Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative,open trial
2019-12-30YounChoiJunWonChungDongKyunParkKyoungOhKimKwangAnKwonYoonJaeKimJaYoungSeo
Youn I Choi, Jun Won Chung, Dong Kyun Park, Kyoung Oh Kim, Kwang An Kwon, Yoon Jae Kim,Ja Young Seo
Abstract BACKGROUND Few studies have compared the efficacy and safety profile of a tailored eradication (TR) strategy based on the presence of a 23S ribosomal RNA point mutation with those of empirical bismuth-based quadruple therapy (EBQT) for first-line eradication of Helicobacter pylori (H. pylori) in Korean patients.AIM To compare the efficacy and safety of a TR strategy and those of EBQT regimen as first-line eradication therapy for H. pylori.METHODS This is an open-label, comparative study in which we prospectively enrolled patients over 18 years of age with H. pylori infection and retrospectively reviewed their data. H. pylori-positive patients diagnosed by rapid urease test, Giemsa staining, or dual priming oligonucleotide polymerase chain reaction (DPO-PCR)were enrolled from May 2016 to September 2018 at Gil Medical Center. Patients with H. pylori infection received either a TR regimen or the EBQT regimen. In the tailored therapy group that underwent DPO-PCR testing, patients with A2142G and/or A2143G point mutations were treated with a bismuth-containing quadruple regimen. The eradication rate, patient-reported side effect rate, and H.pylori eradication success rate were evaluated and compared between the groups.RESULTS A total of 150 patients were assigned to the TR (n = 50) or EBQT group (n = 100).The first-line eradication rate of H. pylori did not differ between the groups(96.0% vs 95.7%, P = 0.9). The rate of eradication-related side effects for TR was 12.0%, which differed significantly from that of EBQT (43.0%) for first-line treatment (P < 0.001).CONCLUSION DPO-PCR-based TR for H. pylori eradication may be equally efficacious, with less treatment-related complications, compared to EBQT in Korea, where clarithromycin resistance is high.
Key words: Helicobacter pylori; Eradication; Tailored; Empirical; Quadruple
INTRODUCTION
Helicobacter pylori(H.pylori) is designated as a class I carcinogen by the International Agency for Research on Cancer and the World Health Organization, and it is a suspected cause of gastric adenocarcinoma or gastric mucosa-associated lymphoid tissue lymphoma[1-4]. Although eradication ofH.pylorihas emerging importance, the eradication success rate forH. pyloriusing an empirical strategy has decreased worldwide[5]. In addition, as the clarithromycin (CAM) resistance rate has surpassed 15% in Korea, and the metronidazole (MTZ) resistance rate has been reported at 30%,the eradication rate of the empirical triple regimen [proton pump inhibitor (PPI),amoxicillin (AMX), and CAM] has decreased to less than 70%[6].
The Maastricht V/Florence Consensus guidelines recommend bismuth-containing quadruple therapy as the empirical treatment choice in countries with high dual resistance to CAM and MTZ[7,8]. Although the 2013 revision of the Korean Clinical Practice Guidelines forH.pylorirecommend triple therapy with PPI, AMX, and CAM or a bismuth-based quadruple regimen if CAM resistance is suspected[9], there are emerging concerns for using a bismuth-based quadruple regimen in that (1) it is too complex a combination of too many antibiotics, which may lead to improper antibiotic overuse; and (2) diverse and enormous cases of treatment-related side effects may occur, resulting in poor patient compliance[7,10-12]. One of the main reasons for the failure ofH. pylorieradication is the increase inH. pyloriantibiotic resistance rates along with improper antibiotic use[13]; thus, a tailored eradication (TR) strategy design has been proposed to improve treatment-related outcomes[14-18].
However, few studies have compared the efficacy, safety profiles, and compliance rates between a TR strategy based on the presence of a 23S ribosomal RNA point mutation and the empirical bismuth-based quadruple therapy (EBQT) as first-line eradication therapy forH. pyloriinfection in Korean patients.
Therefore, in this open-label, and comparative study, we investigated the efficacy(eradication rate), safety profile (treatment-related side effects), and compliance rate of a TR strategy, based on the presence of a 23S ribosomal RNA point mutation,vsthose of EBQT therapy as first-line eradication therapy forH. pyloriinfection in Korean patients.
MATERIALS AND METHODS
Institutional review board approval
The Institutional Review Board of Gil Medical Center (GMC) reviewed the study protocol and ethics. This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of each participating hospital.
Study concept
In this open label, comparative study, we compared the efficacy (measured as eradication success rate) and safety profiles (eradication treatment-related complications) between TR and EBQT as first-line eradication treatment forH. pylori.
Patient characteristics
Patients who visited the GMC for an endoscopic examination between May 2016 and September 2018 and who were over 18 years of age and had aH. pyloriinfection were included in this study.H. pyloriinfection was diagnosed by the rapid urease test,Giemsa staining, or dual priming oligonucleotide polymerase chain reaction (DPOPCR). The exclusion criteria were: (1) < 18 years of age; (2) Not willing to participate in this study; (3) Previous eradication treatment forH. pylori; (4) History of gastric surgery; (5) Complex medical history, such as severe cardiopulmonary dysfunction,liver cirrhosis with Child Pugh classification B or C, or renal failure, or (6) Any allergic history to antibiotics.
The patients withH. pyloriinfection were assigned to either a TR group or an EBQT group in a 1:2 manner. In the tailored therapy group that underwent DPO-PCR testing, patients with A2142G and/or A2143G point mutations were treated with the BQT regiment (PPI + bismuth + MTZ + tetracycline), and patients without A2142G and A2143G point mutations were treated with the PAC regimen (PPI + AMX +CAM).
Clarithromycin resistance test (DPO-PCR)
In the TR group, DPO-PCR was performed using the following steps[19-21]: (1) DNA was extracted from biopsy specimens and DPO-based multiplex PCR (SeeplexH.pylori-ClaR ACE Detection; Seegene Inc., Seoul, South Korea) was performed; (2) Point mutations were identified by PCR amplification of a portion of the 23S ribosomal RNA gene; and (3) When a 194-bp band or a 475-bp band was detected using UV transillumination after gel electrophoresis, the amplified DNA products were determined to have the A2142G or A2143G point mutation.
Eradication regimens for Helicobacter pylori
The PAC regimen consisted of 30 mg lansoprazole + 500 mg CAM + 1000 mg AMX,administered twice daily for 7 or 14 d. PAC regimen was administered for 7 d or 14 d randomly. The EBQT regimen consisted of 30 mg lansoprazole twice daily + 500 mg MYZ twice daily + 300 mg bismuthate four times daily + 500 mg tetracycline four times daily for 14 d.
Outcome measures, efficacy, safety profile, and compliance
The eradication success rate, patient-reported side effect rate, and compliance withH.pylorieradication were evaluated and compared between the groups by intention-totreat (ITT) and per-protocol (PP) analyses. Efficacy, as measured by the eradication success rates of the two eradication regimens, was tested at least 4 wk after treatment using the13C-urea breath test (UBT; UBiTkit; Otsuka Pharmaceutical Co. Ltd., Tokyo,Japan). Each patient was orally administered with 100 mg13C-urea. A cutoff of delta13CO2< 2.5‰ was used to confirm eradication in this study. To avoid a false result on the UBT test, all patients discontinued any PPI, H2blocker, or antibiotic use at least 4 wk before the UBT.
To analyze treatment-related side effects, patient-reported complications and compliance were recorded at the end of the visit. Patients were informed of the type of side effects of the eradication treatment at the time of prescription. Treatmentrelated complications included any case of opportunistic infections, abnormal taste,loss of appetite, dry mouth, sore or dry throat, nausea and vomiting, bloating,belching, abdominal pain, dyspepsia, or general weakness. Each patient evaluated their symptoms on a scale of mild, moderate, or severe. Patient compliance with treatment was defined as consumption of more than 90% of the prescribed therapy.
Statistical analysis
TheH. pylorieradication rate was depicted by ITT and PP analyses. Patients lost to follow-up or those with poor compliance were excluded from the PP analysis.Categorical variables were analyzed as median and interquartile range by theχ2test.Continuous variables are given as the mean ± SD, and were compared between groups using Student'st-test. AP-value < 0.05 was considered significant. The Statistical Package for the Social Sciences (SPSS) software version 20.0 (IBM Corp.,Armonk, NY, United States) was used for statistical analyses.
RESULTS
Characteristics of the study population
From May 2016 to September 2018, a total of 154 patients withH. pyloriinfection were enrolled and assigned to receive either TR (n= 50) or EBQT (n= 104) (Table 1). The mean age of patients in the TR and EBQT groups was 58.3 ± 13.9 years and 57.4 ± 11.6 years, respectively. The most common reason for eradication was peptic ulcer disease(n= 15, 48.4%) in the TR group, and chronic atrophic gastritis with intestinal metaplasia in the EBQT group (n= 71, 68.3%) (Table 2). Among the subjects, four with poor compliance in the EBQT group were excluded from ITT analysis forH. pylorieradication (Figure 1). All the recruited patients received complete follow-up (Figure 1).
Eradication rate
The TR and EBQT groups had comparable results in both ITT analysis (96.0%vs94.2%;P= 0.6) and PP analysis (96.0%vs95.0%;P= 0.8). It was showed thatH. pyloriinfection was cured in 96.0% (48/50) of the patients receiving TR and 95.0% (95/100)of those receiving EBQT treatment. Only two patients failed to eradication in the TR group. One patient receiving the PAC regimen had no mutation detected by DPOPCR, and another patient showed an A2143G mutation on DPO-PCR.
Treatment-related adverse events
In the ITT analysis involving a total of 154H. pyloriinfected patients, 12.0% (6/50) of the TR recipients and 43.7% (45/104) of those treated with EBQT reported at least one adverse event during eradication therapy. In the tailored group, 36 patients were treated with the PAC regimen and 14 treated with the BQT regimen; no patients treated with the PAC regimen (0/36) reported adverse events and two patients treated with the BQT regimen (2/14) had eradication-related adverse events. The EBQT group exhibited a statistically significantly higher frequency of overall adverse events than the TR group (43.7%vs12.0%;P≤ 0.01) (Table 3). None of the patients discontinued treatment because of adverse events.
Treatment compliance
Four patients (all in EBQT group) took less than 80% of the assigned tablets. The EBQT group showed a lower compliance rate, but there was no statistical significance(100.0%vs96.2%,P= 0.2).
DISCUSSION
In this open-label, comparative study, we compared the efficacy and safety profiles between the TR strategy, based on the presence of a 23S ribosomal RNA point mutation (n= 50), and the EBQT strategy (n= 100) as first-line eradication strategies forH. pyloriinfection in Korea. The efficacy of TR was similar to that of EBQT (96.0%vs95.7%,P= 0.9), and the side effect profile of TR was significantly better than that of EBQT (12%vs43.0%,P< 0.001). Given that the eradication rate of the empirical triple regimen (PPI + AMX + CAM) has decreased to less than 70% in Korea, the DPO-PCR-based TR may be an effective first-line eradication therapy with fewer treatmentrelated side effects compared to EBQT.
To our knowledge, this is the first study to make a head-to-head comparison of the efficacy and safety of the TR and EBQT regimens. In this study, we did not consider PPI-based triple therapy as an eradication option, because the CAM resistance rate has surpassed 15% in Korea, and the efficacy of empirical triple therapy is minimal.The latest version of the Korean Clinical Practice Guidelines forH.pylorirecommend either triple therapy with a PPI, AMX, and CAM, or a bismuth-based quadruple regimen if CAM resistance is suspected. In addition, several reports suggest that 9.6%of the strains in Korea have dual resistance to CAM and MTZ[20,22,23]; thus, it may be prudent to avoid choosing an empirical conventional triple regimen as a first-line eradication strategy. Therefore, we did not choose the triple regimen in this study.
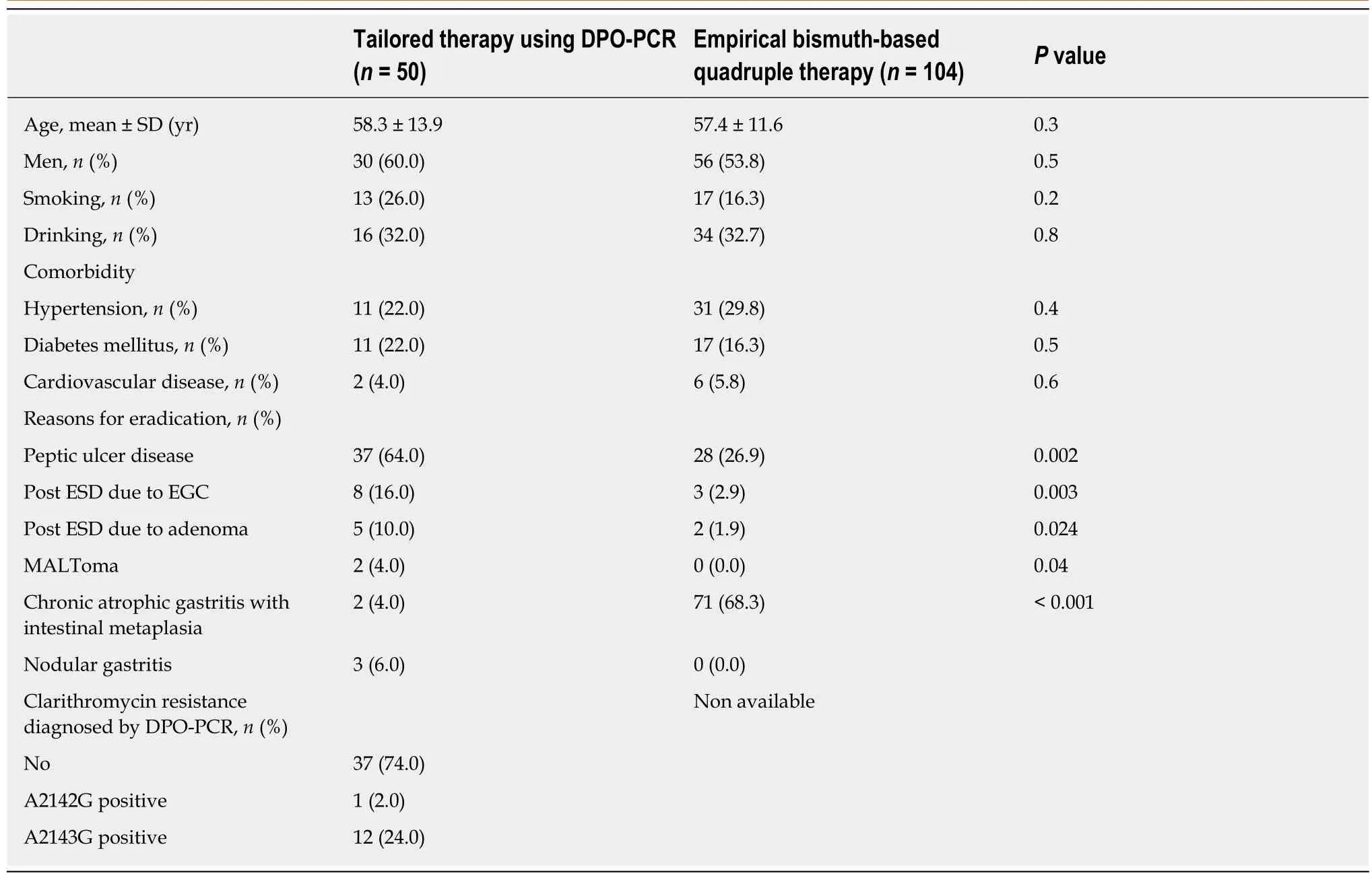
Table 1 Baseline characteristics of the study population
Both the Maastricht V/Florence and Korean guidelines recommend bismuth-based quadruple therapy as the policy for failed first-line therapy, or even as an option for first-line therapy. However, several reports have indicated treatment-related side effects of bismuth-based quadruple therapy, which may directly lead to poor patient compliance. Given that treatment-related side effects might lead to treatment failure,and imperfect eradication is closely associated with increased antibiotic resistance,treatment-related side effects are important factors when consideringH. pyloritreatment. A multicenter study from Italy, where CAM resistance rates are above 15%,reported that 46.6% of patients who received bismuth-based quadruple therapy complained of at least one side effect, including nausea, diarrhea, and vomiting,among 209 patients[7], which was similar to the rate observed in the present study.Danielaet alconducted a randomized controlled trial in Israel, where CAM resistance rates are increasing, and the patients who took the bismuth-containing regimen reported significantly more treatment-related complications (84.0%), such as gastrointestinal discomfort, with less compliance[24]. Although there has been a wide range of complication rates for bismuth-containing quadruple therapy, the complication rates are not negligible, which can lead to poor compliance.
In addition to the treatment-related complications of the bismuth-based quadruple regimen, too many antibiotics have been used to eradicateH. pylori. Because the empirical bismuth-based quadruple regimen includes MTZ and tetracycline, and inappropriate antibiotic use leads to antibiotic resistance, antibiotics should be prescribed more carefully in Korea considering the high CAM (>5%) and MTZ (>30%)resistance rates. AsH. pyloriculture and antibiotic sensitivity testing are not always available in a clinical setting, DPO-PCR-based tailored therapy is a realistic option for eradication in a region with increasing antibiotic resistance.
Several studies have indicated favorable outcomes of DPO-PCR-based tailored therapies in line with our results, even though there are no published comparisons between tailored therapy and bismuth-based quadruple therapy[15,16]. Zhouet al[25]reported that tailored therapy achieved a significantly higher eradication rate (88.7%vs78.3%) and fewer side effects (22.0%vs31.7%) than a concomitant therapy. Parket al[26]reported that personalized tailored therapy based on 23S rRNA genotypes can increase eradication success rates in patients withH. pyloriinfection compared to empirical CAM-based triple therapy, as the 2143G point mutation in the 23S rRNA ofH. pyloriwas found to be an independent risk factor for eradication failure in CAM-based triple therapy. Furthermore, Choet al[27]reported that a tailoredH. pylorieradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes CAM resistance in patients withH. pyloriinfection is more cost-effective than empirical treatment. Kimet al[9]also conducted an economic modeling study comparing TR based on DPO-PCR and empirical treatment.
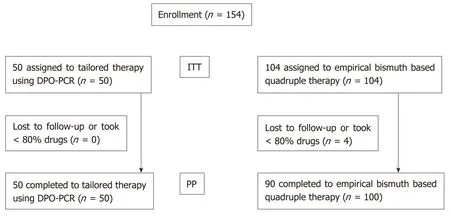
Figure 1 Flow chart. DPO-PCR: Dual priming oligonucleotide polymerase chain reaction; ITT: Intention-to-treat analysis; PP: Per-protocol analysis.
Even though there have been limited reports in which the TRvsEBQT regimens were compared in terms of cost-effectiveness, the cost problem of the TR regimen should be evaluated as compared to EBQT design for the first-line treatment inH.pylorieradication. Choet al[27]reported that a tailoredH. pylorieradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes CAM resistance in patients withH. pyloriinfection is more cost-effective than empirical treatment. In that study, different from ours, the researchers chose the PAC regimen as the empirical treatment[27]. Choet al[27]demonstrated that the average costs per patient for tailored therapy were $307.37, and compared with triple therapy, the incremental cost-effectiveness ratio of tailored therapy was $3.96 per patient for firstline treatments. Since the failure rate of the PAC regimen forH. pylorieradication has been increasing in Korea, the overall medical costs for the PAC regimen might be higher than those of EBQT designs. The medical cost issue should be further evaluated.
Apart from medical cost problem of the TR regimen, given issues on increased prevalence of drug resistance ofH. pyloriworldwide, tailored approaches in treatingH. pyloriinfection should be considered further. The possible reasons for treatment failure in the TR group are as follows[28,29]. First, althoughH. pylorihas traditionally been regarded as a homogenous organism, there is increasing evidence that populations ofH. pyloriin humans show wide diversity[29]. The quasi-species development ofH. pyloriin a single host might result in treatment failure even after a tailored eradication strategy based on the presence of a 23S ribosomal RNA point mutation[28]. Second, a DPO-PCR-based evaluation is limited to detecting mutations of A2142G and A2143G in 23S rRNA, and other mutations, such as the A2144G or A2142C mutation ofH. pylori, cannot be detected[20,30].
This study had several limitations. First, because of the relatively small sample size,the results of this study should be interpreted cautiously. A further larger samplesized, randomized trial should be conducted to verify our results. Second, as this study was conducted at the GMC, a tertiary center in Korea, it may have been subject to selection bias. Third, we did not culture theH. pylorifrom enrolled patients for antibiotic sensitivity testing, but DPO-PCR was performed for CAM. In conclusion,this study showed that TR is a first-line eradication regimen with non-inferior efficacy and a favorable safety profile compared to bismuth-based quadruple therapy. A future eradication regimen could potentially be designed based on these results for areas where CAM resistance rates are increasing.

Table 2 Helicobacter pylori eradication success rates and complication rates, n (%)
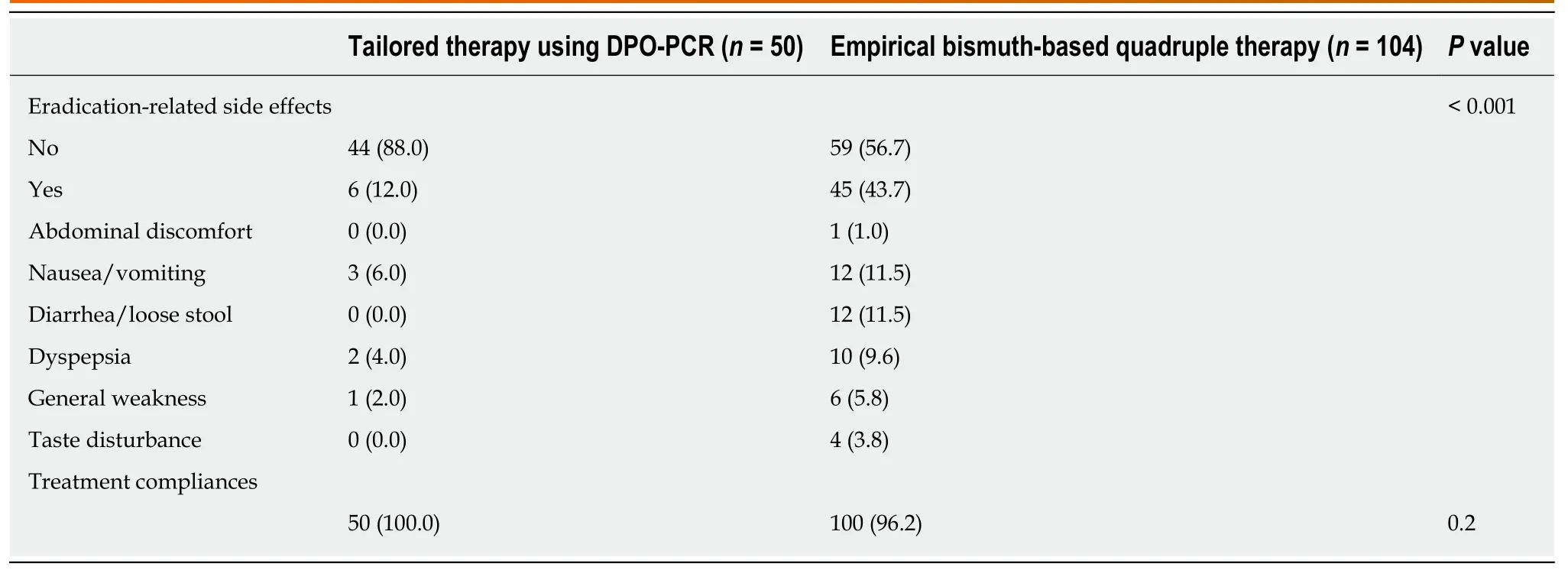
Table 3 Eradication-related adverse events, n (%)
ARTICLE HIGHLIGHTS
clarithromycin (CAM) resistance is high.
Research perspectives
Our study showed that TR is a first-line eradication regimen with non-inferior efficacy and a favorable safety profile compared to bismuth-based quadruple therapy. A future eradication regimen could potentially be designed based on these results for areas where CAM resistance rates are increasing.
杂志排行
World Journal of Gastroenterology的其它文章
- Severe liver injury due to herbal and dietary supplements and the role of liver transplantation
- Long non-coding RNA HULC as a diagnostic and prognostic marker of pancreatic cancer
- Mesenterico-portal vein invasion should be an important factor in TNM staging for pancreatic ductal adenocarcinoma: Proposed modification of the 8th edition of the American Joint Committee on Cancer staging system
- Clinical relevance of fluorodeoxyglucose positron emission tomography/computed tomography and magnifying endoscopy with narrow band imaging in decision-making regarding the treatment strategy for esophageal squamous cell carcinoma
- Transumbilical enterostomy for Hirschsprung's disease with a twostage laparoscopy-assisted pull-through procedure
- Two-week bismuth-containing quadruple therapy and concomitant therapy are effective first-line treatments for Helicobacter pylori eradication: A prospective open-label randomized trial
