Research hotspot and frontier progress of cancer under the background of precision medicine
2020-01-15LiHongZhouYanLiQiLi
Li-Hong Zhou, Yan Li, Qi Li*
Research hotspot and frontier progress of cancer under the background of precision medicine
Li-Hong Zhou1, 2, Yan Li2, Qi Li1*
1Department of Medical Oncology and Cancer Institute, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China.2Department of oncology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
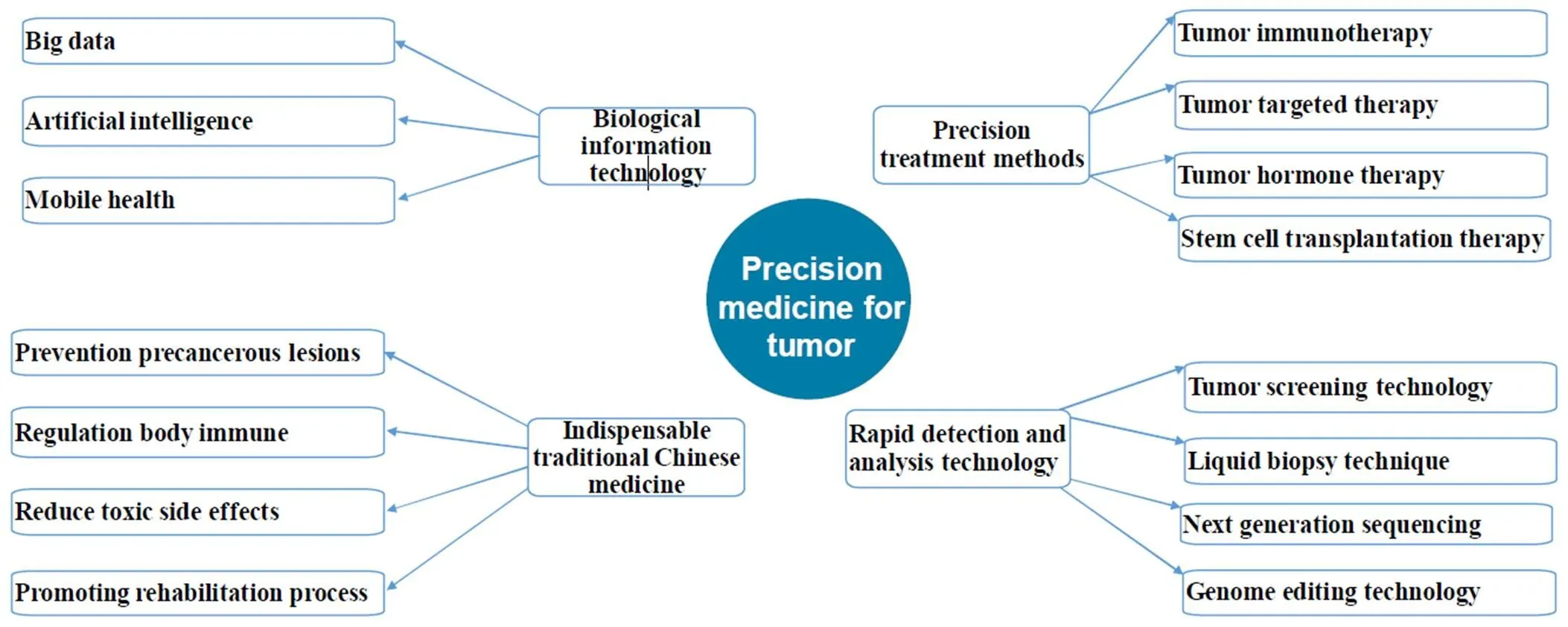
The timely introduction and rapid development of precision medicine have provided strong theoretical support and technical support for tumor research. The treatment methods have been developed from single to multiple; the research technology has been transformed from macro to micro; the treatment drugs have been updated from systemic chemotherapy to targeted therapy and immunotherapy, and cancer has changed from a highly lethal disease to a "chronic disease". Based on the current international cancer research hotspots and treatment frontiers, this paper takes stock from five aspects, namely, treatment methods, detection technology, new drug research and development, information data and traditional Chinese medicine, with a view to "from the point to the surface", "from the outside to the inside", and "the combination of Chinese and western", so as to explore the overall picture of cancer treatment and research.
Precision medicine, Cancer, Treatment methods, Detection technology, Traditional Chinese medicine
This paper takes stock from five aspects, namely, treatment methods, detection technology, new drug research and development, information data and traditional Chinese medicine, with a view to "from the point to the surface", "from the outside to the inside", and "the combination of Chinese and western", so as to explore the overall picture of cancer treatment and research.
Precision medicine is consistent with the treatment philosophy and thought of traditional Chinese medicine. The three factors of "time, place and people" and the dialectical treatment thought of "different treatment for different diseases and the same treatment for different diseases" all reflect the diagnosis and treatment thought of precision medicine.
Background
In January 2015, the US government put forward the "Precision Medicine Initiative", and the concept of precision medicine quickly entered the public vision and international stage, becoming a hot topic of academic concern. Precision medicine is defined as "a new approach to disease prevention and treatment that takes into account differences in an individual's genes, environment and lifestyle" by the National Institutes of Health [1]. The purpose of the "Precision Medicine Initiative" is to transform the concept of precision medicine into scientific evidence for clinical practice, thus ushering in a new era of medicine [1].
The proposal of "Precision Medicine Initiative" is closely related to cancer. Cancer has the highest case-fatality rate in the world, rising with age, and is characterized by "unexplained drug resistance, genetic heterogeneity of tumors, failure to adequately assess efficacy and tumor recurrence.". Precision medicine is a kind of individualized medicine, which can be based on the characteristics of the cancer itself, through the bio-information database, the patient's individualized testing techniques, and the great data analysis techniques, and the big data analysis, to create individualized treatment programs for different patients, which make the tumor treatment appear to be "individualized", "precision" and "optimal".
Precision and innovative tumor treatment methods
In addition to the three main treatment methods of operation, radiotherapy and chemotherapy, the treatment methods of immunotherapy, targeting therapy, hormone therapy and stem cell transplantation have emerged in recent years and has been developed in a single, multi-directional, wide-ranging, and all-round way, in the best of times.
Tumor immunotherapy
Tumor immunotherapy refers to the body by increasing the role of immunity in tumor immune response. The innate immune system produces direct immune cells, which make the body resist tumors, inflammation or other diseases and protect the human body. Adaptive immune system resists specific threats through antigen specific lymphocytes and forms immune memory [2]. Tumor cells can destroy the immune system and turn immune regulation into a favorable mode for tumor cells by influencing the antigen presentation process of the body, destroying the pathways controlling T cell inhibition and activation, collecting immunosuppressive cells and releasing immunosuppressive active factors and other mechanisms [3, 4]. Tumor immunotherapy aims at these mechanisms to develop drugs in the form of antibodies, peptides, proteins, small molecules, cytokines, oncolytic viruses, as well as bispecific molecules and various cellular therapies.
Tumor targeted therapy
Tumor targeted therapy is targeted at specific oncogenic sites at the cellular and molecular level and can be a protein molecule (such as epidermal growth factor receptor [6,7], human epidermal growth factor receptor 2 [8], vascular endothelial growth factor [9–11] and platelet derived growth factor receptor [12, 13] inside tumor cells or a gene fragment (such as cytospecific moroni leukemia toxin insertion site 1 [14]) to design relevant therapeutic drugs. When drugs enter the body, they will specifically select the carcinogenic site to combine with the effect, so that the tumor cells will die specifically without affecting the normal tissue cells around the tumor, thereby improving the efficacy of colorectal cancer, lung cancer, gastric cancer, breast cancer, melanoma, lymphoma and other cancers [15] to reduce toxic and side effects.
Tumor hormone therapy
Tumor hormone therapy (Hormone therapy also called endocrine therapy) refers to a treatment method for slowing or preventing tumor growth. The occurrence and development of tumors are related to the imbalance of hormone secretion in vivo. In the treatment, some hormones or anti-hormone substances can be applied to change the conditions on which tumor growth depends, so as to inhibit the growth of tumors. At present, the clinical application of more hormone treatment options are: (1) thyroid cancer with thyroxine treatment of thyroid cancer [16]; (2) sex hormones and hormone resistance drugs in the treatment of breast cancer [17], prostate cancer [18], endometrial cancer [19]; (3) adrenal cortical hormone and chemotherapy combined application to enhance the effect of chemotherapy, reduce the toxic side effects of chemotherapy. At the same time, endocrine therapy for leukemia [20], malignant lymphoma [21], malignant melanoma [22], ovarian cancer [23] and kidney, seminal vesicle tumor has a certain therapeutic effect.
Stem cell transplantation therapy
Peripheral blood stem cell transplantation has become an effective method to treat solid tumors such as malignant hematopathy, lymphoma and mammary tumor, especially multiple myeloma [24], malignant lymphoma [25] and breast cancer [26]. Stem cell transplantation does not have a direct anti-tumor effect, but can play an anti-tumor role by combining radiotherapy, chemotherapy or concurrent radiotherapy and chemotherapy [27, 28]. Stem cell transplantation includes autologous stem cell transplantation and allogeneic stem cell transplantation [27]. Autologous stem cell transplantation uses the patient's own bone marrow or blood, while allogeneic hematopoietic stem cell transplantation uses bone marrow or blood from a relative donor.
Rapid detection and analysis technology
Clinical detection and analysis of tumors provides strong support and help for the discovery, treatment and prognosis of tumors. Conventional tumor detection and analysis techniques include blood detection, ultrasonic examination, X-ray scanning (including molybdenum target), CT or MRI scanning, endoscopy, radionuclide detection and others, which have been able to provide a strong guarantee for the detection and treatment of clinical tumors. More and more new concepts, technologies and methods have been applied in the detection and analysis of tumors, providing an important technical guarantee for the early detection, diagnosis and treatment of clinical tumors.
Tumor screening technology
Tumor screening refers to the screening of persons with cancer who are actually diagnosed with the disease through simple, convenient and high-throughput screening methods in a large number of "normal" people. Screening through further examination and diagnosis provides information about early clinical intervention and timely treatment. Screening has become an important method of early detection of cancer. The United States recommends screening for: cervical cancer [29], breast cancer [30], colorectal cancer [31], prostate cancer [32]; Japan leads the world in gastric cancer screening [33]; Screening for esophageal, hepatocellular and nasopharyngeal cancers in China has been characterized [34]. Currently, common and effective tumor screening methods include: molybdenum target screening for breast cancer; HPV detection and screening for cervical cancer; screening colorectal cancer with fecal occult blood and colonoscopy; low-dose spiral CT screening for lung cancer; slpha fetoprotein and abdominal ultrasound; screening for liver cancer; gastroscope screening for gastric cancer and esophageal cancer.
Liquid biopsy technique
Liquid biopsy is a kind of method to monitor the status of the tumor in the body or organs for transplant patients through gathering body fluids, including blood, saliva, sweat, and discharge, etc. to analysis of circulating tumor cells, plasma free DNA or exosome. Liquid biopsy has the characteristics of non-invasive, comprehensive, accurate and real-time, and plays an important role in the clinical treatment and research guidance of lung cancer [35], colorectal cancer [36], gastric cancer [37], liver cancer [38], lymphoma [39], myeloma [40] and other malignant tumors.
Next generation sequencing
The next generation sequencing is characterized by high throughput, including whole genome sequencing, whole exome sequencing and targeted region sequencing, which have the advantages of high throughput, high accuracy and abundant information. It has been applied in noninvasive prenatal screening, tumor gene testing, genetic disease diagnosis, drug guidance and other clinical fields. The FDA had approved Foundation One CDx47, Foundation Focus ™ CDx BRCA, Oncomine DX Target Test, Extended the RAS Panel, MSKCC - IMPACT 5 NGS in vitro diagnostic products, to keep up with the latest progress in cancer research, to get more benefit from the latest treatment approaches for patients with tumor.
Genome editing technology
Genome Editing Technology refers to the ability to “edit” target genes by means of technical means and methods to achieve knockout, addition and transformation of specific gene DNA fragments. The first generation of gene editing technology, represented by ZFN (zinc-finger nucleases) and TALEN (transcription activator-like effector nucleases), has shown great potential in basic research, gene therapy and genetic improvement. The second-generation gene editing technology represented by CRISPR/Cas9 can target and repair tumor suppressor genes, restore the function and activity of tumor suppressor genes, inhibit tumorigenesis, break immune tolerance, and increase the ability of T cells to recognize tumor cells; It is applied to the construction of gene-oriented knockout animal models [53–56]. Gene editing technology has broad prospects in the application, development, treatment and prognosis of tumor treatment, and has far-reaching implications for future cancer treatment research.
Update iterative new drug for cancer treatment
In recent years, a prominent feature of tumor research field is that it is guided by clinical treatment and patient demand. With the continuous update of tumor treatment-related drugs and the continuous iteration of similar drugs, it gradually forms a complete research and development process of new tumor clinical drugs for laboratory research and development, clinical trials, department supervision and clinical application.
Monoclonal antibody-based drug
Antibody (Antibody), is produced by a single B lymphocyte clone highly uniform, only for a specific antigen epitope of Antibody, including monoclonal Antibody and polyclonal Antibody. Clinically, the antibody drugs for tumor treatment are mainly monoclonal antibody (also known as monoclonal antibody), which has gone through four stages: mouse monoclonal antibody, chimeric monoclonal antibody, humanized monoclonal antibody and whole-human monoclonal antibody. Currently, there are mainly immune checkpoint inhibitors (e.g. Ipilimumab [57][58], Tremelimumab [59], Nivolumab [60], Pembrolizumab [61]), epidermal growth factor receptor inhibitors (cetuximab [62], gefitiinib [63], erlotinib [64], etc.), vascular endothelial growth factor and receptor inhibitors (bevacizumab [65], sunitinib [66], sorafenib [67], etc.), human epidermal growth factor 2 inhibitors (trastuzumab [47], partuzumab [15], etc.), and CD20 inhibitors (rituximab [39], etc.) can be directly used in the diagnosis, prevention, treatment and immune mechanism research of human diseases. It opens up a broad prospect for the immunological diagnosis and treatment of human malignant tumor.
CAR-T based drugs
Chimeric Antigen Receptor t-cell immunotherapy (CAT-T) is a method for the treatment of tumor cells by artificially synthesizing T cells to express Chimeric proteins which can recognize tumor antigens and then delivering them back to the patient for the elimination of tumor cells after in vitro amplification.CAT-T immunotherapy experienced the first generation (with CD8/CD3γ as the extracellular domain) in 2006 [68], the second generation (adding a CD28 or 4-1BB co-stimulation signal on the basis of CD8/CD3 gamma activation) in 2009 [69] and the third generation (more than or equal to three stimulus signals) currently in clinical use [70]. In 2017, as a treatment drug for CAT-T therapy, FDA approved novartis's Kymriah (tisagenlecleucel-T, CTL019) for children and adolescents with recurrent or refractory acute B lymphocytic leukemia (B-ALL). Gilead's Yescarta (Axicabtagene Ciloleucel) was used in adult patients with recurrent or refractory large b-cell lymphoma (LBCL) who had previously received second-line or multiline systemic therapy. Car-t therapy has been widely studied in hematologic tumors, but its application and research in solid tumors are few [71–74]. Therefore, CAT-T therapy has great potential and broad application prospects.
TCR-T based drugs
T Cell Receptor chimeric T Cell therapy (tcr-t) is to transfer tumor antigen-specific TCR genes into autologous lymphocytes through the carrier and then back into the patient's body to kill tumor cells. This TCR chimeric t-cell can produce a large number of tumor antigen-specific CTL cells in vitro culture, exert cytotoxic effects, and enhance the elimination of tumor cells mediated by the immune system after being returned to the body, targeting and killing tumor cells specifically. In 2006, the first clinical trial of tcr-t for melanoma was conducted [75], followed by clinical trials for melanoma [76], colorectal cancer [77], synovial sarcoma [78], metastatic cervical cancer, esophageal cancer and urinary tract epithelial cancer [79], and the rapid progression of tcr-t-cell therapy, could be an important way to treat malignant tumors.
Cancer vaccines
Cancer Vaccines introduce tumor antigens or genes expressing tumor antigens into a patient to activate the immune system and induce cellular and humoral immune responses to control or eliminate tumors [80]. Tumor vaccine includes tumor therapeutic vaccine [81] and preventive vaccine [78]. Provenge was approved by FDA for treatment of advanced prostate cancer in 2010, which is an important milestone in the immunotherapy of tumor vaccines [82] and provides reference for the development of other tumor vaccines [83, 84]. In 2017, Catherine Wu's team at the dana-farber cancer research institute in the United States developed a personalized cancer vaccine that can mobilize the immune system to target tumor mutated proteins in patients, successfully preventing the risk of cancer recurrence in patients with skin cancer [85]. A team of Ugur Sahin, a professor at the university of Johannes Gutenberg medical center in Germany, found similar results in patients with advanced melanoma [86, 87].
Leading the forefront of biological information technology
Cancer research has become an important topic in the field of natural science. Scientists from various fields such as medicine, biology, pharmacy, information science and statistics have conducted a large number of interdisciplinary studies, ranging from animal tissue level to cellular and molecular level, from the stage of clinical treatment to the stage of basic mechanism, from small-scale individual exploration to big data platform analysis. With the highly developed information society and science and technology, various new bioinformatics means and concepts have been gradually introduced into tumor research and treatment application, providing important support and rich connotation for tumor treatment.
Big data
The popularization of the internet leads human beings into the era of big data. The information storm brought by the internet is changing our life, work and thinking. In the field of biomedicine, big data is abstractly defined as "biomedical big data", "health and medical big data", "medical big data" [88]. It provides an important platform for cancer prevention and treatment research, such as "Human Genome Project" [89–90], "Human Proteome Project (HPP)", "Cancer Genome Atlas" [91] and "The Surveillance, Epidemiology, and End Results of the US national cancer institute" [92] and others. Big data can not only strengthen the integration of clinical information with molecular biology, genomics, metabolomics and other disciplines [93], but also help people discover the secrets behind diseases, improve the research efficiency of targeted drugs, immunotherapy and comprehensive treatment [94], and achieve "same disease but different treatment" and "same treatment of different diseases" for tumors.
Artificial intelligence
Artificial intelligence (AI) refers to the intelligence shown by machines made by human beings, which enables computers to simulate certain thinking processes and intelligent behaviors of human beings. It has wide applications in psychology, philosophy, linguistics, biomedical fields and other disciplines. IBM's Deep Blue, Google's AlphaGo and IBM's Watson are all applications of artificial intelligence in various aspects. In the field of tumor research, AI technology has been widely used in tumor research. In 2016, Watson was used to study thousands of cases of resistance to cancer drugs, hoping to develop a new generation of cancer drugs and therapies by clarifying how resistance develops [95]. In 2017, the accuracy rate of using AI to diagnose colorectal cancer in Japan was as high as 86% [96], and the use of AI in cancer prevention and treatment has become more and more in-depth.
Mobile health
Mobile health is to provide medical services and information through the use of mobile communication technology. From appointment registration, payment follow-up, post-discharge management to medical information acquisition, more and more mobile medical products have come into our lives. In 2013, researchers evaluated 295 mobile apps for cancer [97] and found that understanding of cancer, education information of cancer, early detection and prevention of cancer have become important contents of mobile cancer medicine. In China, mobile medical applications such as "cancer doctor", "tumor chemotherapy assistant" and "tumor chemotherapy manual" provide a very powerful platform for cancer clinicians.
Indispensable traditional Chinese medicine
Traditional Chinese medicine (TCM) refers to the medical science based on the guidance of TCM theory, which has played an important role in safeguarding people's physical health and population reproduction in China's 5,000 years of development. Precision medicine is consistent with the treatment philosophy and thought of traditional Chinese medicine. The three factors of "time, place and people" and the dialectical treatment thought of "different treatment for different diseases and the same treatment for different diseases" all reflect the diagnosis and treatment thought of precision medicine. TCM plays an active role in the comprehensive treatment of malignant tumors and other major and difficult diseases, especially in the prevention of precancerous lesions of tumors, the recuperation of toxic and side effects of chemotherapy, the delay and prevention of postoperative recurrence and metastasis, and postoperative rehabilitation of tumors.
Prevention precancerous lesions
Precancerous lesions is an early stage of cancer, cancer The development process of cancer include precancerous lesions, carcinoma in situ and infiltrating carcinoma three stages. Precancerous lesions are clinically a broad concept, which means to continue to develop certain lesions that are likely to be cancerous, such as lung nodules [98], thyroid nodules [99], chronic atrophic gastritis [100], liver cirrhosis [101], adenomatous intestinal polyps [102], borneas [103], certain benign tumors (hysteromyoma [104], mammary adenoma [105]), and the like. TCM has a good therapeutic effect on the blocking and prevention of precancerous lesions, and can play an all-round, multi-channel and multi-target regulatory role in the treatment of precancerous lesions. It is found that TCM plays an obvious role in preventing the occurrence and development of gastric cancer [106–108], colorectal cancer [109, 110], liver cancer [111], breast cancer [112] and other common malignant tumors. The idea of "treating the disease before cure" in TCM is the concept of preventing the occurrence and development of diseases, and "preventing the disease before cure, namely preventing the disease from changing" can play an active guiding role in the prevention and treatment of precancerous lesions.
Regulation body immune
Immunotherapy has become the most effective method for curing cancer [113, 114], which mainly means to kill tumor cells by activating the body's own immune system [115]. One of the main functions of TCM in the treatment of tumors is to regulate the body's immunity [116] and enhance the body's autoimmune function to achieve the purpose of anti-tumor [117, 118]. The main mechanisms of TCM in the regulation of the body's immune response to tumor include: (1) TCM regulates the body's anti-tumor immune response, such as regulating major histocompatibility complex, Fas/FasL [119], Bcl-2-associated x protein / B-cell lymphoma 2 [120] and inhibiting of stem cells [121, 122]; (2) TCM reverses the immune suppression of the body and regulates the anti-tumor function of immune cells, such as reversing the immune phenotype of T lymphocytes and tumor-related macrophages and regulating Th1/Th2 factor. TCM plays a dual and multi-target role in regulating the immune microenvironment of tumors in the body. It can improve the immune monitoring and clearance function of the body, enhance the killing ability of the body to tumor cells, break the immune tolerance of the body, reverse the immune escape of tumor cells, and enhance the anti-tumor immune efficiency of the body. It is an important method for the comprehensive treatment of tumors.
Reduce toxic side effects
The non-negligible problems in the integrated treatment of tumor radiotherapy, chemotherapy, immunotherapy, target treatment are the adverse side effects of vomiting, alopecia, hands and hands syndrome, and bone marrow rejection caused by the treatment. The toxic and side effects of the above treatment may become an important factor preventing cancer patients from receiving standard treatment, or even the primary reason for patients not to receive standard treatment. TCM therapy is characterized by "combination of disease and syndrome and holistic concept". Considering the overall treatment of patients and diseases, it is combined with radiotherapy and chemotherapy, targeted therapy and immunotherapy. While playing its anti-tumor role, TCM has a significant effect on alleviating the toxic and side effects of treatment on patients. It has been reported that Fufang Kushen Injection [123], Kangai Injection [124], Shenqi Fuzheng Injection [125], Xuefu Zhuyu Decoction [126], Fuzheng Kangai Formula [127], Lophatherum and Gypsum Decoction [128], etc., has a significant deceleration on the toxic and side effect of cancer chemotherapy and radiotherapy patient. Therefore, the comprehensive treatment for various toxic and side effects in the precise treatment by TCM is an indispensable and irreplaceable treatment method.
Promoting rehabilitation process
TCM has both drug and non-drug treatment methods, such as acupuncture (including electroacupuncture), moxibustion (including heat-sensitive moxibustion), Gongfu (Qigong, Taijiquan, eighteen methods of practicing Gongfu, Baduanjin, Wuqinxi, etc.) and massage. These non-drug treatment methods also play an important auxiliary role in the process of tumor treatment and rehabilitation process of tumor patients after surgery and drug treatment. Studies have shown that acupuncture combined with different acupoints has a significant effect on nausea and vomiting caused by chemotherapy [129]; acupuncture combined with western medicine has a definite effect on the treatment of polycystic ovary syndrome [130]; meridian balance method can alleviate chronic pelvic pain caused by gynecological tumors [131], electric acupoint stimulation has a preventive effect on lower limb thrombosis in elderly patients with malignant gastrointestinal tumor postoperative patients [132], Qigong has an improved effect on fatigue and sleep quality of non-hodgkinlymphoma chemotherapy patients [133], Taijiquan has an obvious alleviating effect on cancer-related fatigue of lung cancer chemotherapy patients [134]. It is believed that comprehensive treatment for clinical cancer patients has a good therapeutic effect and plays a positive and positive role in the later rehabilitation of cancer patients.
Summary and analysis
The proposal and implementation of the precision medicine initiative has brought modern medicine into an era of large-scale integration of medical data. Genomics, proteomics, metabolomics, epigenetics, microbiology, environment, behavior, clinical examination, TCM and other information have led to exponential growth of medical data. The knowledge system of precision medicine has exceeded the scope carried by individual doctors, and the speed of knowledge update has also exceeded the capacity of traditional medical education system. The clinical treatment of precision medicine for tumors is increasingly dependent on information technology.
In the context of precision medicine, how to effectively process, integrate, analyze and utilize the health data of cancer patients and make appropriate clinical and production decisions has become a problem that doctors, hospitals, pharmaceutical companies and regulatory departments need to think about and face. Under the background of precision medicine, the treatment of tumors still has a long way to go. The development environment of precision oncology is still immature. The development direction is still unclear. The achievements in clinical practice are also limited. It is necessary to understand and grasp the goals and trends of precision medicine for cancer, conduct in-depth research on the mechanism of tumor development and treatment, better serve the academic, clinical and patient needs, and promote the progress of academic research and clinical treatment of cancer under the background of precision medicine.
1. Rasooly RS, Gossett DR, Henderson MK, et alHigh-throughput processing to preserve viable cells: a precision medicine initiative cohort program workshop. Biopreserv Biobank 2017, 15: 341–343.
2. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013, 39: 1–10.
3. Antonia SJ, Larkin J, Ascierto PA. Immuno-oncology combinations: a review of clinical experience and future prospects. Clin Cancer Res2014, 20: 6258–6268.
4. Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 2012, 23: 6–9.
5. Kintzing JR, Filsinger Interrante MV, Cochran JR. Emerging strategies for developing next-Generation protein therapeutics for cancer treatment. Trends Pharmacol Sci 2016, 37: 993–1008.
6. Bertotti A, Papp E, Jones S, et alThe genomic landscape of response to EGFR blockade in colorectal cancer. Nature 2015, 526: 263–267.
7. Nathanson DA, Gini B, Mottahedeh J, et alTargeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 2014, 343: 72–76.
8. Monteiro Ide P, Madureira P, de Vasconscelos A, et alTargeting HER family in HER2-positive metastatic breast cancer: potential biomarkers and novel targeted therapies. Pharmacogenomics 2015, 16: 257–271.
9. Curtarello M, Zulato E, Nardo G, et alVEGF-targeted therapy stably modulates the glycolytic phenotype of tumor cells. Cancer Res 2015, 75: 120–133.
10. Funakoshi T, Lee CH, Hsieh JJ. A systematic review of predictive and prognostic biomarkers for VEGF-targeted therapy in renal cell carcinoma. Cancer Treat Rev 2014, 40: 533–547.
11. Han KS, Raven PA, Frees S, et alCellular adaptation to VEGF-Targeted antiangiogenic therapy induces evasive resistance by overproduction of alternative endothelial cell growth factors in renal cell carcinoma. Neoplasia 2015, 17: 805–816.
12. Cao Y: Multifarious functions of PDGFs and PDGFRs in tumor growth and metastasis. Trends Mol Med 2013, 19: 460–473.
13. Ho AL, Vasudeva SD, Lae M, et alPDGF receptor alpha is an alternative mediator of rapamycin-induced Akt activation: implications for combination targeted therapy of synovial sarcoma. Cancer Res 2012, 72: 4515–4525.
14. Wu Z, Min L, Chen D, et alOverexpression of BMI-1 promotes cell growth and resistance to cisplatin treatment in osteosarcoma. PLoS One 2011, 6: e14648.
15. Henricks LM, Schellens JH, Huitema AD, et alThe use of combinations of monoclonal antibodies in clinical oncology. Cancer Treat Rev 2015, 41: 859–867.
16. Karls S, Abikhzer G, Tamilia M, et alInterrupted 131I procedures for patients with differentiated thyroid cancer: comparing thyroxine withdrawal with recombinant thyrotropin preparation techniques. Clin Nucl Med 2017, 42: 247–249.
17. Ferreira AR, Palha A, Correia L, et alTreatment adoption and relative effectiveness of aromatase inhibitors compared to tamoxifen in early breast cancer: a multi-institutional observational study. Breast 2017, 37: 107-113.
18. Aguiar PN Jr, Barreto CMN, Gutierres BS, et alCost effectiveness of chemohormonal therapy in patients with metastatic hormone-sensitive and non-metastatic high-risk prostate cancer. Einstein 2017, 15: 349–354.
19. Lee WL, Yen MS, Chao KC, et alHormone therapy for patients with advanced or recurrent endometrial cancer. J Chin Med Assoc 2014, 77: 221–226.
20. Sato M, Harada M, Oishi H, et alVaginal stenosis after gonadotropin-releasing hormone agonist therapy during treatment for acute lymphoblastic leukemia. J Low Genit Tract Dis 2016, 20: e11–13.
21. Brown E. Hormone therapy linked to lower non-Hodgkin lymphoma risk. Womens Health 2014, 10: 235–236.
22. Botteri E, Stoer NC, Sakshaug S, et alMenopausal hormone therapy and risk of melanoma: Do estrogens and progestins have a different role? Int J Cancer 2017, 141: 1763–1770.
23. Voutsadakis IA. On adjuvant hormone therapy in epithelial ovarian cancer. J Clin Oncol 2016, 34: 2070–2071.
24. Mikhael JR. The role of autologous stem cell transplant in patients with multiple myeloma. Clin Adv Hematol Oncol 2017, 15: 604–606.
25. Brockelmann PJ, Zagadailov EA, Corman SL, et alBrentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma who are Ineligible for autologous stem cell transplant: A Germany and United Kingdom retrospective study. Eur J Haematol 2017, 99: 553–558.
26. Nieto Y, Shpall EJ. High-dose chemotherapy with autologous stem cell transplant for breast cancer: what have we learned 25 years later? Biol Blood Marrow Transplant 2012, 18: 3–5.
27. Duong N, Davis H, Robinson PD, et alMind and body practices for fatigue reduction in patients with cancer and hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2017, 120: 210–216.
28. Cessna JM, Pidala J, Jacobsen PB. Relationships between parenting self-efficacy and distress in parents who have school-aged children and have been treated with hematopoietic stem cell transplant or have no cancer history. Psychooncology 2016, 25: 339–346.
29. Greenwald ZR, El-Zein M, Bouten S, et alMobile screening units for the early detection of cancer: a systematic review. Cancer Epidemiol Biomarkers Prev 2017, 26: 1679–1694.
30. Expert Panel on Breast I, Mainiero MB, Moy L, et alACR appropriateness criteria((R)) breast cancer screening. J Am Coll Radiol 2017, 14: S383–S390.
31. McLeod M, Kvizhinadze G, Boyd M, et alColorectal cancer screening: how health gains and cost-effectiveness vary by ethnic group, the Impact on health inequalities, and the optimal age eange to screen. Cancer Epidemiol Biomarkers Prev 2017, 26: 1391–1400.
32. Lawrentschuk N, Daljeet N, Trottier G, et alAn analysis of world media reporting of two recent large randomized prospective trials investigating screening for prostate cancer. BJU Int 2011, 108: E190–195.
33. Hamashima C, Goto R. Potential capacity of endoscopic screening for gastric cancer in Japan. Cancer Sci 2017, 108: 101–107.
34. Shi JF, Dai M. Health economic evaluation of cancer screening in China. Zhonghua Yu Fang Yi Xue Za Zhi 2017, 51: 107–111.
35. Manicone M, Poggiana C, Facchinetti A, et alCritical issues in the clinical application of liquid biopsy in non-small cell lung cancer. J Thorac Dis 2017, 9: S1346–S1358.
36. Tarazona N, Cervantes A. Liquid biopsy: another tool towards tailored therapy in colorectal cancer. Ann Oncol 2017.
37. Shen J, Kong W, Wu Y, et alPlasma mRNA as liquid biopsy predicts chemo-sensitivity in advanced gastric cancer patients. J Cancer 2017, 8: 434–442.
38. Labgaa I, Villanueva A. Liquid biopsy in liver cancer. Discov Med 2015, 19: 263–273.
39. Provencio M, Rodriguez M, Cantos B, et alMRNA in exosomas as a liquid biopsy in non-Hodgkin Lymphoma: a multicentric study by the spanish lymphoma oncology group. Oncotarget 2017, 8: 50949–50957.
40. Almasi M, Sevcikova S, Penka M, et alBiobanking - the first step to successful liquid biopsy experiments. Klin Onkol 2017, 30: 9–12.
41. Lee JJ, Sholl LM, Lindeman NI, et alTargeted next-generation sequencing reveals high frequency of mutations in epigenetic regulators across treatment-naive patient melanomas. Clin Epigenetics 2015, 7: 59.
42. Ekblom R, Wolf JB. A field guide to whole-genome sequencing, assembly and annotation. Evol Appl 2014, 7: 1026–1042.
43. Griebel T, Zacher B, Ribeca P, et alModelling and simulating generic RNA-Seq experiments with the flux simulator. Nucleic Acids Res 2012, 40: 10073–10083.
44. Ong FS, Lin JC, Das K, et alTranslational utility of next-generation sequencing. Genomics 2013, 102: 137–139.
45. Moorcraft SY, Gonzalez D, Walker BA. Understanding next generation sequencing in oncology: a guide for oncologists. Crit Rev Oncol Hematol 2015, 96: 463–474.
46. Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 2016, 107: 1179–1186.
47. Fountzilas G, Giannoulatou E, Alexopoulou Z, et alTP53 mutations and protein immunopositivity may predict for poor outcome but also for trastuzumab benefit in patients with early breast cancer treated in the adjuvant setting. Oncotarget 2016, 7: 32731–32753.
48. Rucaparib approved for ovarian cancer. Cancer Discov 2017, 7: 120–121.
49. Swisher EM, Lin KK, Oza AM, et alRucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017, 18: 75–87.
50. Letovanec I, Finn S, Zygoura P, et alEvaluation of NGS and RT-PCR methods for ALK rearrangement in European NSCLC patients: Results from the ETOP Lungscape Project. J Thorac Oncol 2017, 13: 413–425.
51. Sepulveda AR, Hamilton SR, Allegra CJ, et alMolecular biomarkers for the evaluation of colorectal cancer: guideline from the American society for clinical pathology, college of American pathologists, association for molecular pathology, and American society of clinical oncology. J Mol Diagn 2017, 19: 187–225.
52. Mandelker D, Zhang L, Kemel Y, et alMutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA 2017, 318: 825–835.
53. Niu Y, Shen B, Cui Y, et alGeneration of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 2014, 156: 836–843.
54. Heckl D, Kowalczyk MS, Yudovich D, et alGeneration of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat Biotechnol 2014, 32: 941–946.
55. Matano M, Date S, Shimokawa M, et alModeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 2015, 21: 256–262.
56. Xue W, Chen S, Yin H, et alCRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 2014, 514: 380–384.
57. Nirschl CJ, Drake CG: Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res 2013, 19: 4917–4924.
58. Wang RF, Wang HY. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res 2017, 27: 11–37.
59. Heinrich B, Goepfert K, Delic M, et alInfluence of the oncolytic parvovirus H-1, CTLA-4 antibody tremelimumab and cytostatic drugs on the human immune system in a human in vitro model of colorectal cancer cells. Onco Targets Ther 2013, 6: 1119–1127.
60. Larkin J, Chiarion-Sileni V, Gonzalez R, et alCombined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015, 373: 23–34.
61. Aguiar PN Jr, Tadokoro H, Forones NM, et alMMR deficiency may lead to a high immunogenicity and then an improvement in anti-PD-1 efficacy for metastatic colorectal cancer. Immunotherapy 2015, 7: 1133–1134.
62. Trojan J, Klein-Scory S, Koch C, et alClinical application of liquid biopsy in targeted therapy of metastatic colorectal cancer. Case Rep Oncol Med 2017, 2017: 6139634.
63. Yap TA, Popat S. Toward precision medicine with next-generation EGFR inhibitors in non-small-cell lung cancer. Pharmgenomics Pers Med 2014, 7: 285–295.
64. Zhai H, Zhong W, Yang X, et alNeoadjuvant and adjuvant epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy for lung cancer. Transl Lung Cancer Res 2015, 4: 82–93.
65. Weinberg BA, Hartley ML, Salem ME. Precision medicine in metastatic colorectal cancer: relevant carcinogenic pathways and targets-PART 2: approaches beyond first-line therapy, and novel biologic agents under investigation. Oncology 2017, 31: 573–580.
66. Escudier B, Sharma P, McDermott DF, et alCheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol 2017, 72: 962–971.
67. Miyake H, Miyazaki A, Imai S, et alEarly tumor shrinkage under treatment with first-line tyrosine kinase inhibitors as a predictor of overall survival in patients with metastatic renal cell carcinoma: a retrospective multi-institutional study in Japan. Target Oncol 2016, 11: 175–182.
68. Lamers CH, Sleijfer S, Vulto AG, et alTreatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006, 24: e20–22.
69. Kochenderfer JN, Feldman SA, Zhao Y,et alConstruction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother 2009, 32: 689–702.
70. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013, 3: 388–398.
71. Maude SL, Frey N, Shaw PA, et alChimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014, 371: 1507–1517.
72. Maude SL, Teachey DT, Porter DL, et alCD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015, 125: 4017–4023.
73. Kochenderfer JN, Dudley ME, Kassim SH, et alChemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015, 33: 540–549.
74. Porter DL, Levine BL, Kalos M, et alChimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011, 365: 725–733.
75. Morgan RA, Dudley ME, Wunderlich JR, et alCancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006, 314: 126–129.
76. Burns WR, Zheng Z, Rosenberg SA, et alLack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood 2009, 114: 2888–2899.
77. Robbins PF, Morgan RA, Feldman SA, et alTumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011, 29: 917–924.
78. Tagliamonte M, Petrizzo A, Tornesello ML, et alAntigen-specific vaccines for cancer treatment. Hum Vaccin Immunother 2014, 10: 3332–3346.
79. Lu YC, Parker LL, Lu T, et alTreatment of patients with metastatic cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J Clin Oncol 2017, 35: 3322–3329.
80. Butterfield LH: Cancer vaccines. BMJ 2015, 350: h988.
81. Melero I, Gaudernack G, Gerritsen W, et alTherapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 2014, 11: 509–524.
82. Madan RA, Gulley JL. Sipuleucel-T: harbinger of a new age of therapeutics for prostate cancer. Expert Rev Vaccines 2011, 10: 141–150.
83. Cheever MA, Higano CS. Procenge (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 2011, 17: 3520–3526.
84. Riedmann EM. Two years of provenge. Hum Vaccin Immunother 2012, 8: 505.
85. Kaiser J. Personalized tumor vaccines keep cancer in check. Science 2017, 356: 122.
86. Kreiter S, Vormehr M, van de Roemer N, et alMutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015, 520: 692–696.
87. Kranz LM, Diken M, Haas H, et alSystemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature2016, 534: 396–401.
88. Filipp FV. Precision medicine driven by cancer systems biology. Cancer Metastasis Rev 2017, 36: 91–108.
89. Oliveira-Barros EG, Nicolau-Neto P, Da Costa NM, et alProstate cancer molecular profiling: the achilles heel for the implementation of precision medicine. Cell Biol Int 2017, 41: 1239–1245.
90. Green ED, Watson JD, Collins FS. Human genome project: twenty-five years of big biology. Nature 2015, 526: 29–31.
91. Han J, Puri RK. Analysis of the cancer genome atlas (TCGA) database identifies an inverse relationship between interleukin-13 receptor alpha1 and alpha2 gene expression and poor prognosis and drug resistance in subjects with glioblastoma multiforme. J Neurooncol 2018, 136: 463–474.
92. Wang X, Li X, Su S, et alMarital status and survival in epithelial ovarian cancer patients: a SEER-based study. Oncotarget 2017, 8: 89040–89054.
93. Restifo NP. A "big data" view of the tumor "immunome". Immunity 2013, 39: 631–632.
94. Hinkson IV, Davidsen TM, Klemm JD, et alA comprehensive infrastructure for big data in cancer research: accelerating cancer research and precision medicine. Front Cell Dev Biol 2017, 5: 83.
95. Movassagh R, Shor PW. Supercritical entanglement in local systems: counterexample to the area law for quantum matter. Proc Natl Acad Sci USA 2016, 113: 13278–13282.
96. Nosato H, Sakanashi H, Takahashi E, et alImage retrieval method for multiscale objects from optical colonoscopy images. Int J Biomed Imaging 2017, 2017: 7089213.
97. Bender JL, Yue RY, To MJ, et alA lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res 2013, 15: e287.
98. Wu F, Tian SP, Jin X, et alCT and histopathologic characteristics of lung adenocarcinoma with pure ground-glass nodules 10 mm or less in diameter. Eur Radiol 2017, 27: 4037–4043.
99. Chen L, Zhang J, Meng L, et alA new ultrasound nomogram for differentiating benign and malignant thyroid nodules. Clin Endocrinol 2018, 90: 351–359.
100. Kaji K, Hashiba A, Uotani C, et alGrading of atrophic gastritis is useful for risk stratification in endoscopic screening for gastric cancer. Am J Gastroenterol 2019, 114: 71–79.
101. Lendvai G, Szekerczes T, Gyongyosi B, et alMicroRNA expression in focal nodular hyperplasia in comparison with cirrhosis and hepatocellular carcinoma. Pathol Oncol Res 2018. [Epub ahead of print]
102. Shah R, Jones E, Vidart V, et alBiomarkers for early detection of colorectal cancer and polyps: systematic review. Cancer Epidemiol Biomarkers Prev 2014, 23: 1712–1728.
103. Morkin MI, Kapadia MK, Laver NV. Pigmented spindle cell nevus of reed of the eyelid. Ocul Oncol Pathol 2017, 3: 176–180.
104. Kaneda H, Terao Y, Matsuda Y, et alThe utility and effectiveness of an internal iliac artery balloon occlusion catheter in surgery for large cervical uterine fibroids. Taiwan J Obstet Gynecol 2017, 56: 502–507.
105. Lee SE, Han K, Kwak JY, et alRadiomics of US texture features in differential diagnosis between triple-negative breast cancer and fibroadenoma. Sci Rep 2018, 8: 13546.
106. Xu J, Shen W, Pei B, et alXiao tan he wei decoction reverses MNNG-induced precancerous lesions of gastric carcinoma in vivo and vitro: regulation of apoptosis through NF-kappaB pathway. Biomed Pharmacother 2018, 108: 95–102.
107. Zeng J, Yan R, Pan H, et alWeipixiao attenuate early angiogenesis in rats with gastric precancerous lesions. BMC Complement Altern Med 2018, 18: 250.
108. Liu X, Ji Q, Zhang C, et alMiR-30a acts as a tumor suppressor by double-targeting COX-2 and BCL9 in H. pylori gastric cancer models. Sci Rep 2017, 7: 7113.
109. Bi W, Liu H, Shen J, et alChemopreventive effects of Ku-jin tea against AOM-induced precancerous colorectal lesions in rats and metabolomic analysis. Sci Rep 2017, 7: 15893.
110. Liao W, Wei H, Wang X, et alMetabonomic variations associated with AOM-induced precancerous colorectal lesions and resveratrol treatment. J Proteome Res 2012, 11: 3436–3448.
111. Zeng X, Li X, Xue X, et alActivation of apoptosis in hepatocellular carcinoma by the Chinese traditional medicine Hu Qisan. Exp Ther Med 2013, 5: 695–700.
112. Ma M, Ma Y, Zhang GJ, et alEugenol alleviated breast precancerous lesions through HER2/PI3K-AKT pathway-induced cell apoptosis and S-phase arrest. Oncotarget 2017, 8: 56296–56310.
113. Jerby-Arnon L, Shah P, Cuoco MS, et alA cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 2018, 175: 984–997.
114. Sade-Feldman M, Yizhak K, Bjorgaard SL, et alDefining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 2019, 176: 404.
115. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018, 359: 1350–1355.
116. Nie J, Zhao C, Deng LI, et alEfficacy of traditional Chinese medicine in treating cancer. Biomed Rep 2016, 4: 3–14.
117. Wang CC, Cui L, Zhang ZH, et alResearch thoughts on tumor immune responses by polysaccharide of Chinese medicine via oral administration. Zhongguo Zhong Yao Za Zhi 2016, 41: 1965–1971.
118. Guo Q, Li J, Lin H. Effect and molecular mechanisms of traditional Chinese medicine on regulating tumor immunosuppressive microenvironment. Biomed Res Int 2015, 2015: 261620.
119. Villa-Morales M, Fernandez-Piqueras J. Targeting the Fas/FasL signaling pathway in cancer therapy. Expert Opin Ther Targets 2012, 16: 85–101.
120. Li J, Sun GZ, Lin HS, et alThe herb medicine formula "Yang Wei Kang Liu" improves the survival of late stage gastric cancer patients and induces the apoptosis of human gastric cancer cell line through Fas/Fas ligand and Bax/Bcl-2 pathways. Int Immunopharmacol 2008, 8: 1196–1206.
121. Chang Y, Zhao Y, Zhan H, et alBufalin inhibits the differentiation and proliferation of human osteosarcoma cell line hMG63-derived cancer stem cells. Tumour Biol 2014, 35: 1075–1082.
122. Ponnurangam S, Mammen JM, Ramalingam S, et alHonokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol Cancer Ther 2012, 11: 963–972.
123. Wang S, Lian X, Sun M, et alEfficacy of compound kushen injection plus radiotherapy on nonsmall-cell lungcancer: A systematic review and meta-analysis. J Cancer Res Ther 2016, 12: 1298–1306.
124. He XR, Han SY, Li PP. Injectable Chinese herbal formula Kang'ai for nonsmall cell lung cancer: trial sequential analysis of 2,259 participants from 31 randomized controlled trials. J Cancer Res Ther 2016, 12: 735–743.
125. Dong J, Su SY, Wang MY, et alShenqi fuzheng, an injection concocted from Chinese medicinal herbs, combined with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review. J Exp Clin Cancer Res 2010, 29: 137.
126. Cheng C, Lin JZ, Li L, et alPharmacokinetics and disposition of monoterpene glycosides derived from paeonia lactiflora roots (chishao) after intravenous dosing of antiseptic XueBiJing injection in human subjects and rats. Acta Pharmacol Sin 2016, 37: 530–544.
127. Yang XB, Wu WY, Long SQ, et alFuzheng Kang'ai decoction combined with gefitinib in advanced non-small cell lung cancer patients with epidermal growth factor receptor mutations: study protocol for a randomized controlled trial. Trials 2015, 16: 146.
128. Wang LJ, Lu JZ, Cai BN, et alEffect of compound Zhuye Shigao Granule on acute radiation-induced esophagitis in cancer patients: A randomized controlled trial. Chin J Integr Med 2017, 23: 98–104.
129. Gao L, Chen B, Zhang Q, et alAcupuncture with different acupoint combinations for chemotherapy-induced nausea and vomiting: study protocol for a randomized controlled trial. BMC Complement Altern Med 2016, 16: 441.
130. Wu XK, Stener-Victorin E, Kuang HY, et alEffect of acupuncture and clomiphene in Chinese women with polycystic ovary syndrome: a randomized clinical trial. JAMA 2017, 317: 2502-2514.
131. Chong OT, Critchley HO, Horne AW, et alThe BMEA study: the impact of meridian balanced method electroacupuncture on women with chronic pelvic pain-a three-arm randomised controlled pilot study using a mixed-methods approach. BMJ Open 2015, 5: e008621.
132. Hou LL, Yao LW, Niu QM, et alPreventive effect of electrical acupoint stimulation on lower-limb thrombosis: a prospective study of elderly patients after malignant gastrointestinal tumor surgery. Cancer Nurs 2013, 36: 139–144.
133. Yeh ML, Chung YC. A randomized controlled trial of qigong on fatigue and sleep quality for non-Hodgkin's lymphoma patients undergoing chemotherapy. Eur J Oncol Nurs 2016, 23: 81–86.
134. Zhang LL, Wang SZ, Chen HL, et alTai chi exercise for cancer-eelated fatigue in patients with lung cancer undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manage 2016, 51: 504–511.
:
AI, artificial intelligence; TCM, traditional Chinese medicine.
:
This study is supported by the National Natural Science Foundation of China (Nos.81673784, 81673783, 81520108031, 81830120), the Science Foundation for Shanghai Committee of Science Project (Nos.18QA1404200, 17QA1404100), the Shanghai Health Bureau Science Foundation (No.ZY (2018-2020)-FWTX-4026), and the Xinglin Young Scholar, Shanghai University of Traditional Chinese Medicine.
:
The authors declare that there is no conflict of interests regarding the publication of this paper.
:
Li-Hong Zhou, Yan Li, Qi Li. Research hotspot and frontier progress of cancer under the background of precision medicine. Traditional Medicine Research, 2020, 5 (1): 22–33.
:Xing Zhao
:9 June 2018,
21 December 2018,
: 26 January 2019.
Qi Li, Department of Medical Oncology and Cancer Institute, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China. E-mail: lzwf@hotmail.com.
杂志排行
Traditional Medicine Research的其它文章
- Frequent attacks on health workers in China: social changes or historical origins?
- Application of nanoparticles in the early diagnosis and treatment of tumors: current status and progress
- Complementary and alternative medicine applications in cancer medicine
- Editor-in-Chief of Special Issue on Integrative Oncology
- Clinical distribution and molecular profiling on postoperative colorectal cancer patients with different traditional Chinese medicine syndromes
- Astragalus injection as an adjuvant treatment for colorectal cancer: a meta-analysis
