Study on the Overlapping Characteristics of Fluorescence Signals of Machine Oil and Diesel Mixtures in Soil Based on Iterative Approximation Algorithm
2020-01-08ZUOZhaoluZHAONanjingMENGDeshuoHUANGYaoYINGaofangLIUJianguo
ZUO Zhao-lu, ZHAO Nan-jing, MENG De-shuo,HUANG Yao, YIN Gao-fang, LIU Jian-guo
1. Key Laboratory of Environmental Optics & Technology, Anhui Institute of Optics and Fine Mechanics,Chinese Academy of Sciences, Hefei 230031, China 2. University of Science and Technology of China,Hefei 230026, China 3. Key Laboratory of Optical Monitoring Technology for Environment, Hefei 230031, China
Abstract Petroleum hydrocarbons such as machine oil and diesel are important components of soil pollution, and are of great significance for rapid and accurate detection of organic pollutants such as machine oil and diesel in soil. Laser-induced fluorescence (LIF) technology has the advantages of fast detection speed, high sensitivity and on-site detection. However, when detecting organic pollutants in soil, it faces serious problems such as overlapping fluorescence spectra. In order to study the overlapping characteristic of the fluorescence signals of the machine oil and diesel mixture in the soil, 10 soil samples containing different concentrations of machine oil and diesel mixture were prepared. By establishing the LIF experimental system, the fluorescence signals of different mixing concentrations of machine oil and diesel were obtained, and the inversion relationship between the mixed spectra of machine oil and diesel was established. The iterative approximation algorithm was used to calculate the fluorescence contribution rate of diesel and machine oil samples in soil fluorescence spectra. In the process of calculating the fluorescence contribution rate, the two methods of full spectrum and intercepted characteristic spectrum were compared. When linearly fitting with the machine oil sample concentration, the fitting coefficient R of the intercepted characteristic spectrum method was 0.989, and the average relative error was 3.38%, which was better than the full spectrum of 0.923, 8.79%. At the time of verification, the average relative error of multiple linear regressions was 10.11% compared with the multiple linear regression method, which prove that the intercepted characteristic spectroscopy method is still excellent. There was a good linear relationship between the fluorescence contribution rate of machine oil and diesel in soil and its own concentration, indicating that there is no chemical reaction after mixing machine oil and diesel in soil, and the overlapping characteristic of fluorescence signals in soil are linearly superimposed. The method is equally applicable to the separation of fluorescence spectra of other petroleum hydrocarbon mixtures in the soil. Through the research in this paper, the accuracy of qualitative and quantitative detection of petroleum hydrocarbon pollutants in soil by LIF technology was improved. It provided method support for rapid detection of petroleum hydrocarbons in the soil.
Keywords Soil; Laser-induced fluorescence; Machine oil; Diesel
Introduction
With the rapid growth of economy, the soil environment has suffered from serious pollution of oil pollutant[1-2]. Leakage of oil always happened, when petroleum products were in use, transit or storage. That caused a lot of oil going into the soil, leading to an environmental pollution[3-4]. The pollution is getting worse nowadays, so more and more countries in the world have paid a great attention to studying on how to detect and hold down the oil pollution. The machine oil and the diesel are common petroleum products, which are widely used in the automobile industry and manufacturing industry[5-7]. Both of them are the important components of petroleum pollutants. At present, there are many methods for detecting and analyzing variety oils in soil, such as extraction, ultrasonic extraction, gravimetric, turbidimetry, UV-spectrophotometry, infrared-spectrophotometry, gas chromatography and pyrolysis[8-11]. All of them often waste time cost a lot and even cannot guarantee the precision.Laser-induced fluorescence (LIF) spectroscopy is excellently suited for the in-situ analysis of fluorescent organic compounds in the solid environment. Its theory is that when the surface of the soil sample which is mixed with oils is irradiated by using the laser as a light source, electron transition is excited,then the fluorescence is produced. Because of the high sensitivity, signal-to-noise ratio and the samples for which there are no need to be pretreated complicately, LIF is utilized to detect petroleum products concentrations in water or mass fraction in soils[12-15]e.g. Mbaye OMA et al.[15]and WANG Yu-tian et al.[16]have studied the change in the fluorescence spectra according to the surface loading of PAH on various soil media. The fluorescence spectra revealed that the increasing surface which was loaded of PAH induced excimer emission. They used fluorescence intensity to analyze the mass fraction of PAH, because a good linear relationship exists between the mass fraction and the fluorescence intensity. The concentrations of machine oil, diesel, and lubricating oil were determined by this method, e.g. Feng Wei-wei et al.[17]He also used fluorescence intensity as a characteristic parameter to calculate the concentrations of oils in samples. Although LIF is widely used, it still faces problems like the fluorescence overlap and the difficulty of component identification when people detect the petroleum pollutants in soil. Petroleum hydrocarbon organics like machine oil and diesel are important components of the petroleum pollutants in soils. It’s significant for environmental monitoring to detect their contents in the soils fast and exactly. Laser-induced fluorescence (LIF) technology has the advantages of fast detection speed, high sensitivity and in situ detection, but it still faces the problems such as the overlap of fluorescence and the difficulty of component identification. So, the study on relation of the overlap of fluorescence is urgent.
Similar to all of the petroleum products, the main fluorescent substances in machine oil and diesel oil are polycyclic aromatic hydrocarbons, naphthene, alkane hydrocarbon and non-hydrocarbon[17]. Different kinds of petroleum products have different kinds of fluorescent substances, and each one emit a specific fluorescence spectrum. Each kind of oil in soil offers different fluorescence contribution rate. All of that can be considered as the basis for fluorescence detection of the two oils.
1 Experimental
1.1 Experimental system
According to the experimental purpose and theory,an experimental system was constructed as shown in Fig.1. which contained a laser emitter, a refracting mirror, a collecting mirror, an optical fiber, a spectrograph, and a computer. The laser emitter (Qsmart-850, Quantel)was used as a light source whose fundamental wavelength was 266 nm. After the light was reflected by mirror, the surface of soil samples which were mixed with different mass fractions of the two oils were irradiated by the UV-light, then the fluoresce was excited. Through the collecting mirror and the optical fiber, the fluorescent signal was collected by the spectrograph (AvaSpec-ULS2048L, Avantes), whose scanning wavelength coverage was 200~600 nm. The computer was the control center of the system, which was used for data storage and display of the fluorescence spectrums.

Fig.1 Experimental system
1.2 Experimental samples
When the experimental samples were prepared, Shell-XH3 machine oil and 0#diesel were selected as oil pollutant in soil. The two kinds were mixed in proportion, and then they were mixed with the soil. The concentrations of the mixed two oils were shown in Table 1.

Table 1 The concentrations of the two mixed oils in the soil
2 Results and discussion
All of the spectrums of 10 samples were collected by the system. Each spectrum was removed soil background spectrum. For example three original spectrums were chosen, such as the pure diesel’s spectrum (Sample 0), the pure machine oil’s spectrum (Sample 1) and the mixture spectrum (Sample 5).And by the way the background fluorescence, which was the same to the pure soil fluorescence in the system. The original spectrums were effect from the fluorescence intensity T, which often caused a spectrum fluctuation in experiment. In order to increase the stability of fluorescence intensity of the samples, all the spectrums were normalized. The way was that each spectrum was divided by its corresponding maximum peak,the calculation as equation(1).
(1)
WhereAwas a fluorescence spectrum,max(A) was the max peak value ofA,andA′ was the normalized spectrum.
After normalizing, the normalized spectrums were shown as Fig.3. Every maximum peak of the spectrums was 1. Every point in each spectrum was scaled down by the corresponding maximum peak of each spectrum, while the wave shape of each spectrum was the same to the original one. ThenA′ was used to instead A hereinafter.
In fact each oil fluorescence spectrumAcontained two components: machine oil fluorescence spectrumBand diesel fluorescence spectrumC. So we can get equation(2)
A=qA0+pA1
(2)
Whereqwas the fluorescence contribution rate of machine oil,pwas the fluorescence contribution rate of diesel, andA0was the spectrum of pure diesel,A1was the spectrum of pure machine. The values ofpandqwere unknown. In order to calculate them, we intended to use iterative approximation. As the values ofpandqchanging, the composition spectrumZwas composted. And then the equation (3) and equation (4) could be got.

Fig.2 The normalized spectrums
(3)
E′=E2=[A-qA0-(1-p)A1]2
(4)
WhereEwas the value ofE(residual error, RE) betweenAandZ. WhereE′ was the value of SSE (sum of squares for residuals) betweenAandZ. When the value ofE′ was minimum, the corresponding values ofpandqwas optimal,for the composition spectrumZwas closest toA. We the value ofEcould be calculated as equation(3), and the value ofE′ was calculated as equation(4). According equation(4), the minimum value ofE′ and the correspondingpas the optimal solution (q,E′).
Here two calculation methods were considered to be used to calculateq. The first one was the full spectrum method, which meant the full wavelength coverage 200~600 nm was used to calculate q!according to the steps above and every data in each spectrum should be considered. The second one was intercepted characteristic spectrum method. Its wavelength coverage was 330~460 nm. The intercepted spectrum was including all the characteristic peaks of the spectrums. First, according to approaching the full spectrum algorithm, the method was carried out. The value ofqwas increasing with a step of 0.01 from 0 to 1. And the soil sample 5 was taken for an example. As equation (4), the sum of squares for residualsE′ was the quadratic curve about the fluorescence contribution rate of machine oilq. Whenqwas plotted into the equation(4), the curve was shown as Fig.3.
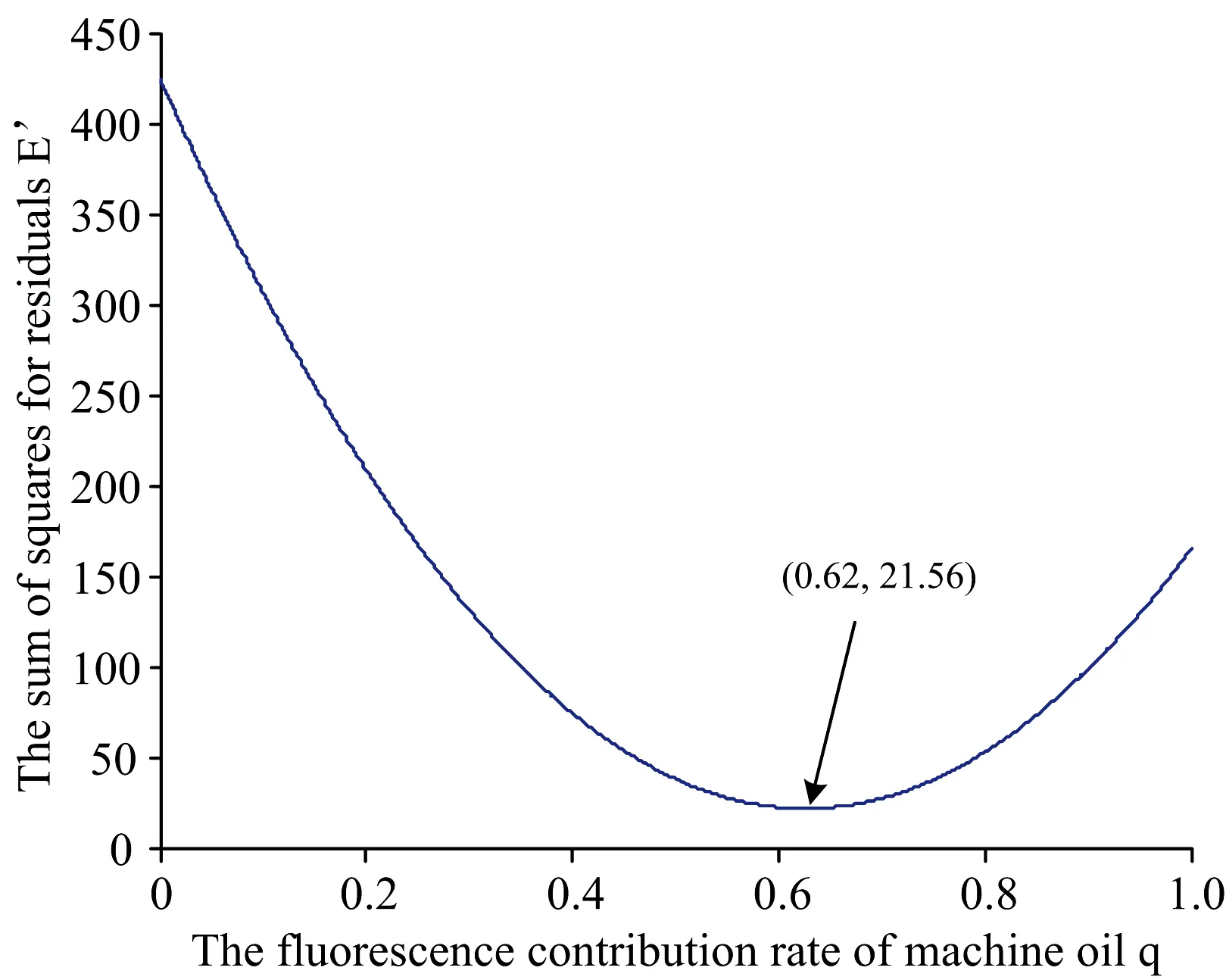
Fig.3 The quadratic curve of q and E′of the full spectrum method
In Fig.3 the curve was a section of a quadratic curve. Obviously, there was a minimum point in the curve, and it was the optimal solution (0.62, 21.56). It meant that whenqwas 0.62,E′ was 21.56, and at this time the composition spectrum was the closest to the normalized spectrum. Fitted linearity ofω1andq, so the fitting equation (5) was acquired.
q=2.06×10-1ω1+9.97×10-2R=0.923
(5)
Whereω1was the concentration of machine oil, the fitting coefficientR=0.923. Then we found that the variation tendency ofqwas consistent withω1. The fitting line was shown as Fig.4.
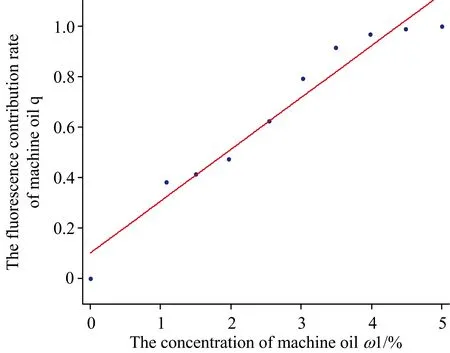
Fig.4 The fitting line of ω1 and q of first method
In Fig.4 the value ofqgradually increased synchronously withω1on the line. The 10 points were all near by the fitting line. Generally,qandω1was a linear relation. The discrete distribution of 10 points was not very uniform on both sides of the calibration curve,especially point (0, 0) was away from the other 9 points. Fluorescence contribution rate of the last three points at high concentration was not significantly changed.
Then the second method was carried out. The wavelength coverage ofA330~460 nm was intercepted,which was contained in all of the characteristic peaks and we repeated the same calculation steps. The quadratic curve ofE′ andqof second method was shown as Fig.5.

Fig.5ThequadraticcurveofE′andqofinterceptedcharacteristicspectrummethodmethod
In Fig.5 the minimum point in the curve also existed, the value of the point was (0.53, 8.92). So the correspondingqwas 0.53,E′ was 8.92. According to equation (2) the spectrum of the sample 5 could be dissociated to two parts by this method.
Then we also fitted linearity ofω1andqand acquired the fitting equation(6),R=0.989. The fitting line was plotted as Fig.6.
q=2.00×10-1ω1+3.90×10-2R=0.989
(6)

Fig.6 The fitting line of ω1 and q of second method
In Fig.6 the value ofqalso gradually increased synchronously withω1. The intercept of the line was 0.039. There were 10 points which are evenly distributed on both sides of the calibration curve. The values ofqandpwere both different from the ones of first method. But the value ofE′ 7.66 was less than the one 21.56 of first method. Furthermore compared equation (5) to equation (6),R=0.989>0.923. That meant the linearity of equation (6) was better than the linearity of equation (5).ω1and q formed a good linear relationship. That meant as the increase of machine oil concentration in the mixed oil, its fluorescence contribution rate in the soil was also increasing. In the mixed oil, the higher concentration of oil, the more similar the fluorescence spectrum of the mixture is to that of the oil. The theory was the same. After mixing soil and oils, their respective chemical properties are stable. Different two petroleum organics also maintained their stable chemical components, for they are all petroleum extracts.
In order to observe the result more directly, the sample 5 was taken as example, and the two composition spectrums were composed according to equation (2), as shown in Fig.7.

Fig.7 Comparison of composition spectrawith original normalized spectrum
In Fig.7 we found that the shape of the composition spectrum of second method was closer to the normalized spectrum than the composition spectrum of first method. In especial, it was obvious that the errors in the wavelength coverage 320~460 nm were higher in the composition spectrum of first method. The maximum residual error was 0.11 at 358 nm which was the position of the maximum peak of the spectrum. That caused a great distortion. While the maximum residual error of second method was 0.03. There was a difference of one-tenth between the two methods. That meant the intercepted characteristic spectrum method was better. The average relative error of the 10 samples was calculated was 3.38% in the way of the intercepted characteristic spectrum method,while the one was 8.79% in the way of the full spectrum method.
The third method MLR(Multiple linear regression)was carried out. Fluorescence intensities at 5 positions 300, 350, 400, 450 and 500 nm were chosen for fitting by MATLAB. For sample 5, its relative error was 9.58%. Other samples were used this method to calculate their relative errors too. In this way, the average relative error of the 10 samples was 10.31%. So according to the value of relative error, the results showed that the intercepted characteristic spectrum method was the most accurate algorithm.
We analyzed the reason that the internal signal noise of the instruments still existed and the soil was not fully same or uniform enough in different samples. They all lead to a reduction of SNR (Signal to Noise Ratio). Furthermore the longer wavelength coverage of the spectrum led to more errors, because the proportion of the errors in the wavelength coverage without characteristic peaks was more outstanding. Because the second method included all the peaks of the spectrums, the fluorescence intensities of second method were more higher than the intensities of noises and that led to an enhancement of SNR. The measurement method and results of the diesel were in a similar way. All above proved that the intercepted characteristic spectrum method was better to calculate the fluorescence contribution rate of the two oils in soil based the iterative approximation. And the concentration of oil and its fluorescence contribution rate formed a good linear relationship.
3 Conclusions
In this paper, we were going to find the overlap relationship of a soil sample mixed with machine oil and diesel. In order to study that, the concentration of each oil and its fluorescence contribution rate were intended to make a linear fitting. When we calculated the fluorescence contribution rate of each oil, two methods as the full spectrum method and the intercepted characteristic spectrum method were carried out respectively. The two methods were both based on iterative approximation. Through comparing, the latter one was better. The results showed that the concentration of each oil and its fluorescence contribution rate formed a good linear relationship in soil. This meant that the fluorescence overlaps characteristics of the machine oil and diesel in the soil were linearly superimposed. This method can also be used for the dissociation of other oil mixtures in soil.
