larger sample sizes, long-term follow-up, standardized outcome measurements, and standardized treatment timing are needed to properly evaluate benef its of WR1065 or any other therapeutic. Finally, among the novel treatments for CIPN there are several manganese chelates. These Mn providing chemicals were noted to be mimetics for mitochondrial manganese superoxide dismutase and efficient in ROS reduction (Karlsson et al., 2017; Glimelius et al., 2018).
Pre-Clinical Models of Chemotherapy-Induced Peripheral Neuropathy
Traditional model systems to study CIPN have mostly relied on animal and cell-line models. Rodent models that mimic the human disease have been used to study pathological changes induced by chemotherapy as well as to investigate potential neuroprotective treatments (Authier et al., 2009). The experimental approach using animals includes drug treatment administered by interperitoneal or intravenous injection followed by behavioral, electrophysiological or morphological analysis. A detailed overview of the current animal models used for CIPN studies has been published recently (Hoke and Ray, 2014). Work with rodent models lead to discovery of morphological changes of DRG neurons and development of their pathology with time. These hallmarks of CIPN include loss of intraepidermal nerve fiber endings, degeneration of sensory neuron axons and demyelination as well as loss of mitochondrial architecture through vacuolation (Chua and Kroetz, 2017). Recently, much effort was placed into development of models that faithfully mimic the human peripheral neuropathy and development of approaches suitable to measure treatment outcomes (Hoke and Ray, 2014). In short — efforts dedicated to growth of animal models of CIPN have been extensive (Renn et al., 2011; Brabb et al., 2014; Guindon et al., 2014; Percie du Sert and Rice, 2014; Lin et al., 2015; Wozniak et al., 2016; Hama et al., 2018) including not only rodents but Drosophila fruit f lies as well (Podratz et al., 2017). Finally, it should be mentioned that rodent models are also used to evaluate etiology and possible treatments for cisplatin complications other than CIPN such as cognitive impairment and ototoxicity (Rainey et al., 2016; Lomeli et al., 2017).
In Vitro Models of Chemotherapy-Induced Peripheral Neuropathy
Animal models are not particularly appropriate for the analysisi of Pt accumulation and distribution at the single cell level, and studies in cultured DRG neurons and neuroblastoma cell lines have been suggested possible molecular mechanisms underlying CIPN (Chua and Kroetz, 2017; Leisengang et al., 2018). These models are suitable for testing potential neuroprotective compounds and analysis of their mechanism of action. Many studies relied on the pluripotent human embryonal carcinoma cell line NT2/D1 (Klajn et al., 2014; Popovic et al., 2014; Drakulic et al., 2015; Jasnic-Savovic et al., 2015). Upon induction with retinoic acid these cells differentiate into post-mitotic, morphologic and phenotypic central nervous system-like neurons (NT2/N) that produce, release and respond to neurotransmitters. In addition, these cells grow neurofilaments and are able to generate action potentials and calcium spikes (Guillemain et al., 2000; Hara et al., 2008). While other cell lines also differentiate into neurons in vitro (for example human neuroblastoma SK-N-SH, rat pheochromocytoma PC12), NT2/D1 yields neurons with complete loss of tumor characteristics (Hara et al., 2008). Recent findings also demonstrate that NT2/N could be used for XFM studies and flow cytometry single cell analysis (Popovic et al., 2019). In particular, Pt distribution imaged in cryogenically prepared NT2/N cell samples was, as far as we know, the first published evaluation of Pt distribution in non-dividing cells by XFM.
Reprogramming of somatic cells into induced pluripotent stem cells (iPSC) followed by redifferentiation of iPSCs into patient specific disease-specific cell types revolutionized the field of “in vitro disease modeling” (Sandoe and Eggan, 2013). Technologies that rely on iPSC hold great potential for understanding CIPN on a molecular level. Protocols to generate human peripheral sensory neurons in vitro (Chambers et al., 2012; Wainger et al., 2015) provide a tool to mimic CIPN target tissue. This tissue model may recapitulate the key neuropathy features and help in developing novel therapeutic approaches to overcome CIPN. iPSC-derived sensory neurons will also enable investigate the role of patient-specif ic genetic variation in CIPN predisposition. Such models may give insights on genetic networks contributing CIPN as well as high throughput screening of potential neuroprotective targets (Chua and Kroetz, 2017; Wing et al., 2017).
Conclusions and Future Perspectives
The many symptoms associated with CIPN reflect the complexity of this condition. Pt compounds injure every component of neuronal cells — from gene expression and mitochondrial homeostasis to cytoskeleton components, subcellular structures and membranes. Consequently — treatment of these endpoints individually, one by one, is almost impossible. Instead, new research is needed — finding first what are the key causes in the chain of events that result in injury of all of these cellular structures and functions. Most of the therapeutic approaches used to address different symptoms had shown some efficiency against CIPN. At the same time, all agents tested in large clinical trials have failed to show efficiency and none of them are currently in widespread use. Therefore, renewed efforts to discover optimal timing and delivery routes for already existing CIPN treatments may be more likely to lead to success then efforts to find new and complete anti CIPN drug alternatives. Better outcomes may come from extended anti-CIPN treatments over long periods of time, or by use of alternate therapeutic approaches in parallel or sequentially etc. Many researchers believe that removal of Pt-DNA adducts could be of the greatest benef it to CIPN patients. Current approaches to fortify repair of DNA carrying DNA adducts include hyperthermia and detoxif ication (Puri et al., 2019). While cell repair may be difficult to accomplish without addition of correctly folded proteins, it is possible that use of hyperthermia may trigger activity of chaperones leading to refolding and re-functionalization of cellular proteins. Detoxif ication — in this case removal of Pt compounds from neuronal cells would additionally support this process. It is not impossible to imagine that treatment regimens alternating hyperthermia and detoxif ication over long periods of time can be devised. Cells affected by CIPN are non-dividing — therefore, as long as they survive they may be able to go through the process of repair. While new agents that oppose CIPN will continue to be developed and tested, we may hope that approaches to boost ability of cells to heal themselves will also gain prominence in the CIPN research and therapy.
Author contributions:Conception and design of the manuscript: AL and JP; critically analyzed and reviewed the existing literature: AL, JP and TP; drafting of the manuscript: AL and TP; critical revision of the manuscript: GEW and MS. All authors approved the final version of the manuscript.
Conf licts of interest:The authors declare no conf licts of interest.
Financial support:This work was supported by grant from the Ministry of Education, Science and Technological Development, Republic of Serbia (173051). JP was also supported by a UICC Yamagiwa-Yoshida Memorial International Cancer Study Grant (YY2/2015/381414).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Randall McKinnon, Rutgers-Robert Wood Johnson Medical School, USA.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of an injured ascending reticular activating system with recovery from a minimally conscious state to normal consciousness in a stroke patient: a diffusion tensor tractography study
- The role of vascularization in nerve regeneration of nerve graft
- New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target?
- Advanced diffusion magnetic resonance imaging in patients with Alzheimer’s and Parkinson’s diseases
- Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury
- Decoding epigenetic codes: new frontiers in exploring recovery from spinal cord injury
