Physicochemical Properties of Poly (Vinyl Alcohol)/Chitosan/Soluble Starch Composite Hydrogel
2020-02-01WANGLei王蕾HUANGYifan黄一凡BAOZelin鲍泽霖LITingxiaoXINBinjie
WANGLei(王蕾),HUANGYifan(黄一凡),BAOZelin(鲍泽霖),LITingxiao,XINBinjie
School of Textiles and Fashion, Shanghai University of Engineering Science, Shanghai 201620, China
Abstract: Fabricating of hydrogels based upon polymers and inorganic matter is an innovative replacement for the generation of adaptable matrices. In this study, the poly(vinyl alcohol) (PVA)/chitosan (CS)/soluble starch (SS) composite hydrogel was prepared by the solution blending method, in which SS was used as a cross-linking agent to cross-link with PVA in order to improve the stability of PVA in the tissue fluid. The composition and the thermal stability of hydrogels were characterized by Fourier transform infrared (FTIR) spectroscopy and thermo gravimetric analysis (TGA). The result shows that the PVA/CS/SS composite hydrogel possesses potentially morphological and thermal stability and can be used for tissue engineering.
Key words: poly (vinyl alcohol) (PVA); chitosan (CS); soluble starch (SS); hydrogel; thermal stability
Introduction
With the development of material science and biomedicine, the increasing polymer materials are applied to the medical field[1-2]. Principles and methods of cell biology and engineering are used in tissue engineering to research and prepare substitutes that can restore or improve the shape and the function of damaged tissues and organs[3]. The crux of tissue engineering scaffolds is to prepare cell scaffolds with biocompatibility, excellent mechanical properties and biodegradability. Hydrogels are proverbially used in the fields of controlled drug release, cell separation and culture, and immobilized enzyme biological systems due to their excellent mechanical strength, moisture content, and stability.
Poly (vinyl alcohol) (PVA) is one of the common water-soluble polymers[4]. Its molecular backbone is a carbon chain, and each repeating unit contains a hydroxyl group[5]. Due to the small size of the hydroxyl group, strong polarity, and easy formation of hydrogen bonds, PVA has favorable water solubility and film-forming properties[6-7]. Owing to its high-water content, low toxicity, excellent biocompatibility, and ease of processing, PVA hydrogels have gradually been diffusely employed in medical and health fields[8]. However, the dehydration rate and the swelling degree of PVA hydrogels cannot meet certain requirements of these scaffold materials[9]. Hence, adding some natural polymer material to change its dehydration rate, swelling degree and other biological properties is a novel and hot spot in the field of tissue engineering[8, 10]. Chitosan (CS) has superior biocompatibility and intrinsic antibacterial activity due to its polycationic characteristic[11]. The antimicrobic activity of CS is accomplished by binding to the negatively charged cytoplasmic membrane of bacterial cells and wrecking its normal functions. Soluble starch (SS) is a biodegradable natural polymer material with good biocompatibility. SS contains a large number of hydroxyl bonds, which can form hydrogen bonds or chemical interactions with many substances, and can interact with PVA. Cross-linking occurs, and it improves the water resistance of the two and the stability of the stent[12]. The disadvantages of CS include poor physiological biodegradability and low mechanical properties, while the disadvantages of PVA are low dehydration rates and high swelling degrees[13-14]. Therefore, the combination of PVA and CS could improve the properties of each other. Adding SS could provide high mechanical properties, low dehydration rates and good swelling performance to composite hydrogels[15].
The aim of this work is to investigate the effect of SS on swelling characteristics and thermal stability of produced PVA/CS/SS composite hydrogels for antibacterial wound dressings.
1 Experiments
1.1 Materials
PVA (a molecular weight of 60 kDa), CS (a medium molecular weight of 190-310 kDa, a deacetylation degree of 75%-85%), SS and deionized water were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd, China.
1.2 Preparation of PVA/CS/SS composite hydrogel
PVA colloid dispersions with a weight percentage of 14% were obtained by dissolving PVA powder at 90 ℃ in deionized water for 2-3 h under vigorous stirring, while CS was dissolved at 50 ℃ in CH3COOH aqueous solution at a weight-volume concentration of 2%. The CS dispersion was slowly added into the PVA colloid dispersion at 50 ℃ (the weight ratio of PVA to CS is 20∶1), which was constantly stirred by a magnetic stirrer. SS was then added, the weight ratio of PVA to SS was 3∶1 and the final dispersions were sonicated for 1 h at 50 ℃. Colloid PVA/CS/SS dispersions were cast onto petri dishes (h=5 mm) and the composite hydrogel was stored at -6 ℃ for 12 h and then 23 ℃ for 4 h. The freeze-thaw cycle was performed twice. The schematic diagram of the hydrogel formation and the molecular structures of the PVA, CS and SS are shown in Fig. 1.
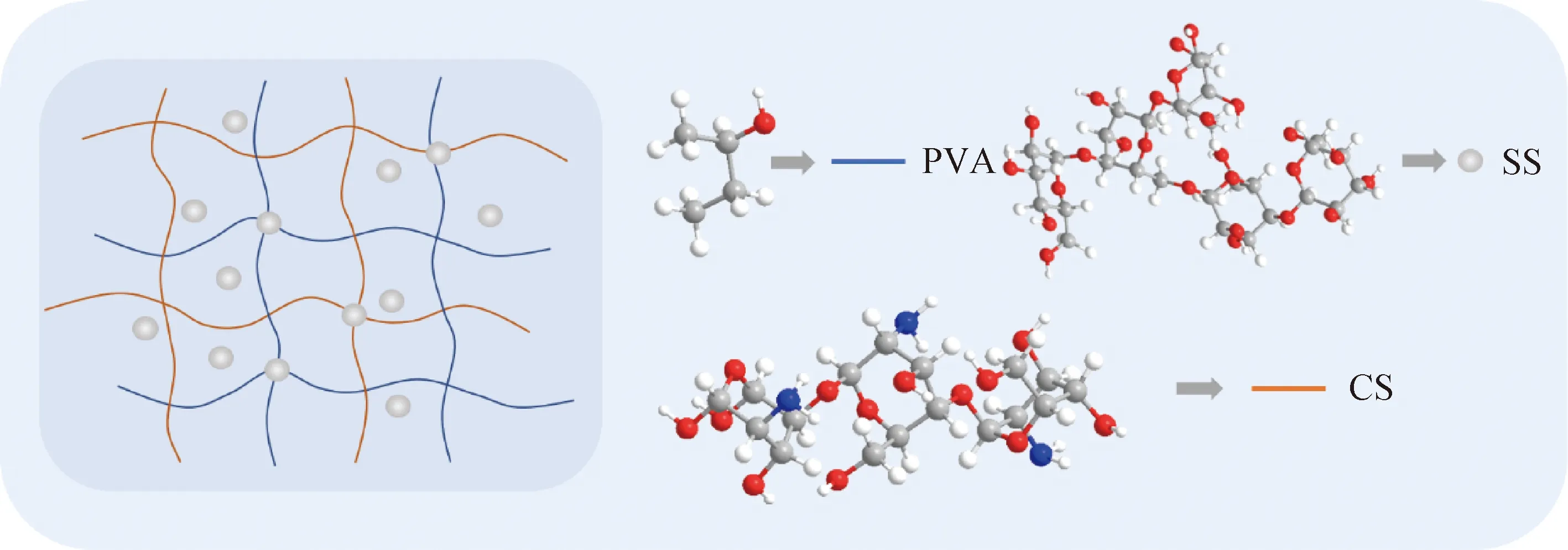
Fig. 1 Schematic illustration of PVA/CS/SS composite hydrogel and molecular structures of PVA, CS and SS
1.3 Characterization of hydrogels
1.3.1Morphologytest
The morphology characterization was performed by a metallographic microscope (BH200M, Huiguang Technology Co., Ltd., Suzhou, China).
1.3.2Fouriertransforminfrared(FTIR)spectroscopy
The FTIR spectra of PVA granules, CS, SS, PVA/CS composite hydrogels, and PVA/CS/SS composite hydrogels were characterized by an FTIR spectrometer (PerkinElmer Spectrum Two, PerkinElmer Co., Ltd., USA) with the testing range of 4 000-600 cm-1.
1.3.3Thermogravimetricanalysis(TGA)
In order to evaluate the mass loss of the material with temperature change, the TGA was carried out by a TGA equipment (Model 4000, PerkinElmer Co., Ltd., USA) in a nitrogen atmosphere, with a flow rate of 50 mL/min, a heating rate of 10 ℃/min and a temperature ranging from 30 ℃ to 800 ℃.
2 Results and Discussion
2.1 Morphology of hydrogels
Figure 2(a) shows the surface and the cross-sectional images of PVA/CS composite hydrogels and PVA/CS/SS composite hydrogels. It can be seen that the inside of the PVA/CS/SS composite hydrogel is relatively uniform, and the surface of the PVA/CS/SS composite hydrogel is smooth and dense, indicating that the components are fully dissolved. Figure 2(b) is the freezing and thawing images during the preparation process. The PVA/CS/SS composite hydrogel is white in the frozen state, and becomes translucent after thawing. Figures 2 (c) and (d) show the stretching, swelling, and drying states of PVA/CS composite hydrogels and PVA/CS/SS composite hydrogels. The volume of the hydrogel increases significantly after water absorption, the shape changes from contraction to flatness, and the color transforms from light yellow to colorless. A favorable stretched morphology indicates that the PVA/CS/SS composite hydrogel has better morphological stability than the PVA/CS composite hydrogel, as shown in Fig. 2(c). Figure 2(e) shows that both hydrogels turned white after being immersed in deionized water for three days.
2.2 FTIR spectra of hydrogels
FTIR spectra of PVA [shown in Fig. 3(a)] show that the characteristic peak of O—H in PVA is at 3 286 cm-1, the absorption peak of aliphatic saturated C—H asymmetric stretching vibration is at 2 933 cm-1, and the absorption peak of C—C skeleton vibration is at 1 092 cm-1. For CS, it has characteristic stretching peaks of the stretching vibration of —NH2and —OH groups at 3 355 cm-1, the stretching vibration of C—H at 2 785 cm-1, the bending vibration of N—H at 1 655 cm-1, the bridge —O stretching vibration at 1 149 cm-1and C—O stretching vibration at 1 069 cm-1[shown in Fig. 3(b)]. The absorption peak at 3 307 cm-1shown in Fig. 3(c) is attributed to the stretching vibration of O—H groups in SS, the absorption peak at 2 926 cm-1is attributed to the methylene C—H bond stretching vibration, the absorption peak at 996 cm-1is attributed to the functional group vibration of the pyranose ring skeleton C—O—C, and the absorption peaks at 860, 763 and 700 cm-1are attributed to the glucose six-membered ring skeleton vibration. The characteristic peaks of the PVA/CS composite hydrogel and the PVA/CS/SS composite hydrogel at 1 069 cm-1(C—O), 1 149, 1 655, 2 933 and 3 286 cm-1(O—H) correspond with the existence of both PVA and CS [shown in Fig. 3(d)]. The characteristic peak of the PVA/CS/SS composite hydrogel at 2 962 cm-1corresponds with the existence of SS in it [shown in Fig. 3(e)].

Fig. 2 Morphology of hydrogels: (a) under microscopes; (b) PVA/CS/SS composite hydrogels during forming process (including freezing and thawing); (c) in stretching state; (d) PVA/CS/SS composite hydrogels in swelling and drying states; (e) images after three days of immersion
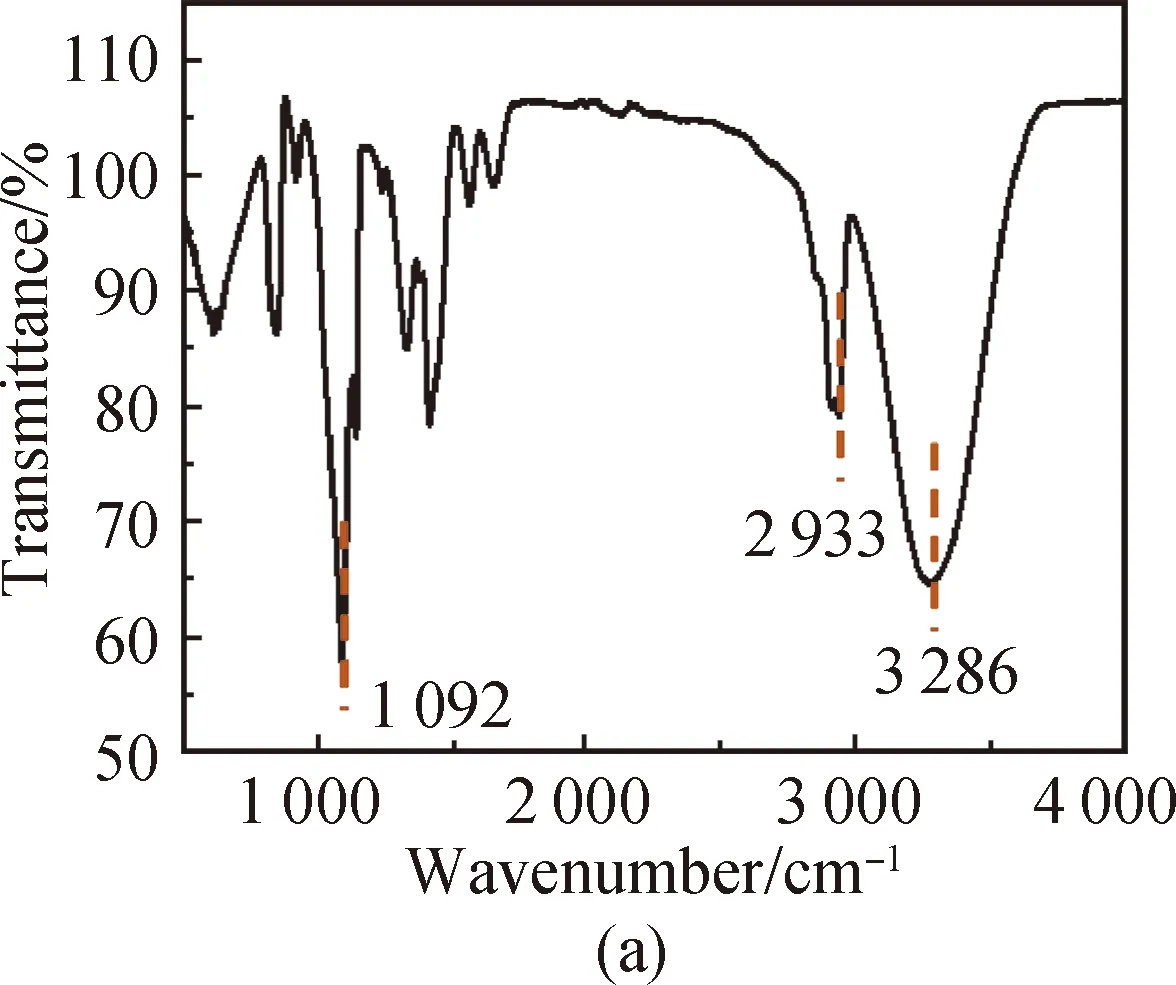
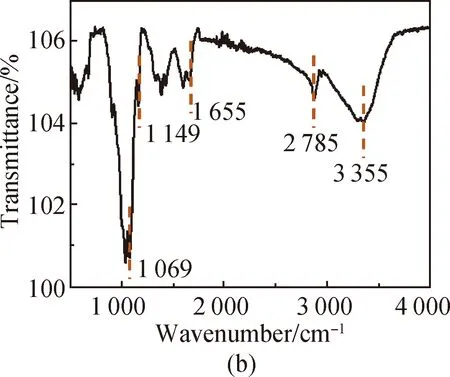
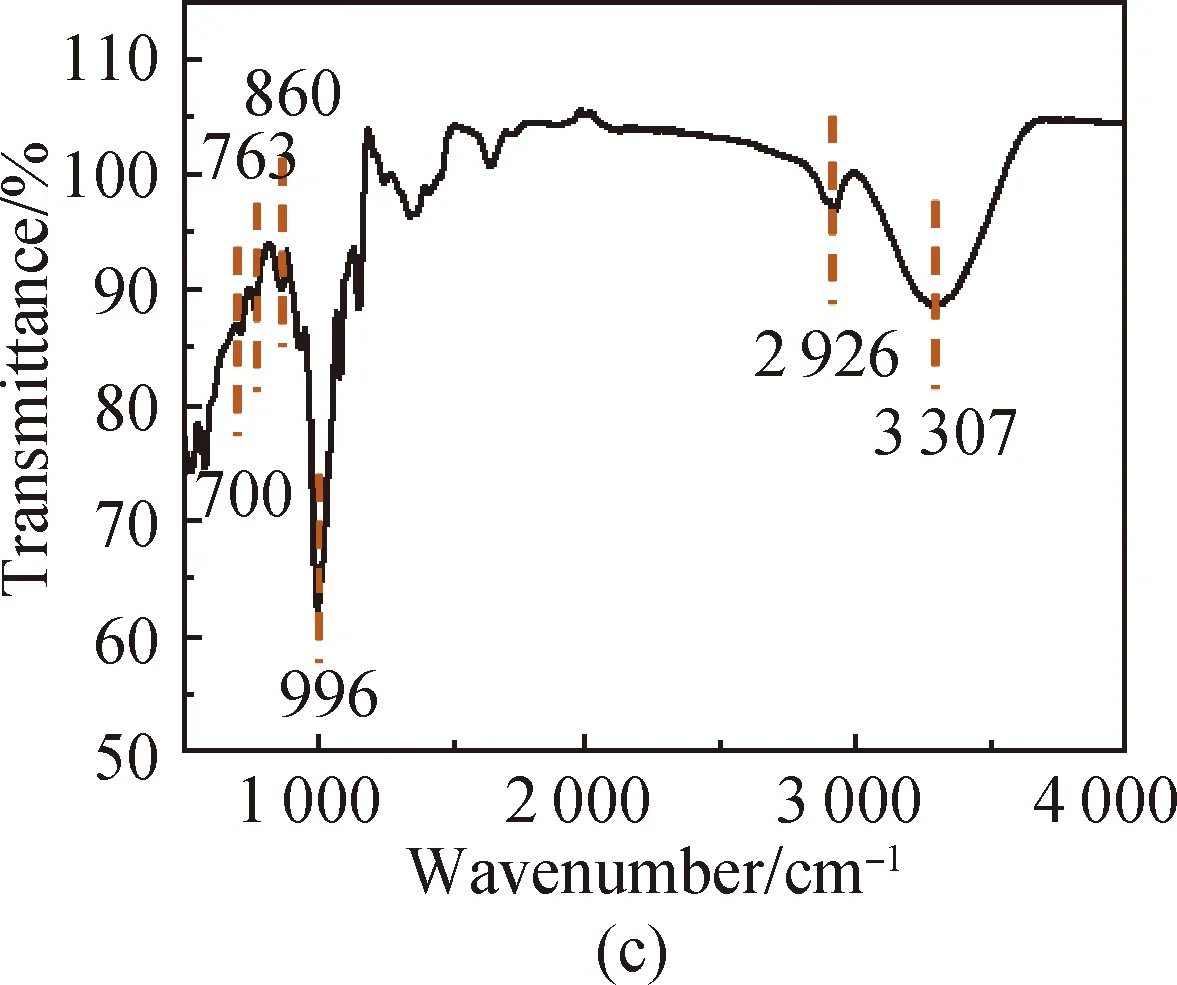
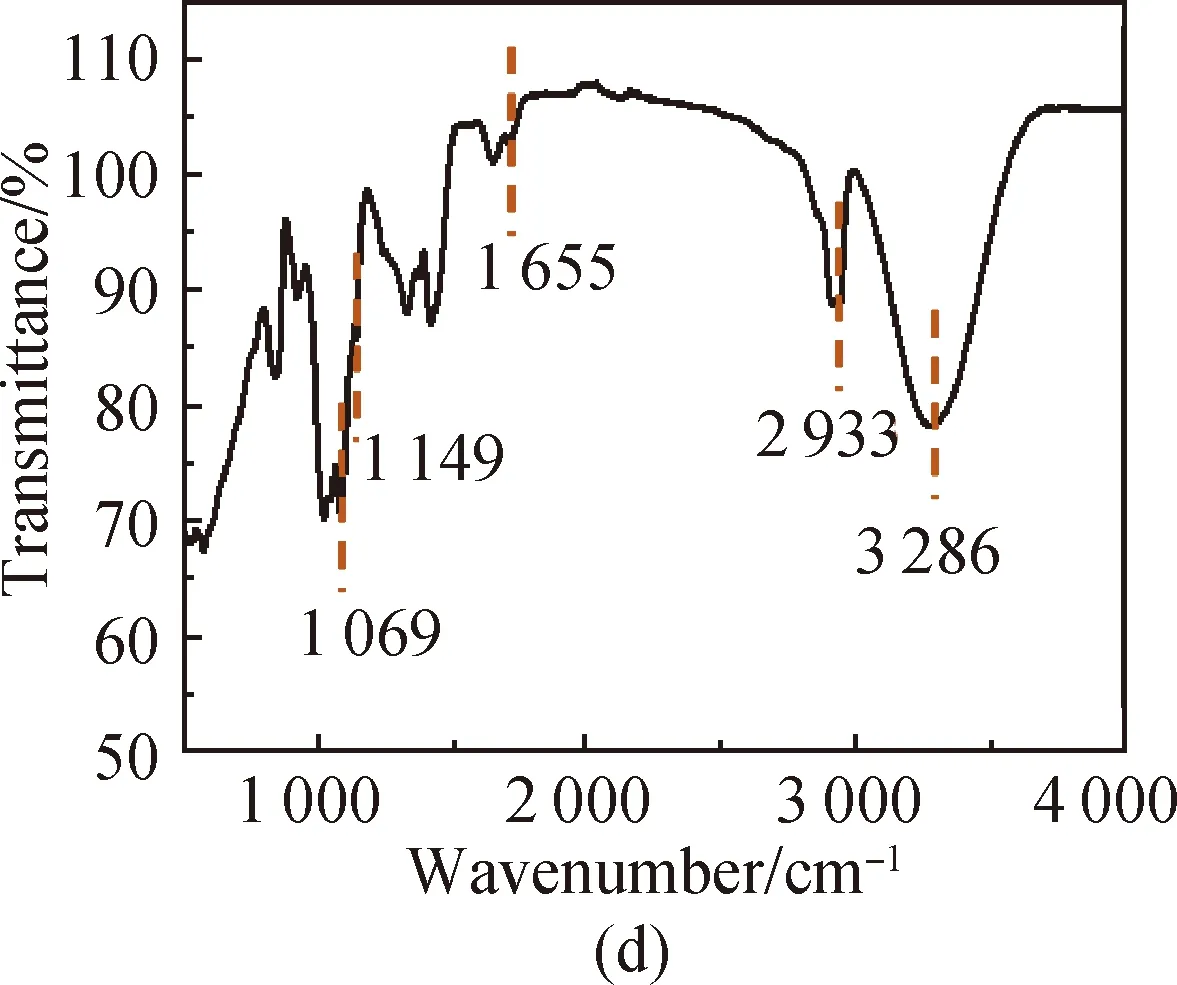
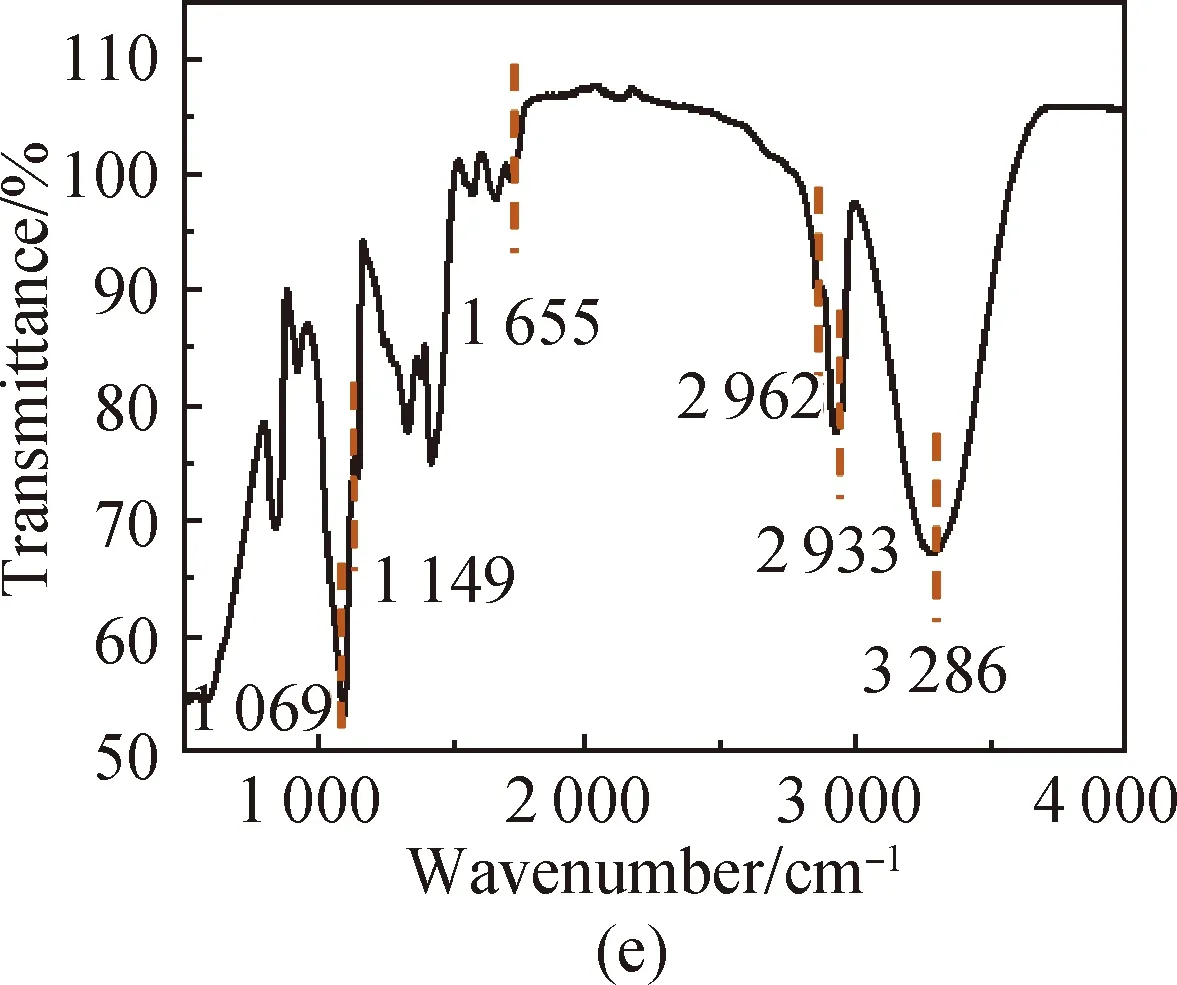
Fig. 3 FTIR spectra of (a) PVA; (b) CS; (c) SS; (d) PVA/CS composite hydrogel; (e) PVA/CS/SS composite hydrogel
2.3 Thermal stability of hydrogels
The thermograms of the PVA/CS composite hydrogel and the PVA/CS/SS composite hydrogel are shown in Fig. 4. The TGA curves of the fabricated PVA/CS/SS composite hydrogels indicate the presence of a weight loss of about 10% at 200 ℃ which is attributed to the loss of water molecules. The second weight loss stage is between 300 ℃ and 450 ℃ and the weight loss is about 70%. This step can be assigned to the cleavage of side chains of the polymeric fiber. The third stage of weight loss ranging from 450 ℃ to 500 ℃ is attributed to the cleavage of nanofibers backbone. The total weight loss is about 97% for the PVA/CS composite hydrogel and about 99% for the PVA/CS/SS composite hydrogel at 800 ℃. It can be seen from these results that both of the fabricated composite hydrogels possess good thermal stability. As shown in the derivative thermo gravimetry (DTG) diagram, the PVA/CS composite hydrogel has an obvious weight loss peak at 350-500 ℃, indicating that PVA decomposes in this temperature range. With the increasing content of SS, the weight loss of PVA/CS/SS composite hydrogel is maximal at 350-400 ℃, and this may be due to partial SS decomposition. Because there are intramolecular and intermolecular hydrogen bonds in the PVA/CS structure, the molecular chain structure is relatively stable. The PVA/CS/SS composite hydrogel reached a maximum decomposition rate of 0.945 9 mg/min at 321.8 ℃, and the PVA/CS composite hydrogel reached a maximum decomposition rate of 0.998 9 mg/min at 393.8 ℃.
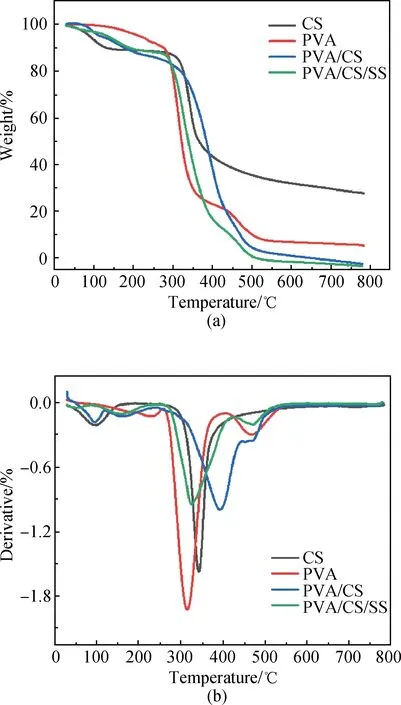
Fig. 4 Thermal behaviours: (a) TGA curves of CS, PVA, PVA/CS composite hydrogel and PVA/CS/SS composite hydrogel; (b) DTG curves of CS, PVA, PVA/CS composite hydrogel and PVA/CS/SS composite hydrogel
3 Conclusions
In summary, the PVA/CS/SS composite hydrogel is successfully fabricated to form a hydrogel tissue engineering scaffold, which facilitates the material exchange during the repair process. FTIR spectra show that PVA can form macromolecular hydrogen bonds with SS, reducing the bending vibration of —OH bending vibration and improving hydrolysis resistance. TGA and DTG analyses demonstrate a good thermal stability because of the strong intermolecular hydrogen bonds between CS and PVA molecules. The results indicate the PVA/CS/SS composite hydrogel is an efficient and potential stuff material in tissue engineering.
猜你喜欢
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Prediction of Logistics Demand via Least Square Method and Multi-Layer Perceptron
- Effect of Different Types of Structural Configuration on Air Distribution in a Compact Purification Device
- Measurement of International Competitiveness of Clothing Industry under the Background of Value Chain Reconstruction
- Counterexample of Local Fractional Order Chain Rule and Modified Definition of Local Fractional Order
- Deep Knowledge Tracing Embedding Neural Network for Individualized Learning
- Design and Application of Coordinator App Based on Needs Survey
