铱磷光配合物的合成、颜色调控方法及应用研究现状
2020-02-02常桥稳王姿奥晏彩先张选东刘伟平
常桥稳,王姿奥,晏彩先,张选东,姜 婧,刘伟平,崔 浩 *
铱磷光配合物的合成、颜色调控方法及应用研究现状
常桥稳1, 2,王姿奥1,晏彩先1,张选东1,姜 婧1,刘伟平1,崔 浩1 *
(1. 昆明贵金属研究所,贵研铂业股份有限公司 稀贵金属综合利用新技术国家重点实验室,昆明 650106;2. 昆明理工大学 材料科学与工程学院,昆明 650093)
铱磷光配合物具有良好的热稳定性,相对短的激发态寿命,发光效率高以及发光颜色容易调节等优势,成为迄今性能最为优异的有机发光材料,在OLED中的应用备受关注。围绕铱磷光配合物的结构、合成方法、颜色调控方法以及应用等方面展开综述,获得了一些铱磷光配合物的构效关系,可用于指导铱磷光配合物的高效研发。阐述了铱磷光配合物发展过程中遇到的问题,并指明了铱磷光配合物的发展趋势和方向。
铱磷光配合物;合成;颜色调控;应用;现状
随着可开采化石能源的日益枯竭和人类生存环境的恶化,能源的有效利用已成为我国乃至全世界面临的一项极为紧迫的任务。有机发光二极管(Organic Light-emitting Diode,OLED)作为一种高效的电光转换技术,可以将电能高效率地转化为光能,应用于显示和照明领域[1-4],从而实现能源的节流,是解决能源和环境问题的有效方案。
OLED技术和产业涉及到一系列新材料的应用,新材料的研究对OLED的发展有着重要的影响。在OLED中使用了诸多有机新材料,有机发光材料是OLED最为核心和重要的关键原材料,它的发光性能直接影响着OLED器件性能。有机发光分子材料主要有荧光材料和磷光材料两大类,其中荧光材料研究较早,应用较多。但是,荧光材料发光效率低,而磷光材料具有高的发光效率,替代荧光材料可以显著提升OLED的效率[5-7]。磷光材料主要是一些过渡金属(Pt、Os、Ir、Au、Ru、Re和Cu等)的配合物分子。在这些配合物中,铱磷光配合物具有良好的热稳定性,相对短的激发态寿命,发光效率高以及发光颜色容易调节等方面的优势,成为迄今性能最为优异的有机发光材料[8-12]。小分子绿光和红光铱磷光配合物已经成功应用在OLED显示产品中,并将有望应用于OLED照明中。而蓝光铱磷光配合物存在光谱不够蓝、效率不够高、稳定性差等问题,仍处于基础研究阶段。尽管红光和绿光铱磷光配合物已成功应用到OLED显示产业中,但它们的广泛应用仍受到效率和发光纯度不足的影响。随着OLED产业的快速发展和人们对高清晰显示的追求,科技和产业界还在继续寻找效率更高和颜色更纯的新型铱磷光配合物。
本文作者根据文献报道,并结合所在实验室对铱磷光配合物的研究工作,对铱磷光配合物的结构、合成方法、颜色调控方法及应用等研究现状进行了综述,并提出了铱磷光配合物所面临的问题和今后的发展趋势。
1 铱磷光配合物的结构及合成方法
铱(III)的价电子构型为d6,采用d2sp3杂化与环金属配体和辅助配体的六个配位原子形成稍微扭曲的八面体配合物。根据分子结构的不同和分子量的大小,铱磷光配合物可以分为小分子铱磷光配合物、树枝状铱磷光配合物和高分子铱磷光配合物。
1.1 小分子铱磷光配合物
小分子铱磷光配合物主要有4类(图1),分别是Ir(C^N)2(LX)型、Ir(C^N)3型、Ir(C^N)2(C`^N`)型和Ir(C^N)2(N^N)离子型铱磷光配合物。
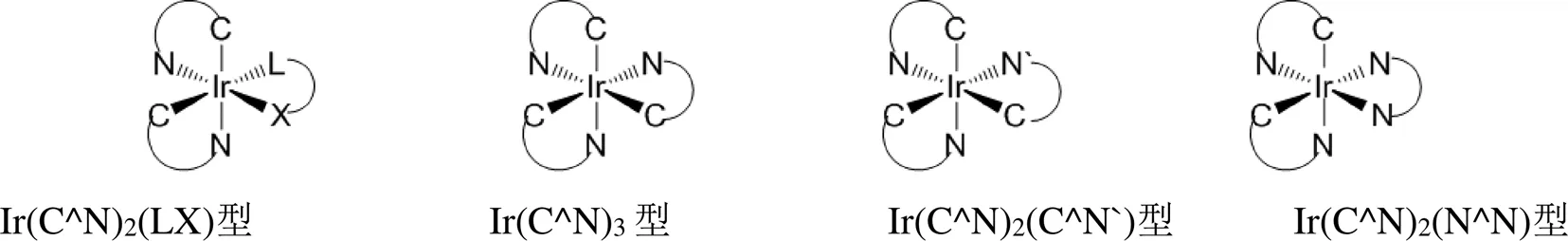
图1 小分子铱磷光配合物的化学结构
1.1.1Ir(C^N)2(LX)型铱磷光配合物
Ir(C^N)2(LX)型铱磷光配合物是由2个环金属配体(C^N)和一个辅助配体(LX)配位构成的中性发光配合物[13-19]。通过改变环金属配体和辅助配体的结构均能调节发光颜色和发射波长,可以获得种类丰富的铱磷光配合物。对于Ir(C^N)2(LX)型铱磷光配合物,其合成路线见图2,合成工艺相对简单,主要有两种方法:第一种是以水合三氯化铱为原料,在乙二醇单乙醚和水(体积比为3:1)的混合溶剂中,与相应的环金属配体反应,得到铱的氯桥二聚体,然后在碱性条件下与辅助配体发生配位反应,合成得到目标铱磷光配合物。该方法是合成这类铱磷光配合物最常用的方法[20-21];第二种方法也是以水合三氯化铱为原料,先合成得到环己二烯氯化铱二聚体,然后和辅助配体的盐反应形成环己二烯和辅助配体的配合物,最后和环金属配体反应,合成得到目标铱磷光配合物[22],该方法目前仅用于Ir(ppy)2(acac)的合成,但有望推广到其它Ir(C^N)2(LX)型铱磷光配合物的合成中。
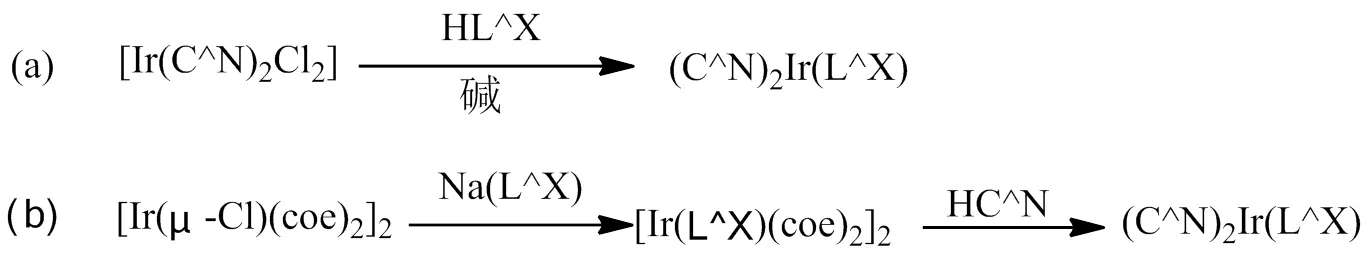
图2 Ir(C^N)2(LX)型铱磷光配合物的合成路线
1.1.2Ir(C^N)3型铱磷光配合物
Ir(C^N)3型铱磷光配合物是由3个相同的环金属配体(C^N)与铱(III)配位形成的均配型的中性分子[23-27],存在面式()和经式()两种异构体(如图3)。在面式异构体中,所有的Ir-C键处于吡啶的反位,Ir-N键处于苯环的反位,所有Ir-C键长和Ir-N键长各自相等。而在经式异构体中,Ir-C键和Ir-N键的键长各不相同。面式和经式异构体的形成与温度有很大的关系。-Ir(C^N)3是热力学稳定产物,一般在高温(≈200℃)条件下形成,-Ir(C^N)3是动力学稳定产物,一般在较低温度(≈150℃)下形成,二者在一定条件下可以相互转化,如-Ir(C^N)3在较高温度下可以转化为-Ir(C^N)3[28]。如果温度控制不好,在合成中往往会反应生成两种异构体,二者之间分离难度大,给后续的提纯工作带来很大的困难。经式和面式在构型上的差异会导致材料光物理性质的不同。通常情况下,与面式构型相比较,经式构型发光光谱红移,发光效率明显降低[29],这也是经式构型的研究较少,面式构型则得到了广泛的研究和应用的主要原因。
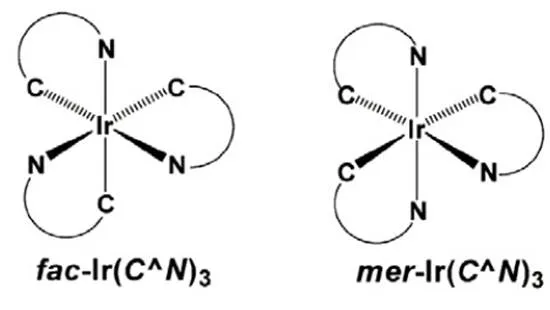
图3 [Ir(C^N)3]的面式和经式立体结构
Ir(C^N)3型铱磷光配合物的合成比Ir(C^N)2(LX)型铱磷光配合物的合成困难,随之也发展了多种多样的合成方法,文献报道的合成方法主要有6种[27-28, 30-35],合成路线见图4。所有合成方法均是以水合三氯化铱为原料,首先合成出相应的铱中间体,再进一步合成得到目标铱磷光配合物。第一种合成方法是铱氯桥二聚体和环金属配体在碱性条件下,以甘油为溶剂,加热回流反应得到目标分子材料;第二种合成方法是通过三氟甲基磺酸银脱除铱氯桥二聚体中的氯离子后,再和环金属配体反应,溶剂为乙二醇单乙醚,反应温度为90~100℃,最终获得目标配合物;第三种合成方法以乙酰丙酮铱和环金属配体在甘油溶剂中加热回流反应一步得到目标配合物;第四种合成方法以Ir(C^N)2(O^O)型铱磷光配合物为原料,在甘油溶剂中和环金属配体反应得到目标配合物;第五种合成方法是通过Na[Ir(acac)2Cl2]和环金属配体在甘油溶剂中加热回流反应得到目标配合物;第六种合成方法是通过Ir(acac-O,O)2(acac- C3)(H2O)和环金属配体在甘油溶剂中加热回流反应得到目标配合物。在这些合成方法中,除第二种方法外,其它方法均在高温下反应。第二种方法中,通过三氟甲基磺酸银脱除氯离子,可以在较低的温度(90~100℃)下实现面式结构的合成,是目前合成Ir(C^N)3型铱磷光配合物最常用的方法。第六种合成方法使用的前驱体Ir(acac-O,O)2(acac-C3)(H2O)是乙酰丙酮铱合成过程中产生的副产物,使用该方法可以实现铱的高效利用,有利于降低Ir(C^N)3型铱磷光配合物的合成成本,也是本文作者的研究成果。
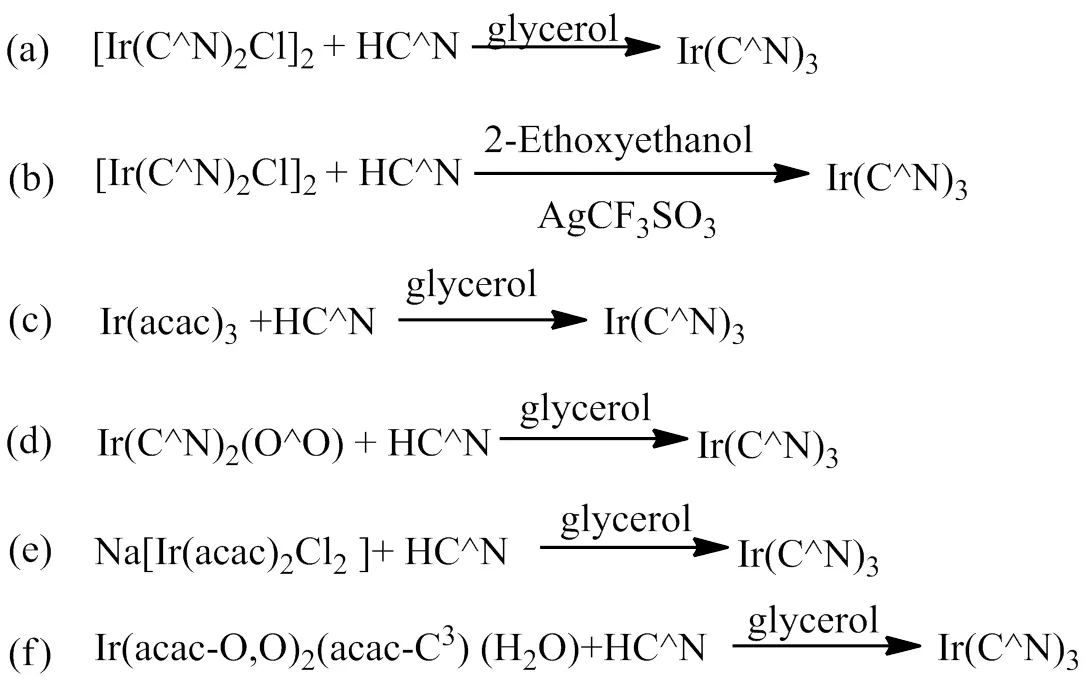
图4 Ir(C^N)3型铱磷光配合物的合成路线
1.1.3Ir(C^N)2(C`^N`)型铱磷光配合物
与Ir(C^N)2(LX)型铱磷光配合物相比较,Ir(C^N)3型铱磷光配合物的三个配体相同,发射波长和发光颜色的调控相对单调,于是发展了Ir(C^N)2(C`^N`)型铱磷光配合物[36-40],使得其中的两个环金属配体相同,改变第3个环金属配体的化学结构,形成了Ir(C^N)2(C`^N`)型铱磷光配合物。第三个环金属配体的引入,使发光颜色调节更容易,同时也可以获得更多结构不同的铱磷光配合物,其中Ir(C^N)2(C`^N`)型铱磷光配合物的绿光材料已在OLED器件中得到广泛应用。与Ir(C^N)3型铱磷光配合物相似,Ir(C^N)2(C`^N`)型铱磷光配合物的合成方法主要有3种[41-43],合成路线见图5。第一种方法是以铱氯桥二聚体和第三环金属配体为原料,在碱性条件下和甘油溶剂中加热回流反应得到目标分子材料;第二种方法是通过三氟甲基磺酸银脱除铱氯桥二聚体中的氯离子,然后和第三环金属配体在90~100℃反应得到目标铱磷光配合物;第三种方法是以甲醇、乙腈、乙醇等为溶剂,通过三氟甲基磺酸银脱除铱氯桥二聚体中的氯离子,形成Ir(C^N)2(solvent)中间体,然后再和第三环金属配体反应,即得到目标分子材料。对比三种合成方法,第一种合成方法反应温度高、容易产生副产物,第二种和第三种合成方法均能在较温和的条件下实现,并且能够获得更高纯度的铱磷光配合物,已在Ir(C^N)2(C`^N`)型铱磷光配合物的合成中得到广泛应用。
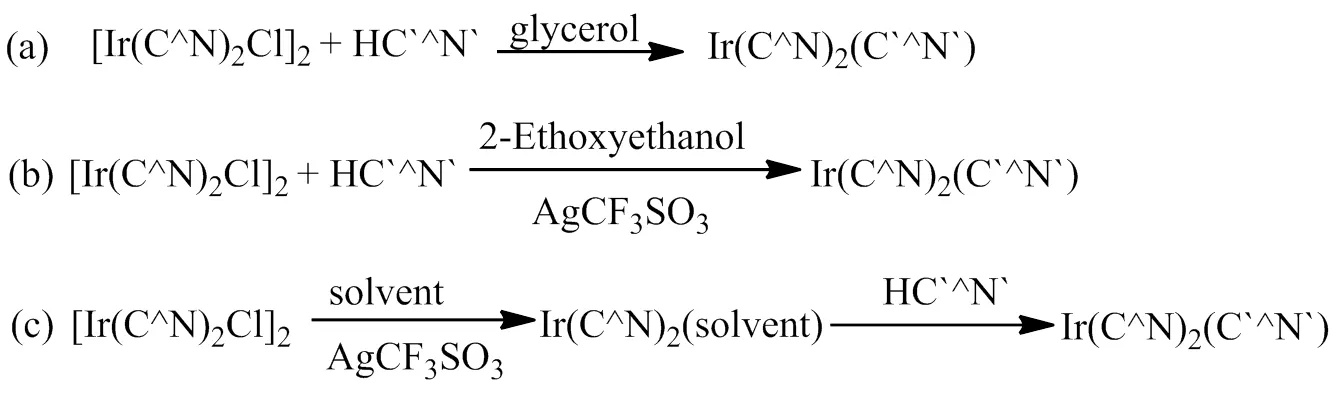
图5 Ir(C^N)3(C`^N`)型铱磷光配合物的合成路线
1.1.4Ir(C^N)2(N^N)离子型铱磷光配合物
离子型Ir(C^N)2(N^N)铱磷光配合物通常是阳离子型铱磷光配合物,中心结构由2个环金属配体和一个中性的N^N辅助配体构成配阳离子,外界为六氟磷酸根阴离子,组成离子型的小分子铱磷光配合物[10, 44-50]。这类铱磷光材料可以通过增加或减小配体的共轭程度、或引入极性基团等结构修饰实现光物理特性的调控,也可以通过改变阴离子的种类改善发光分子的光物理性能。离子型铱磷光材料具有较好的亲水性和生物相容性,在光催化、发光电化学池、化学传感等领域都具有潜在的应用价值。这类材料的合成路线与Ir(C^N)2(LX)型铱磷光配合物的合成路线相似,但不需要像合成Ir(C^N)2(LX)型铱磷光配合物那样在高温条件下进行,铱氯桥二聚体与相应的N^N辅助配体在二氯甲烷和甲醇混合溶剂中,加热回流反应,然后冷至室温,加入含对应的阴离子盐即可制得目标铱磷光配合物。由此可见,离子型铱磷光配合物的合成过程和方法较中性铱磷光分子简单、容易。
1.2 树枝状铱磷光配合物和高分子铱磷光聚合物材料
小分子铱磷光配合物在OLED上已经取得了相当高的器件效率,并实现了商业化应用,但由于小分子铱磷光配合物在发光层容易产生团聚和浓度淬灭现象,人们开始转向大分子铱磷光配合物的研究,于是发展了树枝状铱磷光配合物和高分子铱磷光聚合物材料[51-61]。这两类材料既有小分子铱磷光配合物优异的发光性能,又具备溶解性好、优良的机械性能(如绕曲和加工成型)和良好的成膜性能,容易实现大尺寸和柔性屏体的制备。树枝状铱磷光配合物和高分子铱磷光聚合物材料的合成方法与小分子铱磷光配合物的合成方法类似,最为关键的是树枝状配体和高分子配体的合成。
2 铱磷光配合物的波长和颜色调控方法
铱磷光配合物的发射波长和发光颜色,可以通过改变环金属配体和辅助配体的化学组成和结构在整个可见光区域内调控[62-69]。大量研究表明,对于绝大多数铱磷光配合物,HOMO 能级主要是分布在环金属配体的苯环和中心金属铱上,LUMO能级主要局域在环金属配体的含氮杂环上。通过改变环金属配体的共轭程度、引入不同种类和数量的取代基、引入不同的杂原子或原子团和改变辅助配体等方法都可以调节铱磷光配合物的HOMO-LUMO能级,从而达到调控铱磷光配合物发光波长和发光颜色的目的。
2.1 环金属配体对发光颜色的调控
环金属配体对铱磷光配合物的能级和发光颜色有着最重要的影响[70],通过改变环金属配体的共轭程度、取代基的种类和位置、引入杂原子或原子团等方式,可以实现铱磷光配合物发光波长的调控,从而达到调节发光颜色的目的。
2.1.1共轭效应
对于Ir(C^N)2(acac)型铱磷光配合物,发射波长主要由环金属配体(C^N)决定,随着C^N配体共轭程度的增加和配体刚性增强,降低了配体的能隙,最大发射波长发生红移。如图6中,Ir(ppy)2(acac)、Ir(piq)2(acac)、Ir(niq)2(acac)和Ir(azp)2(acac)的环金属配体的共轭程度越来越大,发射波长分别为516、620、664和932 nm,向长波方向发生了移动[71];对比另外3个铱磷光分子Ir(pbi)2(acac)、Ir(nbi)2(acac)和Ir(fbi)2(acac),随着C^N的刚性和共轭程度越大,对应的发射波长也发生明显红移(530、608和651 nm)[20]。

图6 基于不同共轭程度环金属配体的Ir(C^N)2(acac)型铱磷光配合物
对于Ir(C^N)3型铱磷光配合物,其发射波长完全由环金属配体(C^N)决定,随着C^N配体π共轭程度和配体刚性增强,发射波长发生红移。如图7中[30],改变C^N配体的刚性和共轭程度,实现了最大发射波长从545到652 nm范围内的调控。例如,与Ir(thpy)3相比,Ir(tiq)3、Ir(t-5t-pyhpy)3和Ir(btpy)3的C^N配体的刚性和共轭程度更大,发射波长发生了明显红移,最多的红移了96 nm。Ir(fliq)3的C^N配体的刚性和共轭程度大于Ir(piq)3的C^N配体,发射波长向长波方向移动了32 nm。Ir(fliq)3的C^N配体的刚性和共轭程度明显大于Ir(flpy)3的配体,发射波长红移了107 nm。
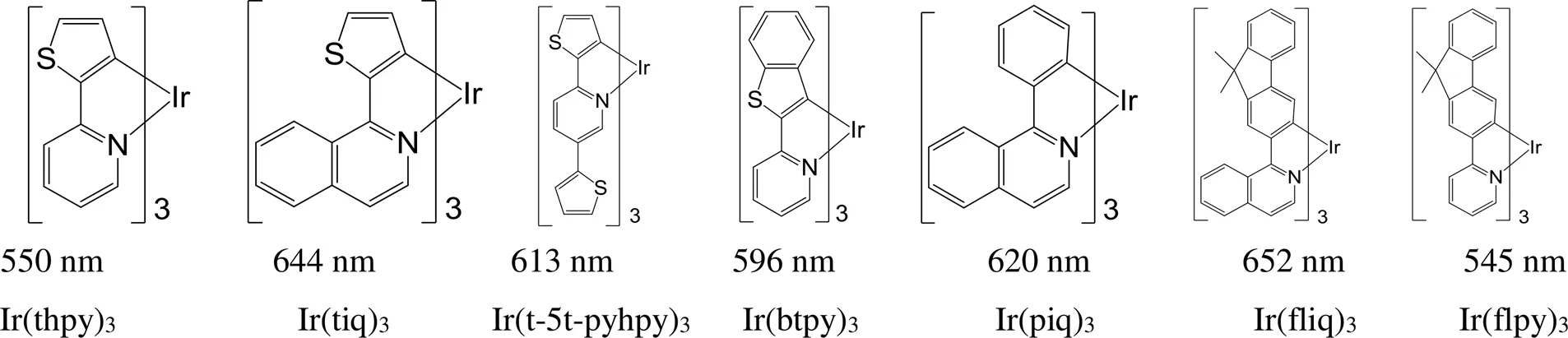
图7 基于不同共轭程度环金属配体的Ir(C^N)3型铱磷光配合物
2.1.2取代基效应
通过改变取代基的种类和位置可以实现铱磷光配合物发射波长的调控。在铱磷光配合物中环金属配体1,2-二苯基苯并咪唑的苯基上引入-OMe或-CN,或者同时引入-OMe和-CN,或将-OMe和-CN引入在不同的位点(图8)[72],都可以改变配合物的发射波长。在没有引入取代基团之前,Ir(pbm)2(acac)的最大发射波长为520 nm。在对位引入-OMe后形成的Ir(p-MeO-pbm)2(acac),最大发射波长红移至555 nm,在间位引入-OMe后得到的Ir(m-MeO- pbm)2(acac),最大发射波长蓝移至507 nm,两者的最大发射波长相差48 nm。与之相反,在对位引入-CN和在间位引入-CN时,得到的两个配合物的最大发射波长相差45 nm;当同时引入-OMe和CN时,且OMe处于对位而-CN处于间位,产生的配合物最大发射波长红移至605 nm;当-OMe和-CN交换取代位点,配合物的最大发射波长则蓝移24 nm至496 nm;OMe处于对位、-CN处于间位的配合物和-OMe处于间位、-CN处于对位的配合物,两者的最大发射波长相差109 nm。由此可见,对于Ir(pbm)2(acac)母体结构,在间位引入-OMe时,最大发射波长红移,在间位引入-CN时,最大发射波长蓝移,在对位引入-OMe时,发射波长蓝移,在间位引入-CN时,最大发射波长红移。
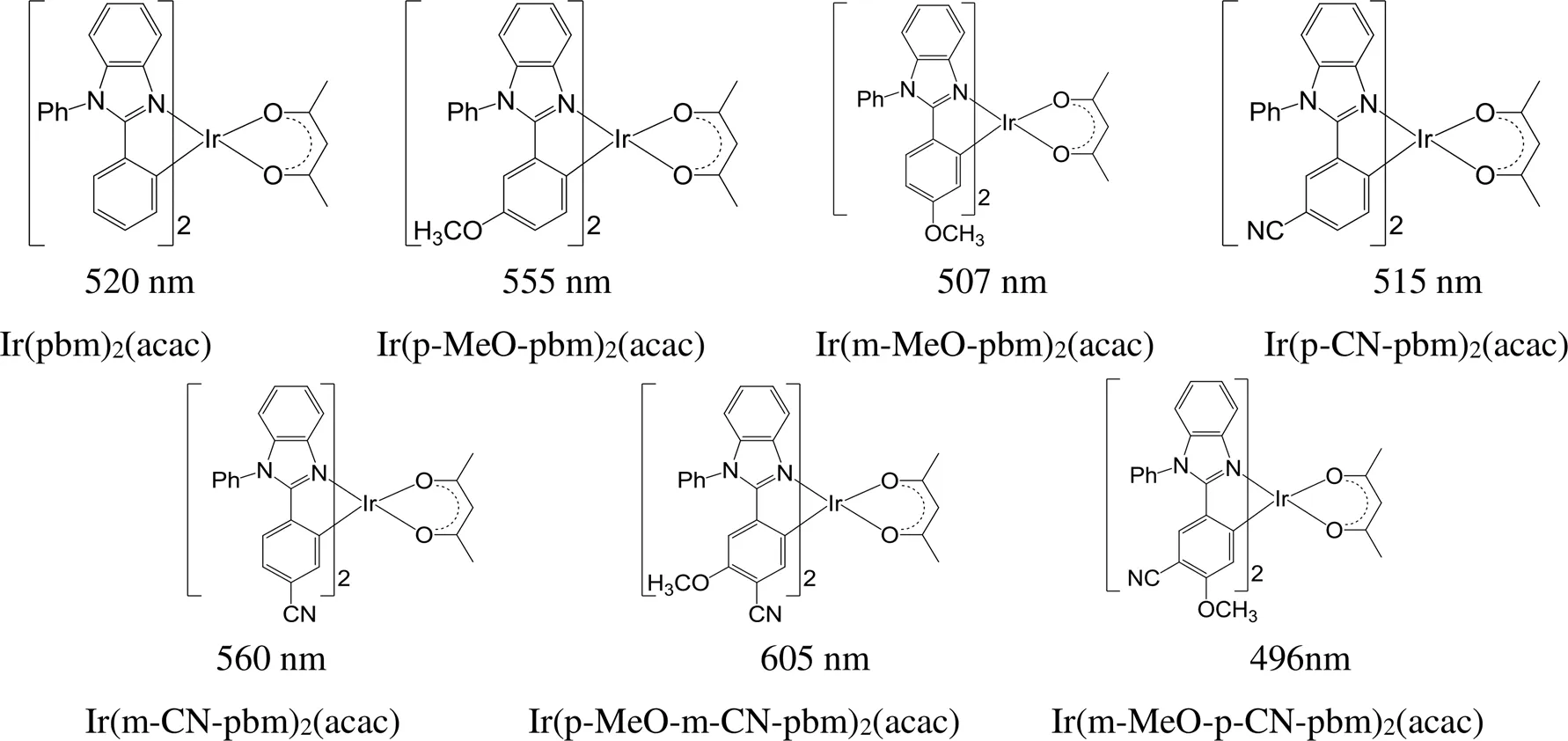
图8 MeO和CN取代的铱磷光配合物
Tsuzuki等[73]在Ir(ppy)2(acac)铱磷光配合物的苯基吡啶环金属配体的不同点位引入具有强拉电子性质的五氟苯基合成了4种铱磷光分子(图9),实现了发射波长在513 nm到578 nm范围内调控,最大外量子效率可达17%。
以2-苯基苯并噻唑为环金属配体,在苯并噻唑环的6位上引入氟原子和在苯基的间位上分别引入三氟甲基、氟、甲基和甲氧基,得到4种铱磷光配合物(图10):Ir(4-CF3-Btp)2(acac)、Ir(4-F-Btp)2(acac)、Ir(4-Me-Btp)2(acac)和Ir(4-MeO-Btp)2(acac),最大发射波长分别为567、544、559和544 nm,与母体分子Ir(Btp)2(acac)的最大发射波长560 nm相比,差异不大,但是量子效率要高出1~2倍[74]。

图9 取代基位置不同的铱磷光配合物
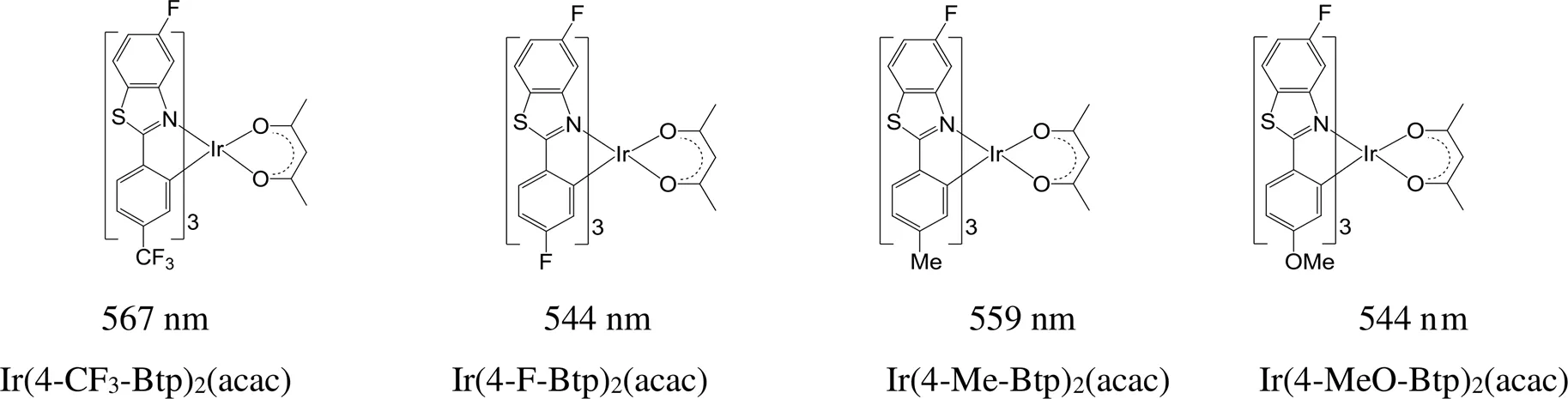
图10 具有不同取代基的铱磷光配位
在2-苯基吡啶的吡啶环的不同位置引入苯环形成喹啉或异喹啉环(图11),得到铱磷光配合物的最大发射波长也不同,Ir(npy)2(acac)[75]、Ir(pq)2(acac)[20]和Ir(piq)2(acac)[76]的最大发射波长分别为551、597和620 nm,对应的发光颜色的变化从黄绿色-橙红色-深红色。
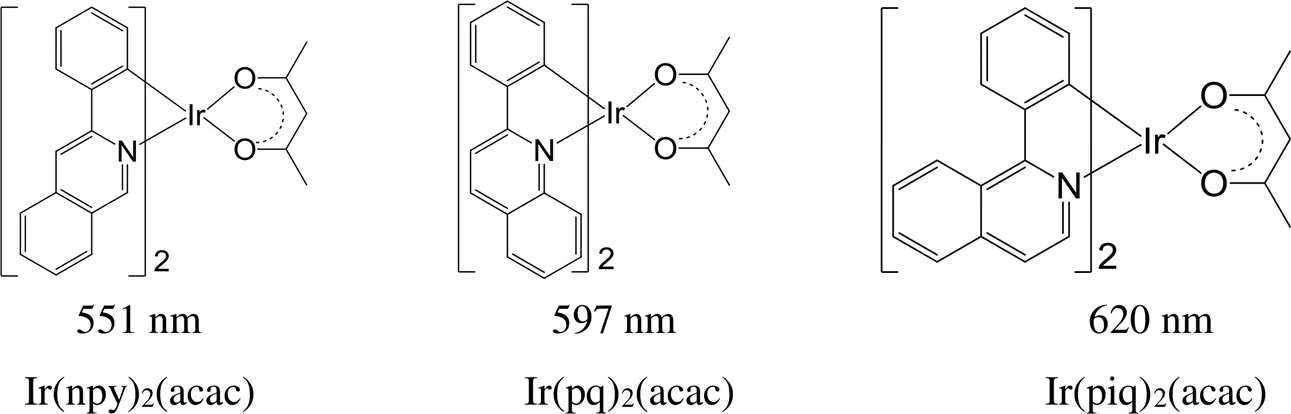
图11 取代基位置不同的铱磷光配合物
在(C^N)2Ir(acac)型铱磷光配合物中,当环金属配体为对称性的2,5-二芳基吡啶,芳基按从苯基、4-氟苯基、4-甲氧基苯基和9,9-二甲基芴的顺序改变时(图12),发射波长红移,从525红移至595 nm,提示当铱磷光配合物的π共轭程度增大时,发射波长会发生红移,实现发射波长和发光颜色调控[77]。
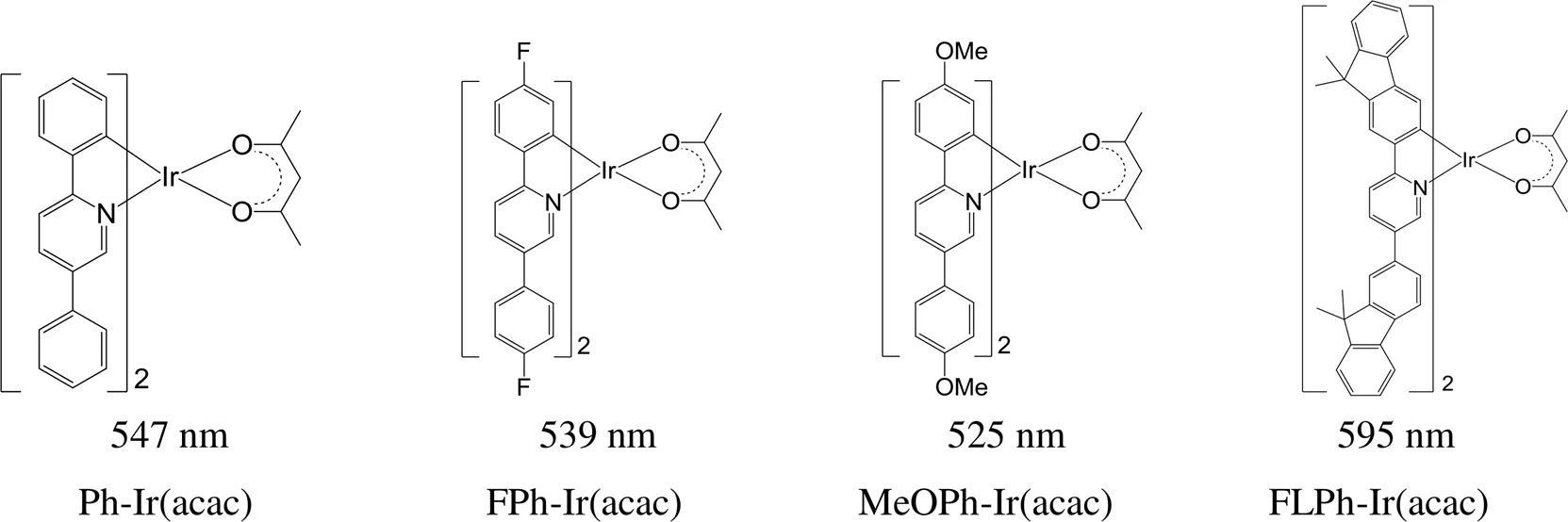
图12 对称性芳烃取代的铱磷光配合物
对于Ir(piq)2(acac),在环金属配体上苯基的同一位置引入不同的取代基或在苯基的不同位置引入相同的取代基(图13),均可以实现发光波长和发光颜色的调控。在苯基异喹啉的苯环的间位分别引入甲基、三氟甲基和甲氧基时,对应的配合物的发射波长分别为628、631和623 nm;在苯基异喹啉的苯环的对位分别引入甲基、三氟甲基和甲氧基时,对应配合物的发射波长分别为645、644和632 nm。由此可以看出,在Ir(piq)2(acac)系列铱磷光配合物中,在同一位置引入不同的取代基,最大发射波长稍有变化,但在不同位置引入同一取代基,最大发射波长的变化更明显[78]。
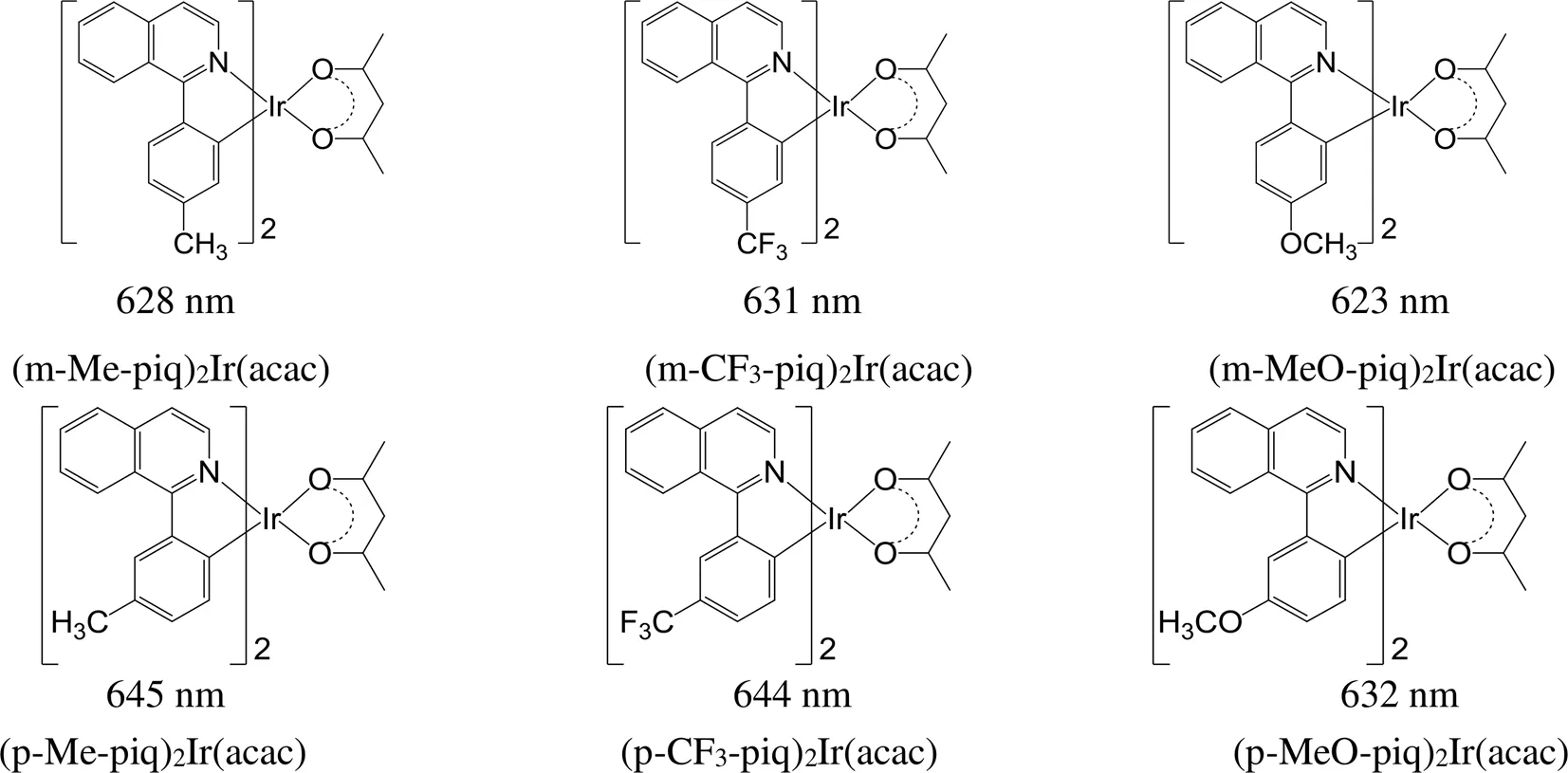
图13 取代基位置不同的异喹啉铱磷光配合物
与(mpq)2Ir(acac)铱磷光配合物相比,在2-苯基-4-甲基喹啉环金属配体苯基的间位和对位上引入甲氧基(图14),并且当甲氧基的位置处于间位时,所对应的配合物发射波长蓝移17 nm,而当甲氧基的位置处于对位时,最大发射波长则红移45 nm。甲氧基位于间位和对位的两种不同的配合物,最大发射波长相差62 nm[79]。
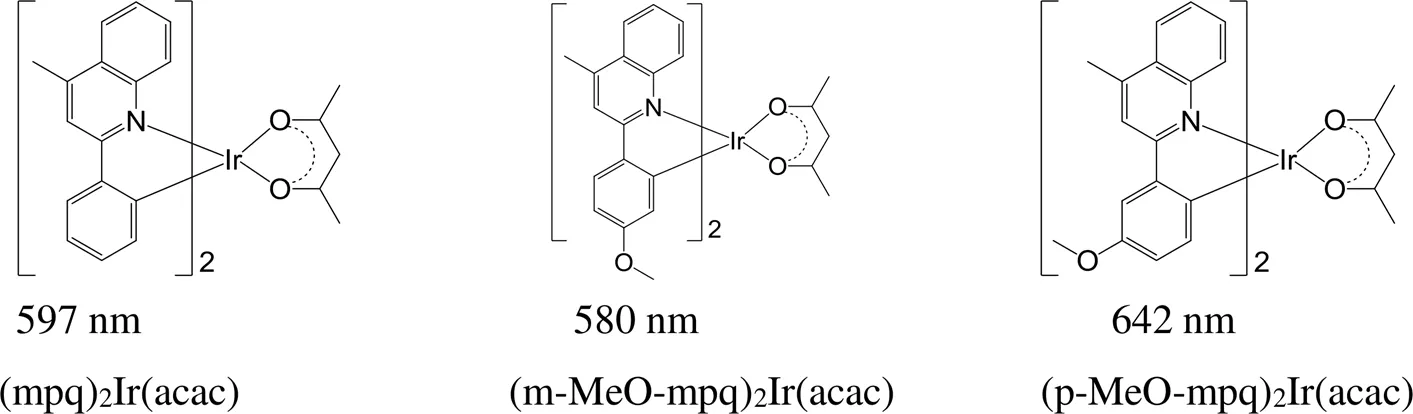
图14 甲氧基处于不同位置的铱磷光配合物
同时,改变取代基数量也可以实现铱磷光配合物发射波长调节。含氟官能团对铱磷光配合物的发射有着显著的影响(图15),由于氟的吸电子诱导效应,与(mpq)2Ir(acac)相比,氟取代后的分子发射光谱发射显著蓝移,随氟原子个数变化,颜色从橙红光至红光[80],(mpq-4F)2Ir(acac)、(mpq-2,4F)2Ir(acac)、(mpq-2,3,4F)2Ir(acac)和(mpq-2,3,4,5F)2Ir(acac)的发射波长分别为574、577、584和564 nm。
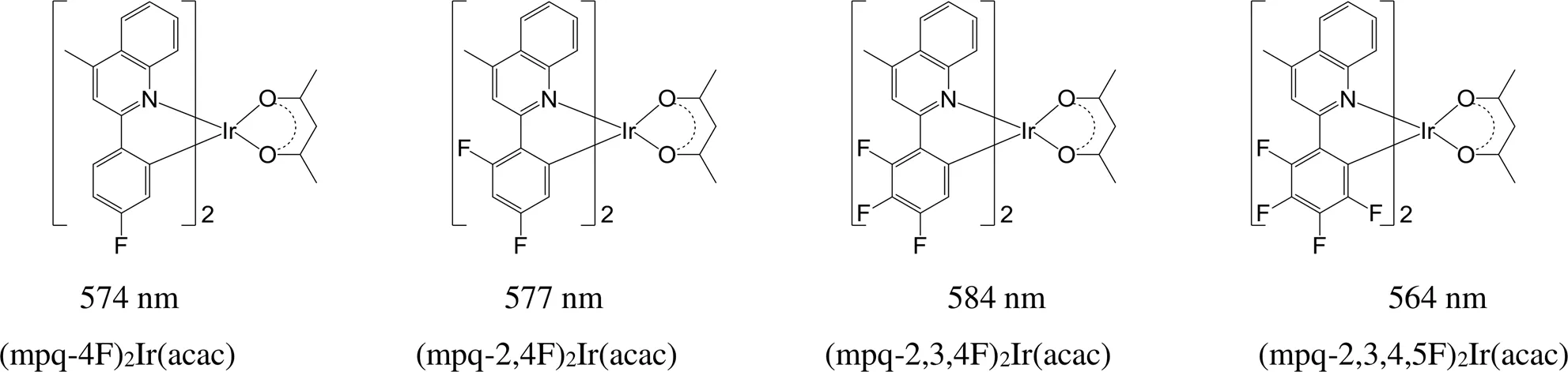
图15 氟原子数量不同的的铱磷光配合物
由以上可见,取代基种类、位置和数量等均对铱磷光配合物的光物理性质调控起着重要的作用。
2.1.3杂原子或原子团效应
杂原子和原子团的引入也可以实现铱磷光配合物最大发射波长的调节。对比图16中(bpo)2Ir(acac)和(bpt)2Ir(acac)的最大发射波长,由硫取代氧后,由于硫原子的可极化性大于氧原子,导致分子最大发射波长红移30 nm;用萘基取代苯基,共轭程度增大,分子的最大发射波长则红移60 nm;如果氧被硫取代的同时苯基也被萘基取代,分子的荧光光谱也相应红移80 nm[81]。Zhou等[82]合成了图17中的7个铱磷光配合物,在Ir(ppy)3的苯基间位引入含主族元素的基团,材料的最大发射波长在497~530 nm范围内变化,发光颜色从蓝绿色到黄绿色的转变。
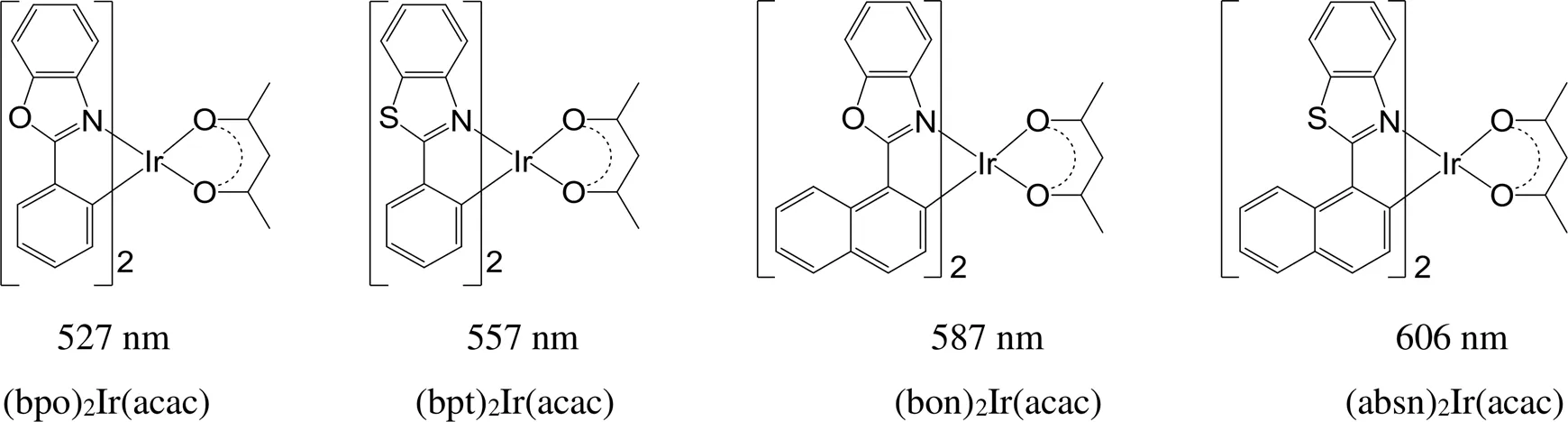
图16 含杂原子的[Ir(ppy-L)2(acac)]型铱磷光配合物
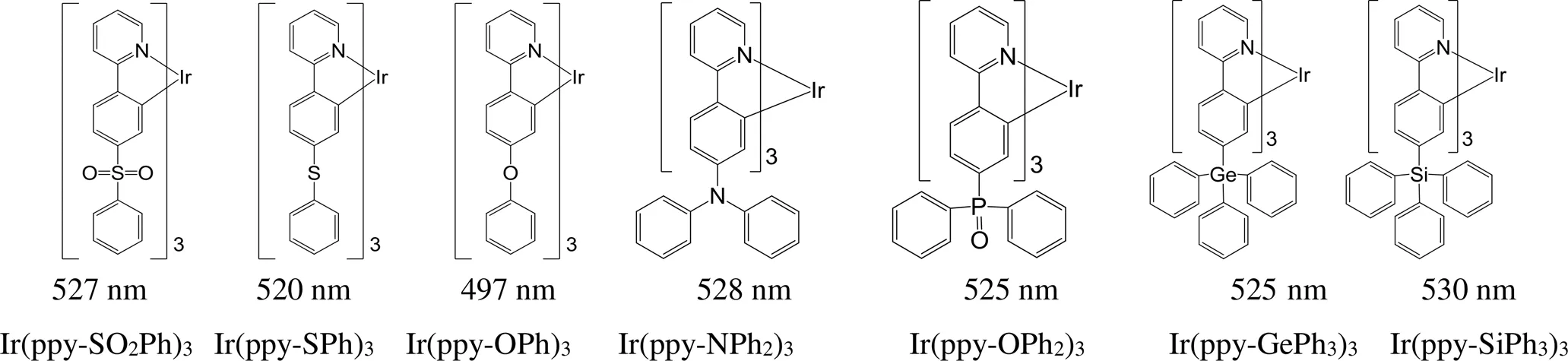
图17 主族元素基团修饰的铱磷光配合物
2.2 辅助配体对能级及发光颜色的调控
环金属配体在铱磷光配合物颜色调控起重要作用,辅助配体对颜色调节也有重要影响[83-84]。在铱磷光配合物中,常见的辅助配体有O^O型[20, 85-87]、N^O型[88-91]、S^S型[92-93]、N^N型[94-95]和P^P型[96]等(图18),其中常用辅助配体为O^O型。β-二酮作为O^O双齿配体,能与铱(III)形成稳定的配合物(图19),是最常见的一类辅助配体。其中乙酰丙酮为最简单的β-二酮,广泛用作铱磷光配合物辅助配体。对Ir(C^N)2(O^O)型铱磷光配合物,β-二酮的结构和三重态能级对铱磷光配合物的发光性能影响较大。β-二酮的三重态能级较高时,在溶液中有较强的磷光发射;当β-二酮配体三重态能级较低时,在溶液中不发光[85, 97]。在掺杂型器件中,要求β-二酮配体具有较高的三重态能级。最近研究表明,对于Ir(dmippiq)2(deacac)[98],3,7-二乙基-4,6-壬二酮的引入可以改善材料的光谱特性,使发射光谱窄化,提高色纯度,还可降低铱磷光配合物的升华温度,改善升华效果,增加OLED器件的最大外量子效率。

图18 不同类型的辅助配体的结构

图19 含不同β-二酮配体的铱磷光配合物
以2-苯基苯并吡喃为环金属配体,改变不同结构的N^O辅助配体可以实现配合物的发光颜色从绿光到橙光的调控,例如Ir(bo)2(pic)、Ir(bo)2(prz)、Ir(bo)2(bop)和Ir(bo)2(btp) (图20)的最大发射波长分别为531、591、539和598 nm[99]。以苯基吡啶为环金属配体,改变不同N^O辅助配体可以实现铱磷光配合物发光颜色从蓝色到橙红色的调控,例如,Ir(ppy)2(ImPyC)、Ir(ppy)2(ImPmC)、Ir(ppy)2(ImPzC)和Ir(ppy)2(ImTzC)(图20)的发射波长分别为501、414、592和504 nm[100]。
以苯基吡啶为环金属配体,不同结构脒基N^N作辅助配体,实现了配合物发光波长在528~548 nm范围内调控[93, 100-102](图21)。
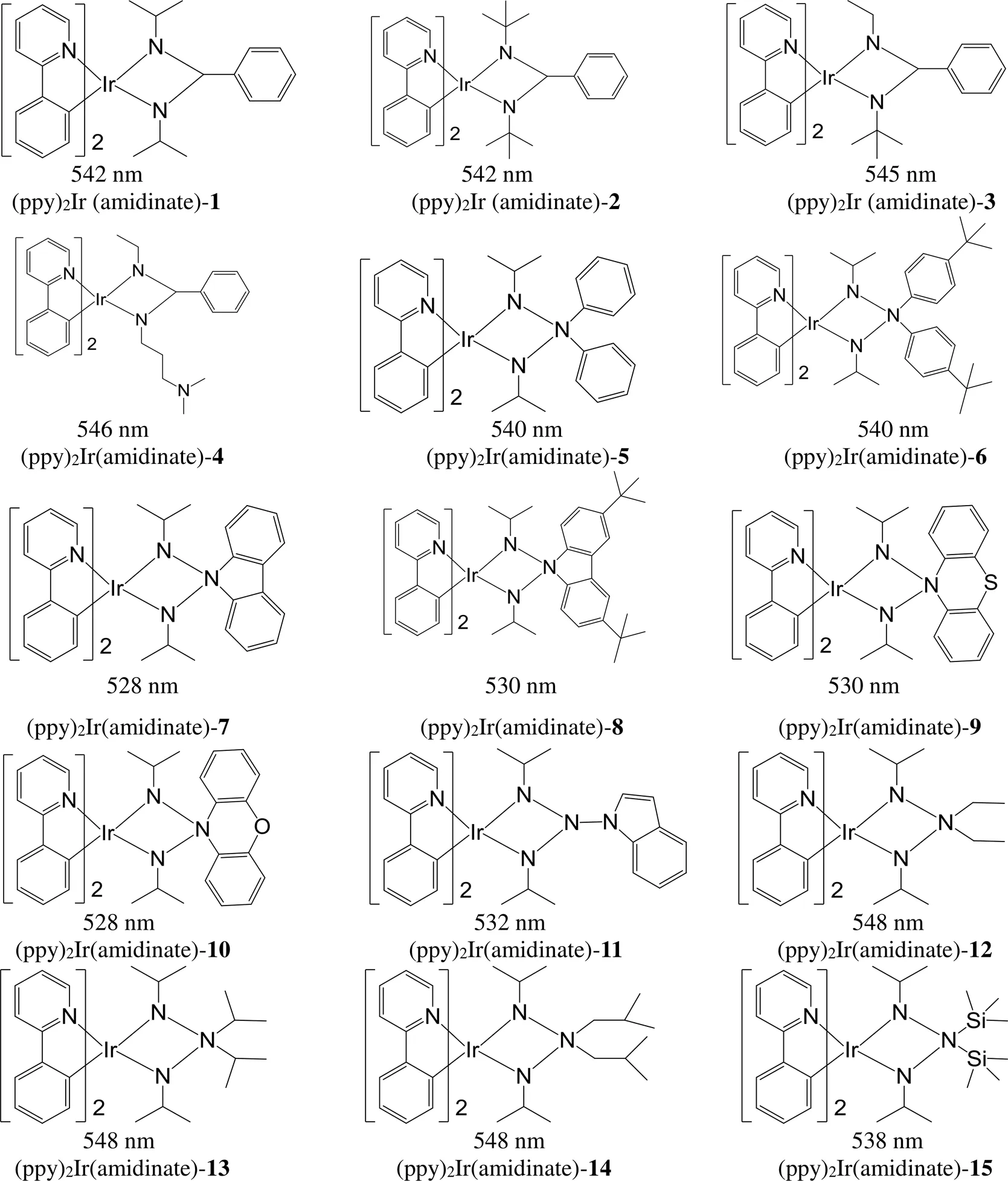
图21 基于N^N辅助配体的铱磷光分子
3 铱磷光配合物的应用研究现状
发光效率高、性能稳定的红、绿、蓝三种颜色的磷光材料是实现全色显示和固态照明必不可少的三基色有机发光分子材料,黄/黄绿光磷光材料可以和蓝光材料组合实现结构简单的白光器件。铱磷光配合物通过改变配体结构可以实现在整个可见光范围内调控,发光颜色主要包括红光、绿光、蓝光和黄光等。
3.1 绿光铱磷光配合物
绿光铱磷光配合物研制的时间最早,现已发展成为最成熟的一类磷光材料,基于这类材料构建的OLED器件具有最好的效率和最长的寿命。最经典的绿光铱磷光配合物是Ir(ppy)3、Ir(ppy)2(acac)及其衍生物(图22),最大发射波长介于510~520 nm,色坐标在(0.32,0.64)左右。1985年[103]Ir(ppy)3被首次合成出来,最大发射波长为510 nm,在甲苯中的量子产率为0.97。1999年[7]Thompson 等将其首次应用到OLED中,经器件结构优化后,获得最大外量子效率21.3%,发光强度为68.1 cd/A,CIE坐标(0.27, 0.63)。2001年[28]发现Ir(ppy)2(acac)与Ir(ppy)3有相似的性质,最大发射波长为516 nm,并有长激发态寿命和高的量子产率,以Ir(ppy)2(acac)为客体材料的OLED器件,获得的最大外量子效率(EQE)为23.7%,发光效率为91.6 cd/A,CIE坐标(0.31,0.64)[104]。在Ir(ppy)3和Ir(ppy)2(acac)的基础上,为了获得更高稳定性、更高效率和更高色纯度等高性能的发绿光材料,通过改变辅助配体和环金属配体研制了许多其他的绿光铱磷光配合物[105-117],其中一些小分子绿光铱磷光配合物已在OLED产业中得到了应用。

图22 经典的绿光铱磷光配合物
3.2 红光铱磷光配合物
与绿光铱磷光配合物相比,受制于能带定律和窄的能隙,红光铱磷光配合物的发展滞后,在色纯度、效率和寿命方面还需进一步提升。这类铱磷光配合物在2-苯基吡啶上增加环金属配体的共轭程度来实现红光发射。但随着环金属配体共轭程度的增大,分子间的π-π相互作用增强,容易引起分子的聚集,产生团聚和浓度淬灭现象,导致器件效率和寿命的双重降低[118]。阻碍红光铱磷光配合物商用的另一个问题是高效率和高色纯度难于同时实现,同时具备高效率、稳定性、亮度、CIE坐标(0.67,0.33)的红光铱磷光配合物非常少。尽管如此,在人们合成的许多红光铱磷光配合物中[119-130],还是筛选出满足OLED产业基本需求的红光铱磷光配合物。
红光铱磷光配合物的环金属配体主要有2-苯基苯并噻唑、1-芳基异喹啉、2-芳基喹啉及其衍生物(图23)。2001年,Lamansky团队[131]报道了第一个红光铱磷光配合物Ir(btp)2(acac),外量子效率在4.6%到6.6%之间、最大发射波长为616 nm、CIEx,y色坐标为(0.68, 0.32)。2003年,Tsuboyama团队[76]合成均配型的异喹啉铱磷光配合物Ir(piq)3,最大发射波长为620 nm、色坐标为(0.68,0.32)、流明效率为8.0 lm/w、外量子效率达到10.3%。通过对Ir(piq)3进行改性,得到Ir(piq)2(acac)[132],色坐标为(0.68,0.32)、最大外量子效率9.7%。Thompson等发展了Ir(pq)2(acac),其最大发射波长为597 nm、在溶液中的量子效率为10%[23]。鉴于红光铱磷光配合物共轭程度大、存在着较强的分子间相互作用力,会产生浓度淬灭,导致发光效率和寿命的双重降低。但是,在环金属配体上引入取代基或引入体积更大的辅助配体,可以改善铱磷光配合物的发光性能。例如,Ir(mpq)2(acac)和Ir(mpmq)2(tmd)[133]的发射波长分别为594和586 nm、CE分别为20.3 cd/A和30.1 cd/A、流明效率分别为23.9和32 lm/w、CIE分别为(0.65,0.34)和(0.65,0.35),基本满足了OLED产业的需求,曾经在OLED产线上得到应用,但是存在色纯度不够高的缺点。为了提高色纯度,对Ir(piq)2(acac)进行结构改造,在异喹啉的6位引入异丙基,将辅助配体乙酰丙酮替换成3,7-二乙基-4,6-壬二酮,研制出一款性能优良的深红光铱磷光配合物,已广泛应用到OLED显示产业中[98]。

图23 经典的红光铱磷光配合物
3.3 蓝光铱磷光配合物
铱磷光配合物发射蓝光主要通过如下两个手段来扩大配合物分子的能隙,增加电子跃迁所需的能量:(1)在2-苯基吡啶的苯环上引入拉电子基团降低HOMO能级,增大HOMO-LUMO能级差,发射波长蓝移;(2)在吡啶环上引入给电子基团提高LUMO能级,增大HOMO-LUMO能级差,发射波长蓝移。蓝光铱磷光配合物能隙大,与之匹配的宽能隙主体材料很少,导致在色纯度、效率、寿命和稳定性等方面的性能尚不能满足OLED产业的需求,仍处于基础研究阶段,开发高性能蓝光铱磷光配合物仍然面临很多挑战。
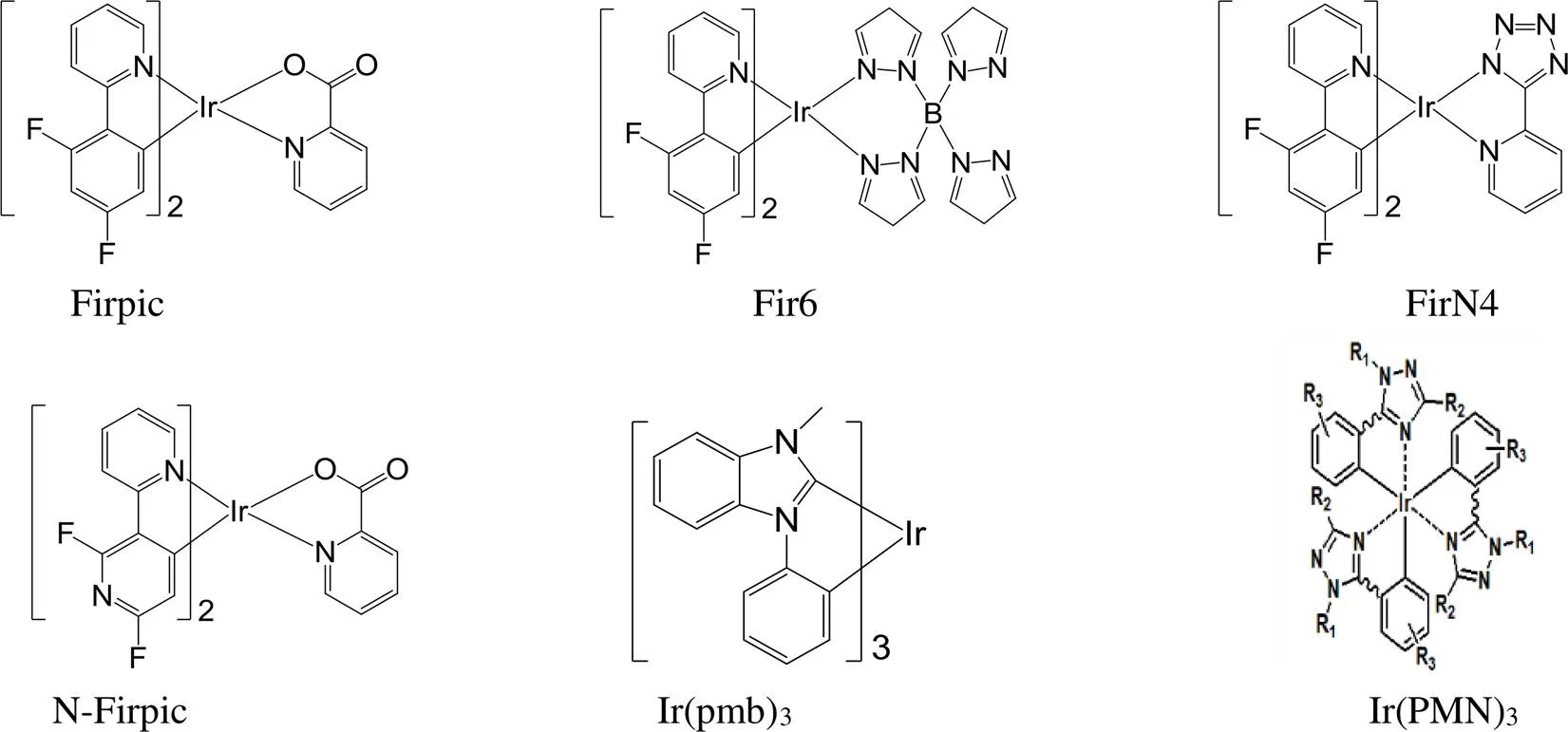
图24 经典的蓝光铱磷光配合物
FIrpic是第一个天蓝色铱磷光配合物[134](图24),最大发射波长为470 nm、CIE色坐标(0.14, 0.30),备受关注[135-136]。Firpic虽然发光性较好,但发射波长太长,不是真正意义上的蓝光。因此,人们更希望得到深蓝光铱磷光配合物[137-149]。对Firpic的化学结构进行改性,增大能隙,可以使其发射波长蓝移。例如,将2,4-二氟苯基吡啶中与氟原子直接键合的两个碳之间的碳原子替换成氮原子,得到深蓝光铱磷光配合物N-Firpic[150],最大发射波长蓝移了25 nm至445 nm;改变Firpic的辅助配体得到Fir6[151]和FirN4[152],发射波长均蓝移。Thompson[153]开发出了第一个含有卡宾结构的深蓝光铱磷光配合物Ir(pmb)3,色坐标(0.17,0.06);我们和北京大学黄春辉院士课题组合作设计合成了一类深蓝光铱磷光配合物Ir(PMN)3,色坐标为(0.15, 0.11)、最大外量子效率达到22.5%[154]。
3.4 黄/黄绿光铱磷光配合物
与三基色铱磷光配合物相比,黄/黄绿光铱磷光配合物的研究较晚。随着OLED照明技术的发展,基于三基色的白光器件性能虽然优异,但是器件结构复杂、制作成本高,不利于产业化和大规模的应用。黄/黄绿光铱磷光配合物可以和蓝光材料互补,实现结构更为简单的白光器件。因此,有很多黄/黄绿光铱磷光配合物被合成出来[157-164],开展了器件性能测试研究。其中,最具代表性的一些黄/黄绿光铱磷光配合物见图25。然而,现有的黄/黄绿光铱磷光配合物的发光性能不及相应的三基色铱磷光配合物,仍需进一步改进。
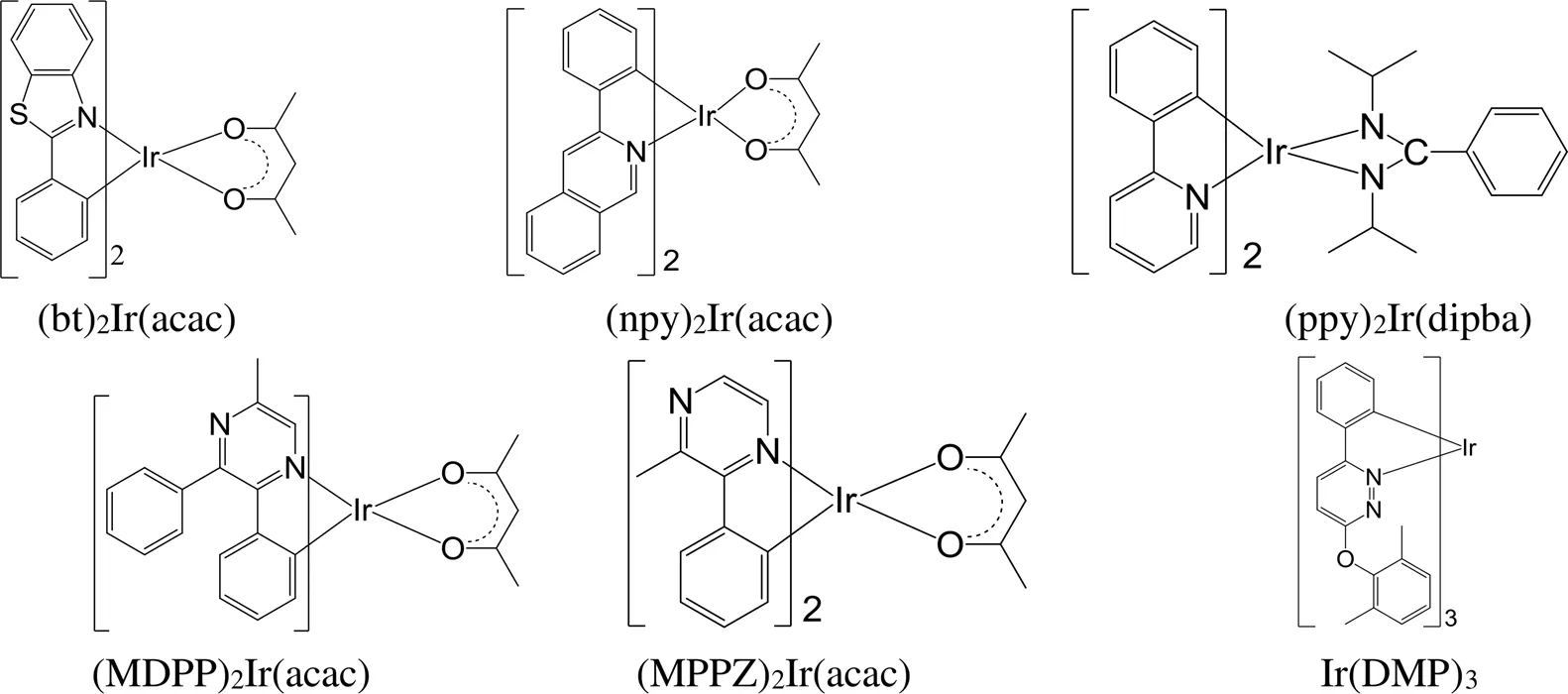
图25 代表性的黄/黄绿光铱磷光配合物
4 结语与展望
铱磷光配合物是性能最优异的有机发光材料,具有较高的发光效率、良好的热稳定性、发光颜色容易调节等特点,是过去20年发光材料领域的研究热点。通过学术界和产业界的共同努力,小分子红光和绿光铱磷光配合物已成功应用到OLED显示产业中,而蓝光铱磷光配合物存在光谱不够蓝、效率不够高、稳定性差等问题,仍处于基础研究阶段。相比红光、绿光和蓝光三基色铱磷光配合物,黄/黄绿光铱磷光配合物的研究仍较少,有待进一步的深入研究。今后应从以下几个方面开展铱磷光配合物的研究开发:
1) 开展原始创新,设计合成具有新颖结构高性能铱磷光配合物,以突破专利封锁,促进我国材料及OLED产业的持续健康发展;
2) 为满足OLED产业需求,仍需继续寻找效率更高和颜色更纯的新型铱磷光配合物;
3) 构建铱磷光分子材料数据库,获得结构与性能之间的构效关系,获得影响发光性能的主要因素,用于指导新型高性能铱磷光配合物的高效研发;
4) 发展低成本、高效率的批量合成新技术,降低铱磷光配合物的成本,有利于促进我国OLED产业的发展。
[1] 陈金鑫, 黄孝文. OLED梦幻显示器-材料与器件[M]. 北京: 人民邮电出版社, 2011: 110.
CHEN J X, HUANG X W. OLED display: materials and devices[M]. Beijing: Posts and Telecom Press, 2011: 110.
[2] 陈金鑫, 陈锦地, 吴忠帜. 白光OLED照明[M]. 上海: 上海交通大学出版社, 2011: 119.
CHEN J X, CHEN J D, WU Z Z. White OLED lighting [M]: Shanghai: Shanghai Jiao Tong University Press, 2011: 119.
[3] TSUJIMURA T. OLED displays fundamentals and application[M]. A John Wiley & Sons, Inc.,2012: 1203.
[4] 晏彩先, 晏廷玺, 李杰, 等. [Ir(ppy)2(dcbpy)]PF6的合成、表征及光物理性能研究[J]. 贵金属, 2019, 40(2): 39-44.
YAN C X, YAN T X, LI J, et al. Synthesis, characteri- zation and light-physical property of [Ir(ppy)2(dcbpy)]PF6[J]. Precious metals, 2019, 40(2): 39-44.
[5] MA Y, ZHANG H, SHEN J, et al. Electroluminescence from triplet metal-ligand charge-transfer excited state of transition metal complexes[J]. Synthetic metals, 1998, 94(3): 245-248.
[6] BALDO M A, O'BRIEN D F, YOU Y, et al. Highly efficient phosphorescent emission from organic electro- luminescent devices[J]. Nature, 1998, 395(6698): 151-154.
[7] BALDO M A, LAMANSKY S, BURROWS P E, et al. Very high-efficiency green organic light-emitting devices based on electrophosphorescence[J]. Applied physics letters, 1999, 75(1): 4-6.
[8] XIAO L, CHEN Z, QU B, et al. Recent progresses on materials for electrophosphorescent organic light-emitting devices[J]. Advanced materials, 2011, 23(8): 926-952.
[9] ULBRICHT C, BEYER B, FRIEBE C, et al. Recent developments in the application of phosphorescent iridium(III) complex systems[J]. Advanced materials, 2009, 21(44): 4418-4441.
[10] MA D, TSUBOI T, QIU Y, et al. Recent progress in ionic iridium(III) complexes for organic electronic devices[J]. Advanced materials, 2017, 29(3): 1603253.
[11] XU F, KIM H U, KIM J H, et al. Progress and perspective of iridium-containing phosphorescent polymers for light- emitting diodes[J]. Progress in polymer science, 2015, 47: 92-121.
[12] YANG X, SUN N, DANG J, et al. Versatile phosphore- scent color tuning of highly efficient borylated iridium(III) cyclometalates by manipulating the electron-accepting capacity of the dimesitylboron group[J]. Journal of materials chemistry C, 2013, 1(20): 3317-3326.
[13] LIU Z, BIAN Z, MING L, et al. Green and blue-green phosphorescent heteroleptic iridium complexes containing carbazole-functionalized β-diketonate for non- doped organic light-emitting diodes[J]. Organic electronics, 2008, 9(2): 171-182.
[14] LIU Z, NIE D, BIAN Z, et al. Photophysical properties of heteroleptic iridium complexes containing carbazole- functionalized β-diketonates[J]. Chem phys chem, 2008, 9(4): 634-640.
[15] WITKOWSKA E, WIOSNA-SALYGA G, GLOWACKI I, et al. Effect of fluorine substitution of the β-ketoiminate ancillary ligand on photophysical properties and electro- luminescence ability of new iridium(III) complexes[J]. Journal of materials chemistry C, 2018, 6(32): 8688-8708.
[16] MEI Q, CHEN C, TIAN R, et al. Highly efficient orange phosphorescent organic light-emitting diodes based on an iridium(III) complex with diethyldithiocarbamate (S^S) as the ancillary ligand[J]. RSC advances, 2016, 6(68): 64003-64008.
[17] LEE S J, PARK J S, SONG M, et al. Synthesis and characterization of red-emitting iridium(III) complexes for solution-processable phosphorescent organic light- emitting diodes[J]. Advanced functional materials, 2009, 19(14): 2205-2212.
[18] YOU Y, PARK S Y. Inter-ligand energy transfer and related emission change in the cyclometalated hetero- leptic iridium complex: facile and efficient color tuning over the whole visible range by the ancillary ligand structure[J]. Journal of the American Chemical Society, 2005, 127(36): 12438-12439.
[19] SINGH A, TEEGARDIN K, KELLY M, et al. Facile synthesis and complete characterization of homoleptic and heteroleptic cyclometalated iridium(III) complexes for photocatalysis[J]. Journal of organometallic chemistry, 2015, 776: 51-59.
[20] LAMANSKY S, DJUROVICH P, MURPHY D, et al. Highly phosphorescent bis-cyclometalated iridium complexes: Synthesis, photophysical characterization, and use in organic light emitting diodes[J]. Journal of the American Chemical Society, 2001, 123(18): 4304-4312.
[21] LAMANSKY S, DJUROVICH P, MURPHY D, et al. Synthesis and characterization of phosphorescent cyclo- metalated iridium complexes[J]. Inorganic chemistry, 2001, 40(7): 1704-1711.
[22] BÖTTCHER H C, GRAF M, SÜNKEL K, et al. [Ir(acac)(η2-C8H14)2]: A precursor in the synthesis of cyclometalated iridium(III) complexes[J]. Inorganica chimica acta, 2011, 365(1): 103-107.
[23] CHEW S, LEE C S, LEE S T, et al. Photoluminescence and electroluminescence of a new blue-emitting homo- leptic iridium complex[J]. Applied physics letters, 2006, 88(9): 093510.
[24] ZHUANG J, LI W, WU W, et al. Homoleptic tris- cyclometalated iridium(III) complexes with phenyli- midazole ligands for highly efficient sky-blue OLEDs[J]. New journal of chemistry, 2015, 39(1): 246-253.
[25] WANG R, LIU D, REN H, et al. Homoleptic tris- cyclometalated iridium complexes with 2-phenylbenzo- thiazole ligands for highly efficient orange OLEDs[J]. Journal of materials chemistry, 2011, 21(39): 15494.
[26] ANTHOPOULOS T D, FRAMPTON M J, NAMDAS E B, et al. Solution-processable red phosphorescent dendri- mers for light-emitting device applications[J]. Advanced materials, 2004, 16(6): 557-560.
[27] COLOMBO M G, BRUNOLD T C, RIEDENER T, et al. Facial tris-cyclometalated rhodium(3+) and iridium(3+) complexes: Their synthesis, structure, and optical spectro- scopic properties[J]. Inorganic chemistry, 1994, 33(3): 545-550.
[28] TAMAYO A B, ALLEYNE B D, DJUROVICH P I, et al. Synthesis and characterization of facial and meridional tris-cyclometalated iridium(III) complexes[J]. Journal of the American Chemical Society, 2003, 125(24): 7377.
[29] IDE N, MATSUSUE N, KOBAYASHI T, et al. Photo- luminescence properties of facial-and meridional-Ir(ppy)3thin films[J]. Thin solid films, 2006, 509(1/2): 164-167.
[30] TSUBOYAMA A, IWAWAKI H, FURUGORI M, et al. Homoleptic cyclometalated iridium complexes with highly efficient red phosphorescence and application to organic light-emitting diode[J]. Journal of the American Chemical Society, 2003, 125(42): 12971-12979.
[31] SCHAFFNER-HAMANN C, VON ZELEWSKY A, BARBIERI A, et al. Diastereoselective formation of chiral tris-cyclometalated iridium(III) complexes: charac- terization and photophysical properties[J]. Journal of the American Chemical Society, 2004, 126(30): 9339-9348.
[32] KONNO H, SASAKI Y. Selective one-pot synthesis of facial tris-ortho-metalated iridium(III) complexes using microwave irradiation[J]. Chemistry letters, 2003, 32(3): 252-253.
[33] STÖSSEL P, BACH I, SPREITZER H. Rhodium and iridium complexes: US 7883785[P]. 2011-02-08.
[34] CUMPSTEY N, BERA R N, BURN P L, et al. Investigating the effect of steric crowding in phosphorescent dendrimers[J]. Macromolecules, 2005, 38(23): 9564-9570.
[35] CHANG Q W, LIE J, YAN C X, et al. A new synthesis method and photophysical properties of Ir((CN)-N- boolean AND)(3) cyclometalated iridium phosphorescent complexes[J]. Bulgarian chemical communications, 2016, 48(3): 532-534.
[36] 马晓宇, 梁洁, 叶玲, 等. 混合配体型铱(III)配合物的合成及电致发光性能[J]. 高等化学学报, 2018, 39(10): 2129-2135.
MA X Y, LIANG J, YE L, et al. Synthesis and electro- luminescence characterization of mixed-C^N ligand tris- cyclometalated Ir(III) complexes[J]. Chemical journal of Chinese universities, 2018, 39(10): 2129-2135.
[37] DEDEIAN K, SHI J, SHEPHERD N, et al. Photophysical and electrochemical properties of heteroleptic triscyclo- metalated iridium(III) complexes[J]. Inorganic chemistry, 2005, 44(13): 4445-4447.
[38] FERNÁNDEZ-HERNÁNDEZ J M, BELTRÁN J I, LEMAUR V, et al. Iridium(III) emitters based on 1,4-disubstituted-1 H-1, 2, 3-triazoles as cyclometalating ligand: Synthesis, characterization, and electro- luminescent devices[J]. Inorganic chemistry, 2013, 52(4): 1812-1824.
[39] NONOYAMA M. Benzo [h] quinolin-10-yl-N iridium(III) complexes[J]. Bulletin of the Chemical Society of Japan, 1974, 47(3): 767-768.
[40] TSUBOYAMA A, MIZUTANI H, OKADA S, et al. Metal coordination compound and organic luminescence device: US6783873[P]. 2004-08-31.
[41] XIA C, KWONG R, LAYEK S. Heteroleptic iridium complex: US8722205[P]. 2014-05-13.
[42] XIA C, ALLEYNE B, KWONG R C, et al. Phospho- rescent materials: US8519384[P]. 2013-08-27.
[43] MA B, BARRON E, DEANGELIS A, et al. Iridium complexes with aza-benzo fused ligands: US8946697[P]. 2015-02-03.
[44] DE ANGELIS F, FANTACCI S, EVANS N, et al. Controlling phosphorescence color and quantum yields in cationic iridium complexes: A combined experimental and theoretical study[J]. Inorganic chemistry, 2007, 46(15): 5989-6001.
[45] TORDERA D, PERTEGÁS A, SHAVALEEV N M, et al. Efficient orange light-emitting electrochemical cells[J]. Journal of materials chemistry, 2012, 22(36): 19264.
[46] SHAVALEEV N M, XIE G, VARGHESE S, et al. Green phosphorescence and electroluminescence of sulfur pentafluoride-functionalized cationic iridium(III) comp- lexes[J]. Inorganic chemistry, 2015, 54(12): 5907-5914.
[47] MA D, DUAN L, WEI Y, et al. Increased phosphorescent quantum yields of cationic iridium(III) complexes by wisely controlling the counter anions[J]. Chemical communications, 2014, 50(5): 530-532.
[48] LI X, YIN Y, YAN H, et al. Novel phosphorescent cationic iridium(III) complexes with o-carboranylation on the ancillary N^N ligand[J]. Dalton transactions, 2017, 46(30): 10082-10089.
[49] WONG W Y, ZHOU G J, YU X M, et al. Efficient organic light-emitting diodes based on sublimable charged iridium phosphorescent emitters[J]. Advanced functional materials, 2007, 17(2): 315-323.
[50] HUANG S F, SUN H Z, SHAN G G, et al. Rational design and synthesis of cationic Ir(III) complexes with triazolate cyclometalated and ancillary ligands for multi- color tuning[J]. Dyes and pigments, 2017, 139: 524-532.
[51] 郭远辉, 王玲霞, 翁洁娜, 等. 高分子铱配合物磷光材料的制备技术进展[J]. 科学通报, 2011, 56(31:): 2548.
GUO Y H, WANG L X, WENG J N, et al. Technical development in the synthesis of polymer iridium complex electrophosphorescent materials[J]. Chinese sci bull, 2011, 56(31): 2548.
[52] QIN T, DING J, WANG L, et al. A divergent synthesis of very large polyphenylene dendrimers with iridium(III) cores: molecular size effect on the performance of phosphorescent organic light-emitting diodes[J]. Journal of the American Chemical Society, 2009, 131(40): 14329-14336.
[53] LAI W Y, LEVELL J W, JACKSON A C, et al. A phosphorescent poly(dendrimer) containing iridium(III) complexes: Synthesis and light-emitting properties[J]. Macromolecules, 2010, 43(17): 6986-6994.
[54] LIANG B, WANG L, ZHU X, et al. Application of heteroleptic iridium complexes with fluorenyl-modified 1-phenylisoquinoline ligand for high-efficiency red polymer light-emitting devices[J]. Journal of organo- metallic chemistry, 2009, 694(19): 3172-3178.
[55] CHO Y J, HONG S A, SON H J, et al. Efficient light harvesting and energy transfer in a red phosphorescent iridium dendrimer[J]. Inorganic chemistry, 2014, 53(24): 13136-13141.
[56] ZHANG K, CHEN Z, YANG C, et al. First iridium complex end-capped polyfluorene: Improving device performance for phosphorescent polymer light-emitting diodes[J]. The journal of physical chemistry C, 2008, 112(10): 3907-3913.
[57] SHI H F, NAKAI Y, LIU S J, et al. Improved energy transfer through the formation of the β phase for polyfluorenes containing phosphorescent iridium(III) complexes[J]. The journal of physical chemistry C, 2011, 115(23): 11749-11757.
[58] YING L, ZOU J, ZHANG A, et al. Novel orange-red light-emitting polymers with cyclometaled iridium complex grafted in alkyl chain[J]. Journal of organo- metallic chemistry, 2009, 694(17): 2727-2734.
[59] CARLISE J R, WANG X Y, WECK M. Phosphorescent side-chain functionalized poly (norbornene) s containing iridium complexes[J]. Macromolecules, 2005, 38(22): 9000-9008.
[60] LIANG A H, ZHANG K, ZHANG J, et al. Supra- molecular phosphorescent polymer iridium complexes for high-efficiency organic light-emitting diodes[J]. Chemistry of materials, 2013, 25(6): 1013-1019.
[61] ZHAO Q, LIU S J, HUANG W. Promising optoelectronic materials: polymers containing phosphorescent iridium(III) complexes[J]. Macromolecular rapid commu- nications, 2010, 31(9/10): 794-807.
[62] LI T Y, WU J, WU Z G, et al. Rational design of phosphorescent iridium(III) complexes for emission color tunability and their applications in OLEDs[J]. Coordi- nation chemistry reviews, 2018, 374: 55-92.
[63] TAVASLI M, MOORE T N, ZHENG Y, et al. Colour tuning from green to red by substituent effects in phosphorescent tris-cyclometalated iridium(III) comp- lexes of carbazole-based ligands: synthetic, photophysical, computational and high efficiency OLED studies[J]. Journal of materials chemistry, 2012, 22(13): 6419-6428.
[64] SEO J H, KIM I J, KIM Y S, et al. Color tuning of organic light-emitting diodes by adjusting the ligands of heteroleptic iridium(III) complexes[J]. Journal of crystal growth, 2011, 326(1): 113-115.
[65] CHANG W C, HU A T, DUAN J P, et al. Color tunable phosphorescent light-emitting diodes based on iridium complexes with substituted 2-phenylbenzothiozoles as the cyclometalated ligands[J]. Journal of organometallic chemistry, 2004, 689(26): 4882-4888.
[66] CHAU N Y, HO P Y, HO C L, et al. Color-tunable thiazole-based iridium(III) complexes: Synthesis, charac- terization and their OLED applications[J]. Journal of organometallic chemistry, 2017, 829: 92-100.
[67] WANG L, WANG N, ZHANG Y, et al. Color tuning from red to green of bis-cyclometalated iridium(III) emitters based on benzoimidazole ligands in OLEDs: A DFT and TD-DFT investigation[J]. Synthetic metals, 2014, 194: 160-169.
[68] KAJJAM A B, VAIDYANATHAN S. Tuning the photo- physical properties of heteroleptic Ir(III) complexes through ancillary ligand substitution: Experimental and theoretical investigation[J]. Journal of photochemistry and photobiology A: Chemistry, 2018, 350: 130-141.
[69] KWON T H, CHO H S, KIM M K, et al. Color tuning of cyclometalated iridium complexes through modification of phenylpyrazole derivatives and ancillary ligand based on ab initio calculations[J]. Organometallics, 2005, 24(7): 1578-1585.
[70] HAN D, LIU C, LV S, et al. The effect of different conjugated structures in main ligand on the photophysical properties for a series of iridium(III) complexes from a theoretical perspective[J]. Polyhedron, 2018, 144: 234.
[71] ZYSMAN-COLMAN E. Iridium(III) in optoelectronic and photonics applications[M]. John Wiley & Sons Ltd, 2017: 206-274.
[72] JIAO Y, LI M, WANG N, et al. A facile color-tuning strategy for constructing a library of Ir(III) complexes with fine-tuned phosphorescence from bluish green to red using a synergetic substituent effect of -OCH3and -CN at only the C-ring of C^N ligand[J]. Journal of materials chemistry C, 2016, 4(19): 4269-4277.
[73] TSUZUKI T, SHIRASAWA N, SUZUKI T, et al. Color tunable organic light-emitting diodes using pentafluoro- phenyl- substituted iridium complexes[J]. Advanced materials, 2003, 15(17): 1455-1458.
[74] LASKAR I R, CHEN T M. Tuning of wavelengths: Synthesis and photophysical studies of iridium complexes and their applications in organic light emitting devices[J]. Chemistry of materials, 2004, 16(1): 111-117.
[75] LAI S L, TAO S L, CHAN M Y, et al. Efficient white organic light-emitting devices based on phosphorescent iridium complexes[J]. Organic electronics, 2010, 11(9): 1511-1515.
[76] SU Y J, HUANG H L, LI C L, et al. Highly efficient red electrophosphorescent devices based on iridium isoquino- line complexes: remarkable external quantum efficiency over a wide range of current[J]. Advanced materials, 2003, 15(11): 884-888.
[77] GRISORIO R, SURANNA G P, MASTRORILLI P, et al. Aryl 5-substitution of a phenyl-pyridine based ligand as a viable way to influence the opto-electronic properties of bis-cyclometalated Ir(III) heteroleptic complexes[J]. Dalton transactions, 2013, 42(24): 8939-8950.
[78] FANG K H, WU L L, HUANG Y T, et al. Color tuning of iridium complexes- Part I: Substituted phenylisoquino- line-based iridium complexes as the triplet emitter[J]. Inorganica chimica acta, 2006, 359(2): 441-450.
[79] 陶鹏. 高效磷光铱(III)配合物的设计、合成、激发态调控及光电应用研究[D]. 山西: 太原理工大学, 2017: 41-127.
TAO P. Design, synthesis, and excited states tuning of highly efficient iridium(III) complexes for applications in optoelectronics[D]. Shanxi: Taiyuan University of Technology, 2017: 41-127.
[80] TAO P, LI W L, ZHANG J, et al. Facile synthesis of highly efficient lepidine-based phosphorescent iridium(III) complexes for yellow and white organic light-emitting diodes[J]. Advanced functional materials, 2016, 26(6): 881-894.
[81] 赵强. 光功能铱配合物的分子设计、合成极其光电性质研究[D]. 上海: 复旦大学, 2007: 6-8.
ZHAO Q. Molecular design, synthesis and photoelectric properties of iridium complexes[D]. Shanghai: Fudan University, 2007: 6-8.
[82] ZHOU G, WANG Q, HO C L, et al. Robust tris- cyclometalated iridium(III) phosphors with ligands for effective charge carrier injection/transport: Synthesis, redox, photophysical, and electrophosphorescent behavior [J]. Chemistry - an Asian journal, 2008, 3(10): 1830.
[83] LEPELTIER M, XIAO P, GRAFF B, et al. Wide bandgap iridium complexes varying by their ancillary ligands: Influence on their electroluminescence properties[J]. Synthetic metals, 2015, 204: 48-56.
[84] HAN D, ZHAO L, PANG C, et al. The effect of substi- tuted pyridine ring in the ancillary group on the electronic structures and phosphorescent properties for Ir(III) complexes from a theoretical viewpoint[J]. Polyhedron, 2017, 126: 134-141.
[85] WU H, YANG T, ZHAO Q, et al. A cyclometalated iridium(III) complex with enhanced phosphorescence emission in the solid state (EPESS): Synthesis, characterization and its application in bioimaging[J]. Dalton transactions, 2011, 40(9): 1969-1976.
[86] HAN H B, WU Z G, ZHENG Y X. Efficient bluish green electroluminescence of iridium complexes with good electron mobility[J]. New journal of chemistry, 2018, 42(16): 13351-13357.
[87] PENCONI M, CAZZANIGA M, KESARKAR S, et al. β-Diketonate ancillary ligands in heteroleptic iridium complexes: a balance between synthetic advantages and photophysical troubles[J]. Photochemical & photobio- logical sciences, 2018, 17(9): 1169-1178.
[88] SEO J A, GONG M S, LEE J Y. High efficiency yellowish green phosphorescent emitter derived from phenylbenzothienopyridine ligand[J]. Organic electronics, 2014, 15(9): 2068-2072.
[89] GRAF M, SÜNKEL K. First cyclometalated iridium(III) complex containing methyl 2-amino-2-deoxy-β-d- glucopyranoside as N,O-chelate[J]. Inorganica chimica acta, 2012, 387: 81-85.
[90] LIU Y I, LI M, ZHAO Q, et al. Phosphorescent iridium(III) complex with an N^O ligand as a Hg2+- selective chemodosimeter and logic gate[J]. Inorganic chemistry, 2011, 50(13): 5969-5977.
[91] ZHANG S, WU F, YANG W, et al. Synthesis, characte- rization and photophysical properties of two heteroleptic 2-(4,6-difluorophenyl) pyridyl iridium(III) complexes with amidate ancillary ligands[J]. Inorganic chemistry communications, 2011, 14(9): 1414-1417.
[92] TONG B, ZHANG M, HAN Z, et al. Novel colorimetric phosphorescent chemodosimeter for Hg2+based iridium (III) complex with phosphonodithioate auxiliary ligand[J]. Journal of organometallic chemistry, 2013, 724: 180-185.
[93] MEI Q, SHI Y, HUA Q, et al. Phosphorescent chemo- sensor for Hg2+based on an iridium(III) complex coordinated with 4-phenylquinazoline and carbazole dithiocarbamate[J]. RSC advances, 2015, 5(91): 74924.
[94] LIU Y, YE K, FAN Y, et al. Amidinate-ligated iridium(III) bis (2-pyridyl) phenyl complex as an excellent phosphorescent material for electrolumine- scence devices[J]. Chemical communications, 2009(25): 3699-3701.
[95] RAI V K, NISHIURA M, TAKIMOTO M, et al. Guanidinate ligated iridium(III) complexes with various cyclometalated ligands: Synthesis, structure, and highly efficient electrophosphorescent properties with a wide range of emission colours[J]. Journal of materials chemistry C, 2013, 1(4): 677-689.
[96] TAKAHIRA Y, MUROTANI E, FUKUDA K, et al. Design, synthesis, and properties of a series of charged iridium(III) complexes with a neutral bidentate ligand for deep-blue phosphorescent emitter[J]. Journal of fluorine chemistry, 2016, 181: 56-60.
[97] HUANG K, WU H, SHI M, et al. Reply to comment on ‘aggregation-induced phosphorescent emission (AIPE) of iridium(III) complexes’: Origin of the enhanced phosphorescence[J]. Chemical communications, 2009(10): 1243-1245.
[98] BOUDREAULT P L T, DYATKIN A, LI D Z, et al. Ancillary ligands for organometallic complexes: US13/ 932508[P]. 2015-01-01.
[99] LI C F, YONG G P, LI Y Z. Phosphorescent iridium(III) 2-phenylpyridine complexes: Efficient color tuning by novel ancillary ligands[J]. Inorganic chemistry communi- cations, 2010, 13(1): 179-182.
[100]LI G N, GAO C W, CHEN H H, et al. Color tuning of cyclometalated 2-phenylbenzo [d] oxazole-based iridium (III) complexes through modification of different N^O ancillary ligands[J]. Inorganica chimica acta, 2016, 445: 22-27.
[101]RAI V K, NISHIURA M, TAKIMOTO M, et al. Substituent effect on the electroluminescence efficiency of amidinate-ligated bis (pyridylphenyl) iridium(III) complexes[J]. Journal of materials chemistry C, 2014, 2(27): 5317-5326.
[102]RAI V K, NISHIURA M, TAKIMOTO M, et al. Bis- cyclometalated iridium(III) complexes bearing ancillary guanidinate ligands: Synthesis, structure, and highly efficient electroluminescence[J]. Inorganic chemistry, 2011, 51(2): 822-835.
[103]KING K A, SPELLANE P J, WATTS R J. Excited-state properties of a triply ortho-metalated iridium(III) complex [J]. Journal of the American Chemical Society, 1985, 107(5): 1431-1432.
[104]YANG X, ZHOU G, WONG W Y. Functionalization of phosphorescent emitters and their host materials by main- group elements for phosphorescent organic light-emitting devices[J]. Chemical society reviews, 2015, 44(23): 8484-8575.
[105]TAO Y, WANG Q, YANG C, et al. Multifunctional triphenylamine/oxadiazole hybrid as host and exciton- blocking material: high efficiency green phosphorescent OLEDs using easily available and common materials[J]. Advanced functional materials, 2010, 20(17): 2923-2929.
[106]BOLINK H J, CORONADO E, SANTAMARIA S G, et al. Highly phosphorescent perfect green emitting iridium (III) complex for application in OLEDs[J]. Chemical communications, 2007 (31): 3276-3278.
[107]LI H Y, ZHOU L, TENG M Y, et al. Highly efficient green phosphorescent OLEDs based on a novel iridium complex[J]. Journal of materials chemistry C, 2013, 1(3): 560-565.
[108]JUNG S O, ZHAO Q, PARK J W, et al. A green emitting iridium(III) complex with narrow emission band and its application to phosphorescence organic light-emitting diodes (OLEDs)[J]. Organic electronics, 2009, 10(6): 1066-1073.
[109]JAYABHARATHI J, THANIKACHALAM V, SATHI- SHKUMAR R. Highly phosphorescent green emitting iridium(III) complexes for application in OLEDs[J]. New journal of chemistry, 2015, 39(1): 235-245.
[110]KIM H U, PARK H J, JANG J H, et al. Green phosphorescent homoleptic iridium(III) complexes for highly efficient organic light-emitting diodes[J]. Dyes and pigments, 2018, 156: 395-402.
[111]LIANG X, ZHANG F, YAN Z P, et al. Fast synthesis of iridium(III) complexes incorporating a bis(diphenyl- phorothioyl) amide ligand for efficient pure green OLEDs[J]. ACS applied materials & interfaces, 2019, 11(7): 7184-7191.
[112]HAN H B, TU Z L, WU Z G, et al. Green iridium complexes based on pyrimidine derivatives for efficient electroluminescence with EQE near 30%[J]. Dyes and pigments, 2019, 160: 863-871.
[113]WEI W, LIMA S A M, DJUROVICH P I, et al. Synthesis and characterization of phosphorescent isomeric iridium complexes with a rigid cyclometalating ligand[J]. Polyhedron, 2018, 140: 138-145.
[114]ZHAO J H, HU Y X, LU H Y, et al. Progress on benzimidazole-based iridium(III) complexes for appli- cation in phosphorescent OLEDs[J]. Organic electronics, 2017, 41: 56-72.
[115]BENJAMIN H, LIANG J, LIU Y, et al. Color tuning of efficient electroluminescence in the blue and green regions using heteroleptic iridium complexes with 2-phenoxyoxazole ancillary ligands[J]. Organometallics, 2017, 36(9): 1810-1821.
[116]TONG B, WANG H, CHEN M, et al. High efficiency green OLEDs based on homoleptic iridium complexes with steric phenylpyridazine ligands[J]. Dalton trans- actions, 2018, 47(35): 12243-12252.
[117]LIU X, LIU Y, YU T, et al. Efficient green phosphorescent Ir(III) complexes with β-diketonate ancillary ligands[J]. Inorganic chemistry frontiers, 2018, 5(9): 2321-2331.
[118]XU H, CHEN R, SUN Q, et al. Recent progress in metal- organic complexes for optoelectronic applications[J]. Chemical society reviews, 2014, 43(10): 3259-3302.
[119]PENG T, BI H, LIU Y, et al. Very high-efficiency red- electroluminescence devices based on an amidinate- ligated phosphorescent iridium complex[J]. Journal of materials chemistry, 2009, 19(43): 8072-8074.
[120]OKADA S, OKINAKA K, IWAWAKI H, et al. Subs- tituent effects of iridium complexes for highly efficient red OLEDs[J]. Dalton transactions, 2005(9): 1583-1590.
[121]GRAF M, THESEN M, KRÜGER H, et al. Synthesis and X-ray crystal structure of a bis-cyclometalated phospho- rescent red-emitting iridium(III) complex[J]. Inorganic chemistry communications, 2009, 12(8): 701-703.
[122]DING J, GAO J, FU Q, et al. Highly efficient phosphorescent bis-cyclometalated iridium complexes based on quinoline ligands[J]. Synthetic metals, 2005, 155(3): 539-548.
[123]THOMAS K R J, VELUSAMY M, LIN J T, et al. Efficient red-emitting cyclometalated iridium(III) comp- lexes containing lepidine-based ligands[J]. Inorganic chemistry, 2005, 44(16): 5677-5685.
[124]LEPELTIER M, DUMUR F, WANTZ G, et al. Red phosphorescent organic light-emitting diodes (PhOLEDs) based on a heteroleptic cyclometalated iridium(III) comp- lex[J]. Journal of luminescence, 2013, 143: 145-149.
[125]PARK G Y, HA Y. Red phosphorescent iridium(III) complexes containing 2,3-diphenylquinoline derivatives for OLEDs[J]. Synthetic metals, 2008, 158(3/4): 120-124.
[126]SCHNEIDENBACH D, AMMERMANN S, DEBEAUX M, et al. Efficient and long-time stable red iridium(III) complexes for organic light-emitting diodes based on quinoxaline ligands[J]. Inorganic chemistry, 2009, 49(2): 397-406.
[127]GAO J, YOU H, FANG J, et al. Pure red electrophospho- rescent organic light-emitting diodes based on a new iridium complex[J]. Synthetic metals, 2005, 155(1): 168.
[128]LAI P N, BRYSACZ C H, ALAM M K, et al. Highly efficient red-emitting bis-cyclometalated iridium complexes[J]. Journal of the American Chemical Society, 2018, 140(32): 10198-10207.
[129]KIM H U, JANG J H, PARK H J, et al. Highly efficient and spectrally stable white organic light-emitting diodes using new red heteroleptic iridium(III) complexes[J]. Dyes and pigments, 2018, 149: 363-372.
[130]LEE S Y, NA OH Y, SHIN D M, et al. Effects of the methyl group on the emission efficiency of the red phosphorescent iridium(III) complexes for OLEDs[J]. Journal of nanoscience and nanotechnology, 2018, 18(10): 7137-7141.
[131]ADACHI C, BALDO M A, FORREST S R, et al. High-efficiency red electrophosphorescence devices[J]. Applied physics letters, 2001, 78(11): 1622-1624.
[132]YANG C H, TAI C C, SUN I W. Synthesis of a high- efficiency red phosphorescent emitter for organic light- emitting diodes[J]. Journal of materials chemistry, 2004, 14(6): 947-950.
[133]KIM D H, CHO N S, OH H Y, et al. Highly efficient red phosphorescent dopants in organic light-emitting devices [J]. Advanced materials, 2011, 23(24): 2721-2726.
[134]ADACHI C, KWONG R C, DJUROVICH P, et al. Endo- thermic energy transfer: A mechanism for generating very efficient high-energy phosphorescent emission in organic materials[J]. Applied physics letters, 2001, 79(13): 2082.
[135]HOLMES R J, FORREST S R, TUNG Y J, et al. Blue organic electrophosphorescence using exothermic host- guest energy transfer[J]. Applied physics letters, 2003, 82(15): 2422-2424.
[136]LIN M S, YANG S J, CHANG H W, et al. Incorporation of a CN group into mCP: a new bipolar host material for highly efficient blue and white electrophosphorescent devices[J]. Journal of materials chemistry, 2012, 22(31): 16114-16120.
[137]KESSLER F, WATANABE Y, SASABE H, et al. High- performance pure blue phosphorescent OLED using a novel bis-heteroleptic iridium(III) complex with fluori- nated bipyridyl ligands[J]. Journal of materials chemistry C, 2013, 1(6): 1070-1075.
[138]YI S, KIM J H, CHO Y J, et al. Stable blue phosphorescence iridium(III) cyclometalated complexes prompted by intramolecular hydrogen bond in ancillary ligand[J]. Inorganic chemistry, 2016, 55(7): 3324-3331.
[139]FELDMAN J, VO G D, MCLAREN C D, et al. Highly quantum efficient phosphorescent sky blue emitters based on diastereomeric iridium(III) complexes of atropiso- meric 5-aryl-4 h-1, 2, 4-triazole ligands[J]. Organo- metallics, 2015, 34(15): 3665-3669.
[140]YANG C H, MAURO M, POLO F, et al. Deep-blue- emitting heteroleptic iridium(III) complexes suited for highly efficient phosphorescent OLEDs[J]. Chemistry of materials, 2012, 24(19): 3684-3695.
[141]YANG L, OKUDA F, KOBAYASHI K, et al. Syntheses and phosphorescent properties of blue emissive iridium complexes with tridentate pyrazolyl ligands[J]. Inorganic chemistry, 2008, 47(16): 7154-7165.
[142]KWON Y, HAN S H, YU S, et al. Functionalized phenylimidazole-based facial-homoleptic iridium(III) complexes and their excellent performance in blue phosphorescent organic light-emitting diodes[J]. Journal of materials chemistry C, 2018, 6(16): 4565-4572.
[143]CHOI H J, HYUN M H, PARK H J, et al. Synthesis of efficient blue phosphorescent heteroleptic Ir(III) complexes containing 4-alkoxy-or 4-alkylaminopicoli- nates as ancillary ligands[J]. Journal of luminescence, 2017, 188: 323-330.
[144]OMAE I. Application of the five-membered ring blue light-emitting iridium products of cyclometalation reactions as OLEDs[J]. Coordination chemistry reviews, 2016, 310: 154-169.
[145]LEE J H, SARADA G, MOON C K, et al. Finely tuned blue iridium complexes with varying horizontal emission dipole ratios and quantum yields for phosphorescent organic light-emitting diodes[J]. Advanced optical materials, 2015, 3(2): 211-220.
[146]LO S C, SHIPLEY C P, BERA R N, et al. Blue phospho- rescence from iridium(III) complexes at room tempe- rature[J]. Chemistry of materials, 2006, 18(21): 5119.
[147]CHANG C F, CHENG Y M, CHI Y, et al. Highly efficient blue-emitting iridium(III) carbene complexes and phosphorescent OLEDs[J]. Angewandte chemie international edition, 2008, 47(24): 4542-4545.
[148]LEE S J, PARK K M, YANG K, et al. Blue phospho- rescent Ir(III) complex with high color purity: fac-tris(2′,6′-difluoro-2,3′-bipyridinato-N,C4′)iridium(III) [J]. Inorganic chemistry, 2008, 48(3): 1030-1037.
[149]KIM J B, HAN S H, YANG K, et al. Highly efficient deep-blue phosphorescence from heptafluoropropyl- substituted iridium complexes[J]. Chemical communi- cations, 2015, 51(1): 58-61.
[150]KANG Y, CHANG Y L, LU J S, et al. Highly efficient blue phosphorescent and electroluminescent Ir(III) compounds[J]. Journal of materials chemistry C, 2013, 1(3): 441-450.
[151]LI J, DJUROVICH P I, ALLEYNE B D, et al. Synthetic control of excited-state properties in cyclometalated Ir(III) complexes using ancillary ligands[J]. Inorganic chemistry, 2005, 44(6): 1713-1727.
[152]YEH S J, WU M F, CHEN C T, et al. New dopant and host materials for blue-light-emitting phosphorescent organic electroluminescent devices[J]. Advanced materials, 2005, 17(3): 285-289.
[153]HOLMES R J, FORREST S R, SAJOTO T, et al. Saturated deep blue organic electrophosphorescence using a fluorine-free emitter[J]. Applied physics letters, 2005, 87(24): 243507.
[154]LI X, ZHANG J, ZHAO Z, et al. Deep blue phosphorescent organic light-emitting diodes with CIEy value of 0.11 and external quantum efficiency up to 22.5%[J]. Advanced materials, 2018, 30(12): 1705005.
[155]KUMAR S, SURATI K R, LAWRENCE R, et al. Design and synthesis of heteroleptic iridium(III) phosphors for efficient organic light-emitting devices[J]. Inorganic chemistry, 2017, 56(24): 15304-15313.
[156]FAN C, ZHU L, JIANG B, et al. High power efficiency yellow phosphorescent OLEDs by using new iridium complexes with halogen-substituted 2-phenylbenzo [d] thiazole ligands[J]. The journal of physical chemistry C, 2013, 117(37): 19134-19141.
[157]YU X M, ZHOU G J, LAM C S, et al. A yellow-emitting iridium complex for use in phosphorescent multiple- emissive-layer white organic light-emitting diodes with high color quality and efficiency[J]. Journal of organometallic chemistry, 2008, 693(8/9): 1518-1527.
[158]NIU Z G, CHEN J, TAN P, et al. Efficient yellow electroluminescence of four iridium(III) complexes with benzo[d] thiazole derivatives as main ligands[J]. Dalton transactions, 2018, 47(24): 8032-8040.
[159]GE G, ZHANG G, GUO H, et al. Yellow organic light-emitting diodes based on phosphorescent iridium(III) pyrazine complexes: Fine tuning of emission color[J]. Inorganica chimica acta, 2009, 362(7): 2231-2236.
[160]XU F, KWON J Y, KIM J H, et al. Highly efficient yellow and white phosphorescent organic light-emitting diodes using a benzothiazole-liganded new iridium complex[J]. Synthetic metals, 2012, 162(15/16): 1421.
[161]SLINKER J D, GORODETSKY A A, LOWRY M S, et al. Efficient yellow electroluminescence from a single layer of a cyclometalated iridium complex[J]. Journal of the American Chemical Society, 2004, 126(9): 2763.
[162]PARK Y S, KANG J W, KANG D M, et al. Efficient, color stable white organic light-emitting diode based on high energy level yellowish-green dopants[J]. Advanced materials, 2008, 20(10): 1957-1961.
[163]SUNESH C D, CHANDRAN M, MATHAI G, et al. Highly luminescent yellow and yellowish-green light- emitting electrochemical cells based on cationic iridium complexes with phenanthroline based ancillary ligands[J]. Optical materials, 2013, 35(3): 407-413.
[164]MULANI S, XIAO M, WANG S, et al. Structure properties of a highly luminescent yellow emitting material for OLED and its application[J]. RSC advances, 2013, 3(1): 215-220.
Synthesis, Color-Controlling Methods and Application Research of Iridium Phosphorescent Complexes
CHANG Qiao-wen1, 2, WANG Zi-ao1, YAN Cai-xian1, ZHANG Xuan-dong1, JIANG Jing1, LIU Wei-ping1, CUI Hao1 *
(1. Kunming Institute of Precious Metals, State Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Group Metals, Sino-Platinum Metals Co. Ltd., Kunming 650106, China; 2. Faculty of Material Science and Engineering, Kunming University of Science and Technology, Kunming 650093, China)
Iridium phosphorescent complexes have good thermal stability, relatively short excited state lifetime, high luminous efficiency and easy adjustment of luminous color, and they has become the most excellent organic luminescent materials, and their applications in OLED have attracted much attention. In this paper, the structure, synthesis, color control and application of iridium phosphorescent complexes were reviewed. The structure-activity relationships of several iridium phosphorescent complexes have been obtained, which can be used to guide the efficient research and development of iridium phosphorescent complexes. The problems encountered in the development of iridium phosphorescent complexes were also described, and the development trend and direction of iridium phosphorescent complexes were pointed out.
iridium phosphorescent complexes; synthesis; color regulation; application; development.
O614.82+5
A
1004-0676(2020)03-0094-21
2019-12-30
国家自然科学基金(21861023)、云南省应用基础研究项目(2019FA047和2018FD141)、云南省转制院所技术开发研究专项(202004AR040001) 、稀贵金属综合利用新技术国家重点实验室开放课题(SKL-SPM-201807)
常桥稳,男,博士,高级工程师,研究方向:贵金属功能配合物研究开发。E-mail:changqiaowen@126.com
崔 浩,男,高级工程师,研究方向:贵金属新材料研究开发。E-mail:cuihao@ipm.com.cn
