Imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumors of the liver: Two case reports and literature review
2019-12-16HaiLanLiHuaPingLiuGraceWenJunGuoZhiHongChenFuQingZhouPengLiuJianBinLiuRenWanZhiQunMao
Hai-Lan Li, Hua-Ping Liu, Grace Wen-Jun Guo, Zhi-Hong Chen, Fu-Qing Zhou, Peng Liu, Jian-Bin Liu,Ren Wan, Zhi-Qun Mao
Abstract BACKGROUND Inflammatory pseudotumor-like follicular dendritic cell (IPT-like FDC) tumors of the liver is an uncommon tumor with extremely low incidence. To date, the radiologic findings of this tumor in multiphase computed tomography (CT) and magnetic resonance imaging (MRI) imaging have not been described.CASE SUMMARY Patient 1 is a 31-year-old Chinese female, whose complaining incidentally coincided with the finding of multiple liver masses. In the local hospital, an abdominal enhanced CT found two hypo-dense solid lesions, with heterogeneous sustained hypoenhancement, in the upper segment of the liver’s right posterior lobe. In our hospital, enhanced magnetic resonance imaging (MRI) with hepatocyte-specific contrast agents showed a similar enhanced pattern of lesions with patchy hyperintensity in the hepatobiliary phase (HBP). The patient underwent surgery and recovered well. The final pathology confirmed an IPTlike FDC tumor. No recurrence was found on the regular re-examination. Patient 2 is a 48-year-old Chinese male admitted to our hospital for a huge unexpected hepatic lesion. A dynamic enhanced abdominal CT revealed a huge heterogeneous enhanced solid tumor in the right lobe of the liver with a size of 100 mm × 80 mm, which showed a heterogeneous sustained hypoenhancement.In addition, enlarged lymph nodes were found in the hilum of the liver. This patient underwent a hepatic lobectomy and lymph node dissection. The final pathology confirmed an IPT-like FDC tumor. No recurrence was found upon regular re-examination.CONCLUSION When a hepatic tumor shows heterogeneous sustained hypoenhancement with a patchy enhancement during HBP, an IPT-like FDC tumor should be considered in the differential diagnosis.
Key words: Magnetic resonance imaging; Computed tomography; Inflammatory pseudotumor-like follicular dendritic cell tumor; Liver
INTRODUCTION
The inflammatory pseudotumor-like follicular dendritic cell (IPT-like FDC) tumor is a variant subset of a follicular dendritic cell (FDC) tumor[1]. FDC tumors most commonly occur in the cervical lymph nodes, which are extremely rare in the liver and represent < 0.1% of all primary hepatic tumors[2]. The IPT-like FDC tumors of the liver are exceptionally rare, and are different from FDC tumors by female predilection. They have nearly exclusive hepatic and splenic involvement, with low aggressivity and an association with the Epstein-Barr virus (EBV)[3]. As far as we know, only 26 cases of IPT-like FDC liver tumors have been reported in the Englishlanguage literature[4,5], but their radiologic findings have rarely been described.Herein, we report the imaging features of two histopathologically proven cases of IPT-like FDC tumors of the liver. This report is helpful for understanding hepatic IPTlike FDC tumors. The study was approved by the institutional review board of our hospital, which waived the need for informed consent.
CASE PRESENTATION
Chief complaints
Patient 1: A 31-year-old Chinese female was incidentally reported to have multiple liver masses.
Patient 2: A 48-year-old Chinese male was incidentally found to have a massive liver mass for 7 d during health examination.
History of present illness
Patient 1: One month ago, the patient was found to have multiple hepatic lesions by chest computed tomography (CT), due to an upper respiratory tract infection in the local hospital. Two hypodense solid lesions, with sizes of 36 mm × 31 mm and 21 mm× 16 mm, respectively, were detected in the upper segment of the right posterior lobe(SVII) of the liver with enhanced abdominal CT (Figure 1, Table 1). The local hospital gave an imaging diagnosis of hepatocellular carcinoma or metastatic lesions, and recommended further MRI examination. The patient came to our hospital for further evaluation.
Patient 2: The patient was admitted to the local hospital for a health examination, and a massive hepatic lesion was accidentally discovered by ultrasound 7 d ago. An enhanced abdominal CT showed a huge solid mass (100 mm × 80 mm) and suspected a hepatic carcinoma (HCC). The patient was admitted to our hospital for further evaluation and treatment.
History of past illness
Patient 1: The patient had a 10-year history of chronic hepatitis B virus infection, and had not received any antiviral therapy. The patient underwent two caesarean sections 7 years ago and 3 years ago.
Patient 2: Unremarkable.
Personal and family history
Patient 1: Unremarkable.
Patient 2: Unremarkable.
Physical examination upon admission
Patient 1: Vital signs, such as temperature, respiration, heart rate, and blood pressure,were within normal limits. There were no positive signs upon abdominal examination.
Patient 2: Vital signs, such as temperature, respiration, heart rate, and blood pressure,were within normal limits. There were no positive signs upon abdominal examination.
Laboratory examinations
Patient 1: Blood routine: neutrophil absolute counts and neutrophil percentages were low, at 1.53 × 109/L and 40.6%, respectively; the white blood cell counts, red blood cell counts, platelet counts, and hemoglobin assays were normal. Liver function tests, such as serum proteins, transaminases and bilirubin, were normal. Quantitative analysis of hepatitis B markers showed three small positives: Hepatitis B virus surface antigen(586.9 IU/mL), hepatitis B virus e antibody (0.07 COI), and hepatitis B virus core antibody (5.99 COI). The levels of tumor markers, including α-fetoprotein (AFP),carcinoembryonic antigen (CEA), cancer antigen (CA) 125 and CA19-9, were within normal ranges.
Patient 2: Blood routine examination: high white blood cell counts (12.27 × 109/L),high platelet counts (571 × 109/L), low hemoglobin levels (116 g/L), and low hematocrit levels (36.4%). Clinical biochemistry: low apolipoprotein A-1 and prealbumin were 0.76 g/L and 151 mg/L, respectively; high apolipoprotein B, 1.09 g/L; high lipoprotein (a), 1448 mg/L, total protein, 90.8 g/L, globulin, 47.70 g/L,alkaline phosphatase, 219 U/L and γ-glutamyltransferase, 109.0 U/L. A thyroid function test showed high total thyroxine (255.444 nmol/L), as well as normal triiodothyronine, thyroid stimulating hormone, free triiodothyronine and free thyroxine. The levels of tumor markers, including AFP, CEA, CA125, and CA19-9,were within normal ranges.
Imaging examinations
Patient 1: The unenhanced ultrasound (US) showed two heterogeneous hypoechoic lesions without detectable internal blood flow in the SVII of the liver, which was suspected for metastasis (Figure 2).
A gadoxetic acid [gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid(Gd-EOB-DTPA)]-enhanced liver MRI was performed using the Dutch Philips Achieva 3.0T superconducting MR scanner, with a 16-channel phase array coil. The no-contrast images, including axial and coronal T1- and T2-weighted with/without fat saturation images, were obtained in sequence[6]. Multiphase dynamic imaging was scanned after a rapid bolus of Gd-EOB-DTPA (0.025 mmol/kg) at a rate of 1.5 mL/s,immediately followed by a 30 mL saline flush using a power injector[7]. Arterial phase,portal venous phase, equilibrium phase and hepatobiliary phase (HBP) images were performed sequentially, and scanned at 20-30 s, 60-70 s, 120-150 s, and 20 min after the injection.
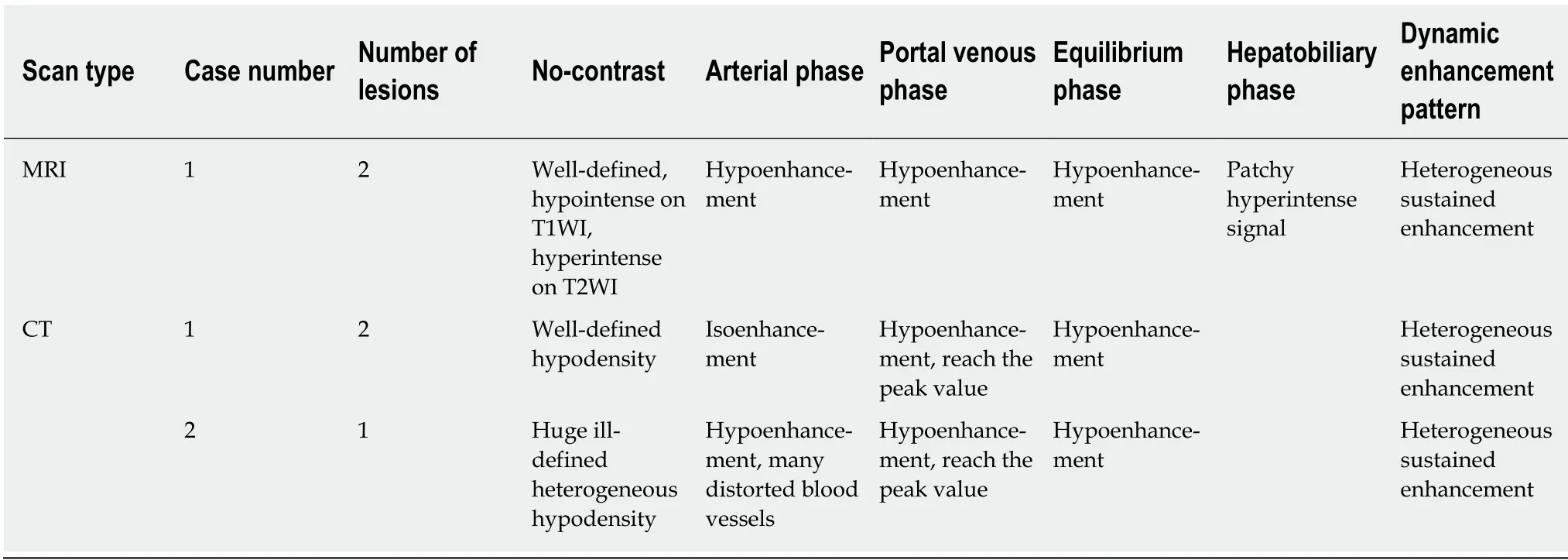
Table 1 Computed tomography and magnetic resonance imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumors
Enhanced MRI with hepatocyte-specific contrast agents showed two masses of 36 mm × 32 mm and 20 mm × 16 mm, respectively, in the SVII of the liver (Figure 3,Table 1). It was diagnosed as a metastatic tumor.
Patient 2: The US revealed one massive heterogeneous hypoechoic lesion with internal blood flow located in the right lobe of the liver, and was diagnosed as HCC(Figure 4).
The upper abdominal scan was performed by using Philips Brilliance 256-slices spiral CT. The patient was placed in the supine position. The scan ranged from the upper edge of the xiphoid to the lower pole of the kidney. The tube voltage was 120 kV, the tube current was 200 mA, and the section thickness was 5 mm. A non-ionic contrast agent (Omnipaque 350 mg I/mL) was injected at a dose of 0.9-1.5 mL/kg by using a power injector. A triphasic liver protocol was sequentially scanned at 30 s(arterial phase), 60 s (portal venous phase), and 120 s (equilibrium phase) after injection[8].
The enhanced CT revealed a huge heterogeneous enhancement solid tumor in the right lobe of the liver with a size of 100 mm × 80 mm. Multiple enlarged lymph nodes were seen in the hepatic hilar region (Figure 5, Table 1). The imaging diagnosis was a large HCC of the right lobe, with multiple lymph node metastasis in the hilar region.
FINAL DIAGNOSIS
Patient 1
Microscopically, the tumors were composed of spindle cells, abundant small lymphocytes, eosinophils and plasmocytes (Figure 6), mixed with multifocal coagulative necrosis and granulomas. Molecular pathology results: Epstein-Barrencoded-RNA (EBER)(+)(Figure 6). The immunohistochemical test results included the following: CD21(+), CD23(+) (Figure 6), CK (pan)(-), CD163(+), CD68(+), CD3(+),CD30(-), CD20(+), Ki-67 (20%+), S-100(-), CD138(+), CD2(+), CD7(+), CD5(+), CD56(-),Granzyme B(-), TIA-1(+), CD1a(-), and SMA (scattered +); Special staining results:Acid-fast(-), PAS(-). The primary IPT-like FDCS tumors of the liver were diagnosed pathologically after operation.
Patient 2
Microscopically, the tumors were composed of spindle cells, abundant small lymphocytes and plasmocytes, and scattered eosinophils mixed with multifocal coagulative necrosis. Molecular pathology results: Epstein-Barr-encoded-RNA(EBER)(+). The immunohistochemical test results included the following:CD21(+), CK (pan)(-), CD163(+), CD68(+), CD3(+), CD20(+), Ki-67 (20%+), S-100(-),CD138(+), CD38(+), kappa(+), lambda(+), IgG(+), IgG4(-), and PAS(-). Primary IPTlike FDCS tumors of the liver with lymph node involvement were diagnosed
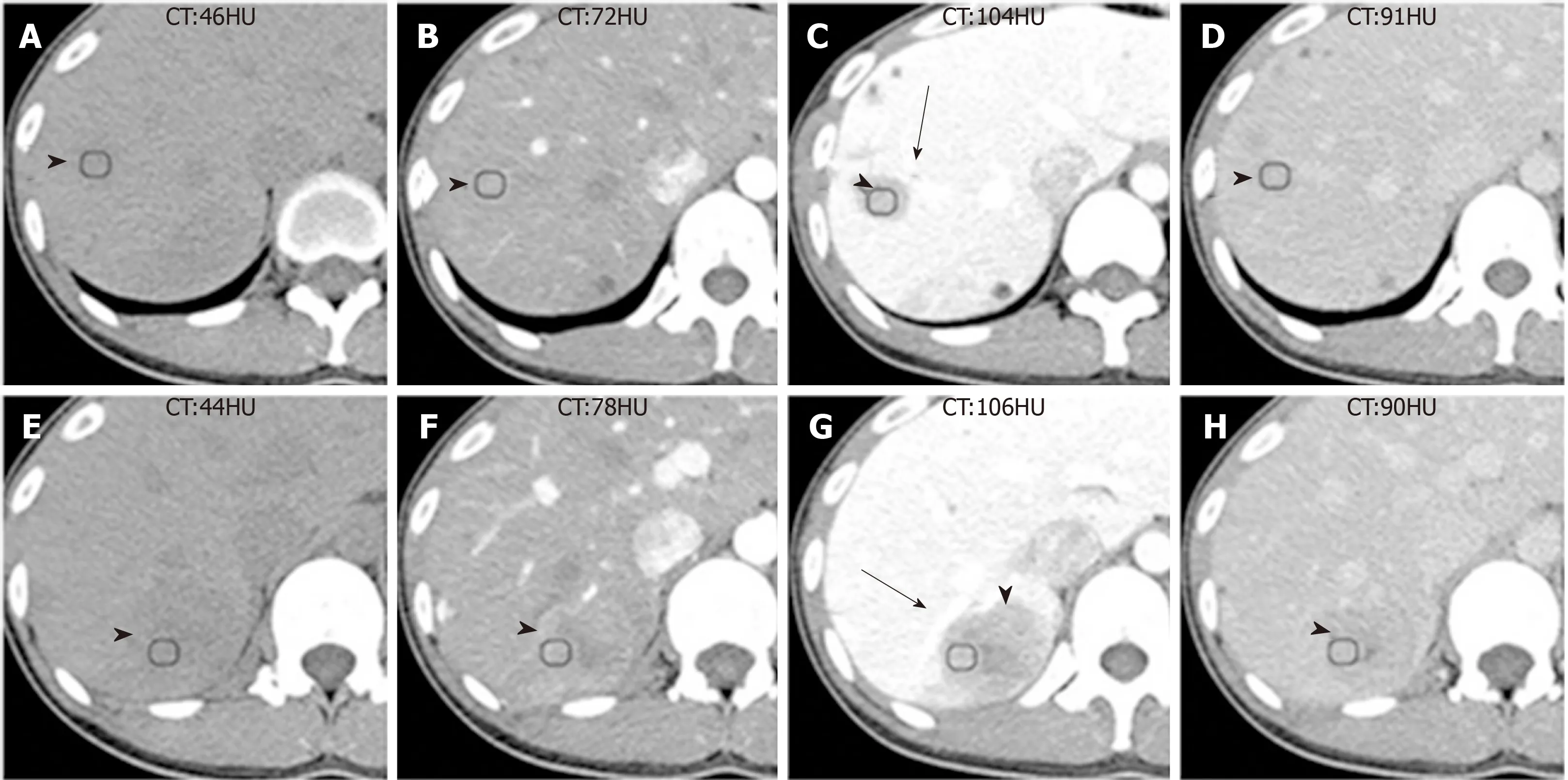
Figure 1 Computed tomography images of Case 1.
pathologically after operation. No tumor involvement was found in the liver surgical margin.
TREATMENT
Patient 1
Considering that the primary cancer was not found in the chest and abdomen CT, and the patient was eager to remove the liver lesions, the right hepatectomy was planned to provide an optimized method for the complete resection of the two hepatic tumors.After operation, the patient was reviewed regularly.
Patient 2
Right hepatectomy and lymph node dissection were planned to provide an optimized method for the complete resection of the large mass. After operation, the patient accepted regular reexamination.
OUTCOME AND FOLLOW-UP
Patient 1
The surgery was successful and both tumors were completely removed. Postoperative regular review was performed until now, the patient recovered well, and no recurrence or metastasis was seen during the 28 mo follow-up.
Patient 2
The huge tumor and enlarged lymph nodes were completely removed without major intraoperative complications. After operation, the patient was followed-up until now,and no recurrence or metastasis appeared during the 10 mo follow-up.
DISCUSSION
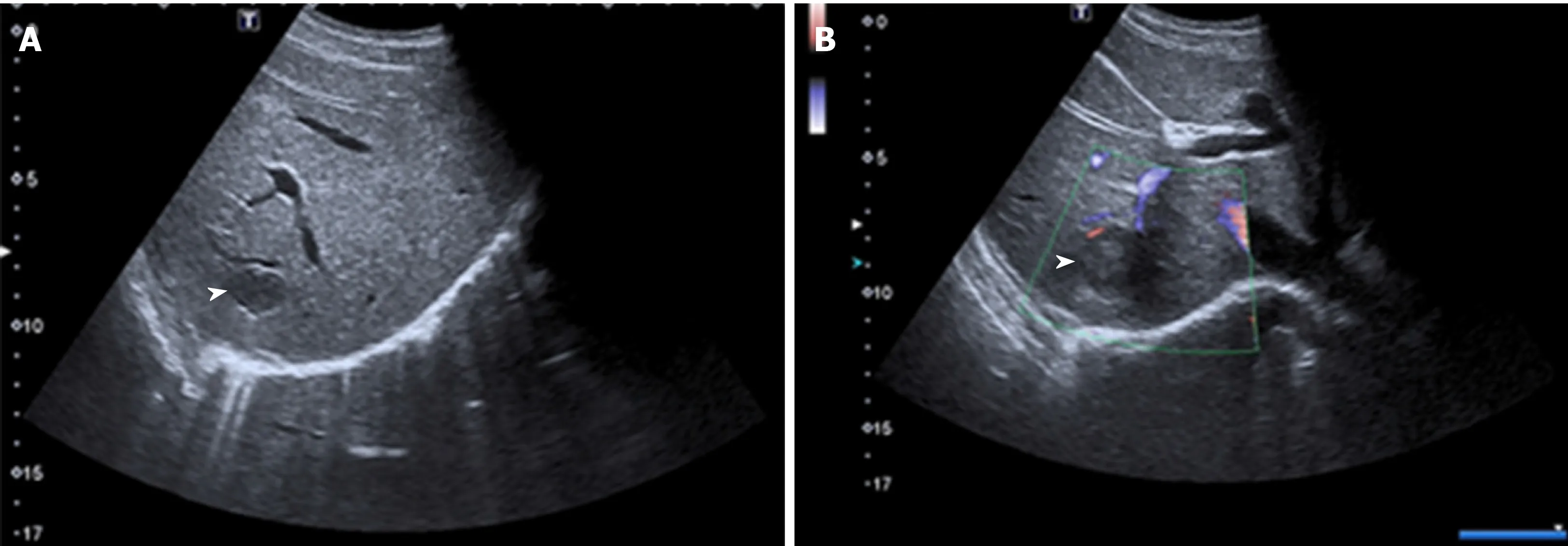
Figure 2 Unenhanced ultrasound images of Case 1.
The IPT-like FDC tumor is an extremely rare and low-grade malignant soft tissue sarcoma[1]. It presents with indolent clinical course, and is typically asymptomatic like in our cases, except for the side effects of weight loss and fever, and is associated with EBV[3]. In contrast to the female patient population predominance reported in the literature[1,3,9], the sex ratio of patients was equal in our series. This difference might be caused by the small number of cases. Previous studies have shown that the age range of the disease was 28-68 years[1]. In our series, the mean age at diagnosis is 39.5 years(range, 31-48 years). A strong relationship between FDC tumors and Castleman disease or autoimmune conditions have been reported in the literature[10,11]. This may be the reason why the case 2 patient developed a thyroid dysfunction. However, the exact mechanism remains unclear.
IPT-like FDC tumors are diagnosed based on variable amounts of spindle tumor cells with intense lymphoplasmacytic infiltrates, coupled with a heavy inflammatory background and supportive immunohistochemistry[1,12]. Moreover, the positive expression of EBV-encoded small RNA is helpful for diagnosis[1,3,12]. However, many IPT-like FDC tumors were previously diagnosed as IPT because specific immunohistochemical markers are not recognized to represent FDCs. The best available immunohistochemical markers for IPT-like FDC tumors are CD21, CD23 and CD35[1,12,13].
To the best of our knowledge, the radiologic findings of IPT-like FDC tumors of the liver have rarely been described. We only found two reports that briefly mention the imaging features of IPT-like FDC tumors of the liver (see Wu et al[14]), where the tumor appeared as a well-defined, heterogeneous arterial-enhanced mass without significant portovenous washout. However, this study did not provide the delay phase images;in another case report study[3], they only showed portal phase images without a summary of imaging appearances. Therefore, the enhancement patterns in the multiphase CT and MRI imaging findings of IPT-like FDC tumors have not been previously reported.
The imaging features of IPT-like FDC tumors of the liver aid in making correct diagnoses before treatment when a neoplasm is detected. In unenhanced US, the lesions showed well- or ill-defined heterogeneous hypoechoic tumors with or without detectable internal blood flow. Correlating to CT imaging, there is no calcification.The findings of unenhanced US imaging were non-specific and difficult to distinguish from other cystic and solid liver tumors[15-17]. In CT images (Table 1), all three tumors showed heterogeneous soft-tissue density with sustained hypoenhancement. This finding is consistent with the findings of Li et al[18]and Bui et al[19], who summarized the CT imaging findings of abdominal FDC sarcomas. Both of the cases we reported here had low-density areas after intravenous administration of contrast medium, but their pathological components were different. In case 1, the low-attenuating areas decreased during the multiphase images. This area histologically corresponded to the tumor’s spindle cells and fibrotic components, which showed centripetally persistent enhancement. These imaging features were also reported in IPT-like FDC tumors of the spleen[19,20]. However, in case 2, the hypodense parts were due to necrosis and hemorrhage in enhancement scanning, which might be related to the relatively large size[18]. On MRI (Table 1), with hepatocyte-specific contrast agents (Gd-EOB-DTPA) in case 1, the most characteristic feature was the central stellate enhancement during HBP. The mechanistic explanation for the retention of gadoxetic acid on HBP images remains elusive, but it may have something to do with the transporter’s located on either the sinusoidal (OATP1B1/3 and MRP3) or canalicular (MRP2) membranes of the cell[21]. Moreover, the stellate enhancement areas during HBP within the masses was T1 and T2 hypointense. Furthermore, the enhancement pattern in multiphase MRI was similar to that in CT.
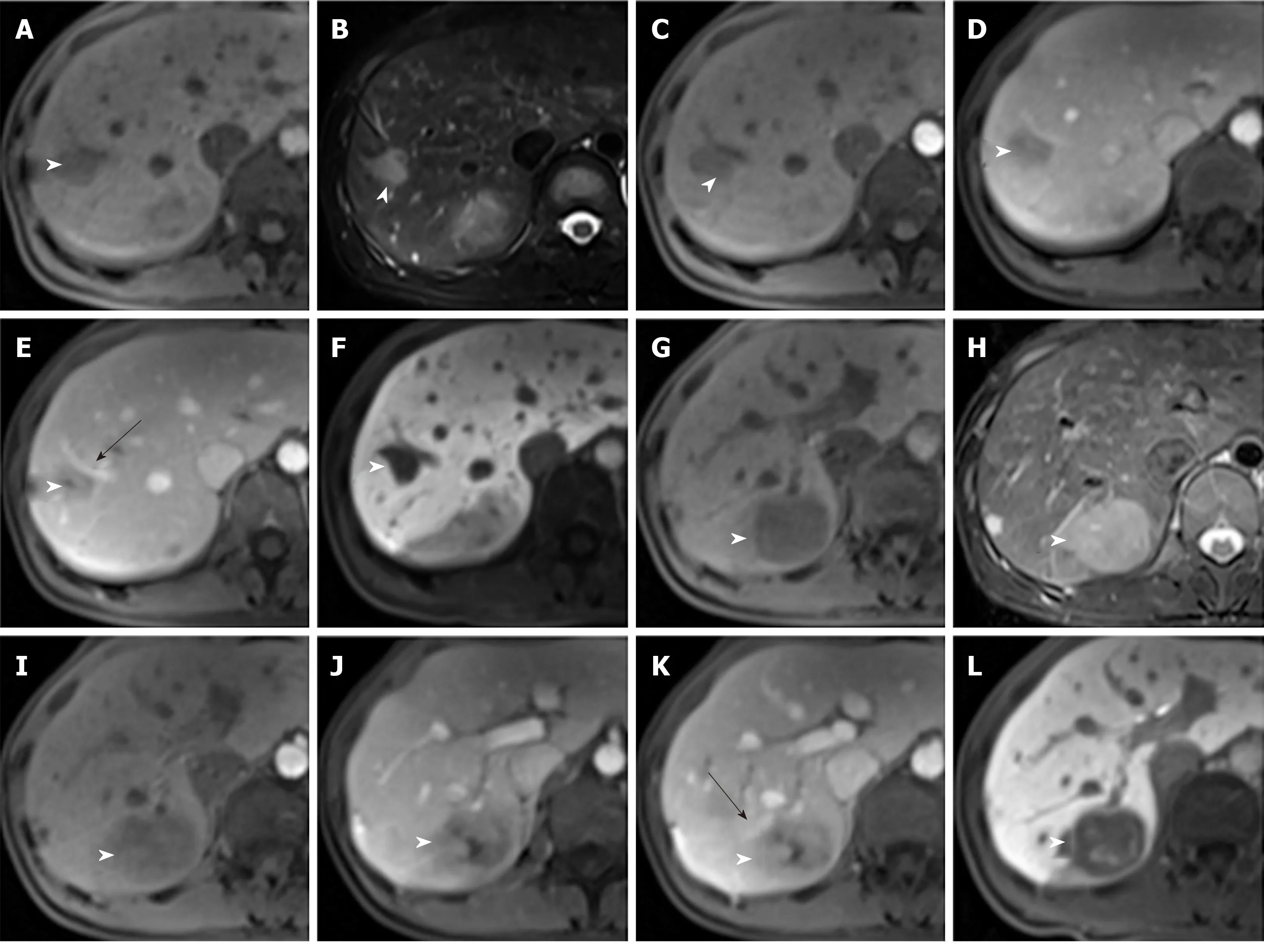
Figure 3 Magnetic resonance images of Case 1.
Differential diagnosis of IPT-like FDC tumors of the liver should include many tumors, such as IPTs, HCCs, metastases, leiomyosarcomas, malignant fibrous histiocytomas, fibrosarcomas, extragastrointestinal stromal tumors, solitary fibrous tumors, and neurogenic tumors[18]. The imaging features of the above tumors overlap with IPT-like FDC tumors, and the first three are the main differential diagnosis. IPTs of the liver usually appears as heterogeneous enhancing tumors with uneven peripheral enhancements[22]. Suspicion should be high for IPT in patients with low grade fever, jaundice, hepatomegaly, weight loss or leukocytosis[23]. Typical HCC is characterized by portal phase washout, elevated AFP and threshold growth at followup imaging[22,24]. However, atypical HCC is sometimes difficult to distinguish from sarcomas and metastases[24,25]. Metastasis is most common in colon cancer[16], with typical peripheral rim enhancements and hypoavascular internal necrotic regions[15].However, different primary tumors have different imaging characteristics upon metastasis to the liver[16,23]. Thus the differential diagnosis is very difficult. Given the rarity of IPT-like FDC tumors of the liver, preoperative imaging diagnosis of these tumors can be challenging; correct final diagnoses depend upon histopathological testing and immunohistochemical markers.
There is no standard treatment for IPT-like FDC tumors of the liver. If the lesion is confined to the liver, surgical excision is the treatment of choice[12]. There is no evidence to prove that adjuvant therapy is effective for this tumor[20]. However, given the potential of recurrence after hepatic lobectomy, surveillance is currently suggested after resection[1,19].
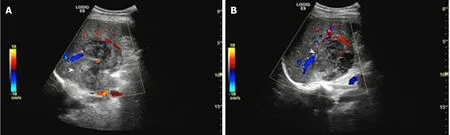
Figure 4 Unenhanced ultrasound images of Case 2.
CONCLUSION
In conclusion, the IPT-like FDC tumor of the liver is an extremely rare tumor. When hepatic tumors show heterogeneous sustained hypoenhancement with patchy enhancement during HBP, IPT-like FDC tumors should be considered in the differential diagnosis.
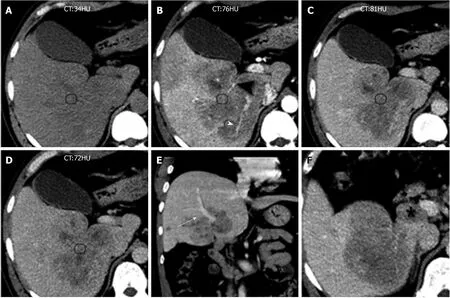
Figure 5 Computed tomography images of Case 2.
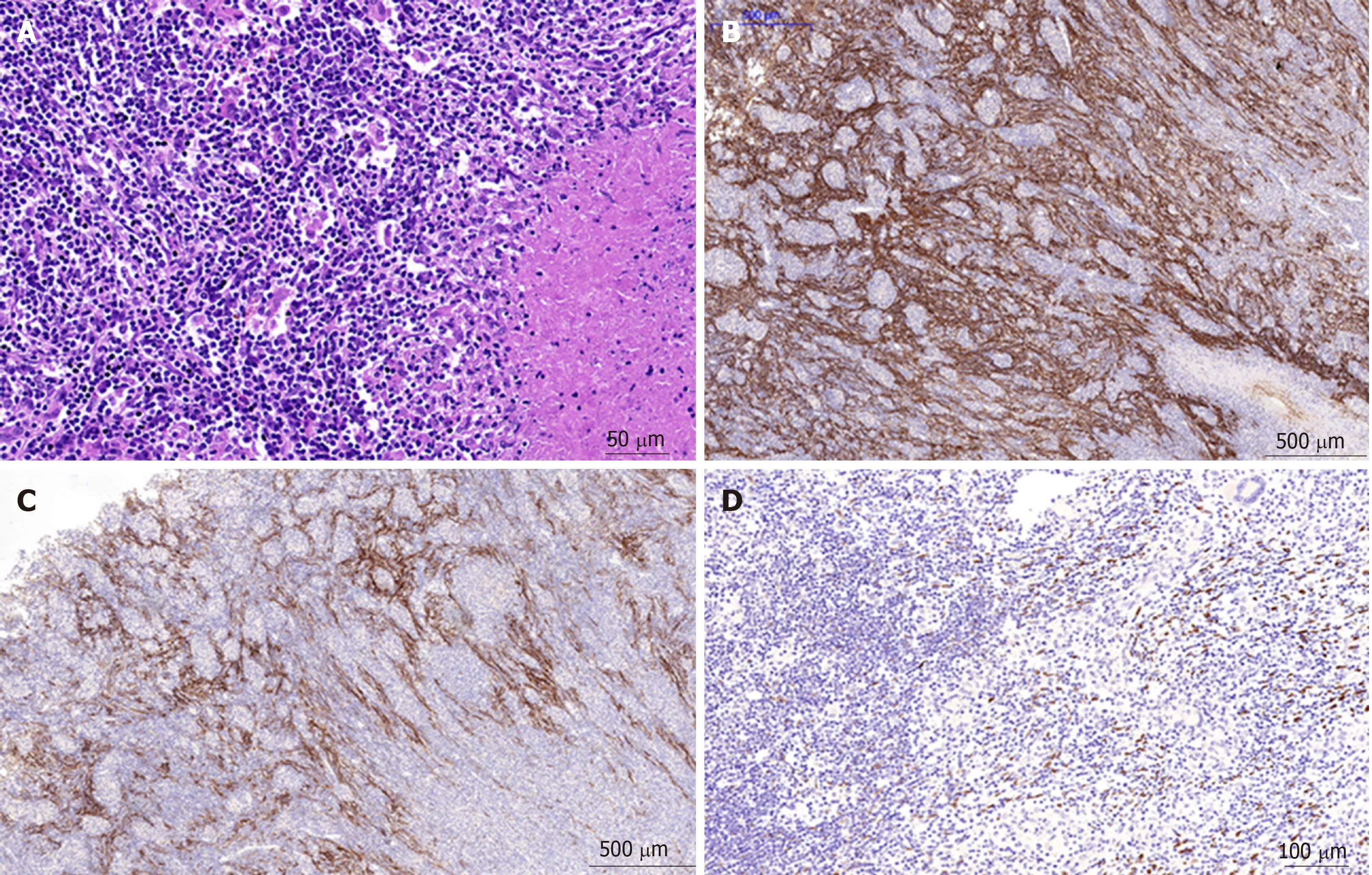
Figure 6 Inflammatory pseudotumor-like follicular dendritic cell tumor from the liver of a 31-year-old Chinese woman.
杂志排行
World Journal of Gastroenterology的其它文章
- Gastrointestinal discomforts and dietary intake in Chinese urban elders: A cross-sectional study in eight cities of China
- Prognostic value of risk scoring systems for cirrhotic patients with variceal bleeding
- Irisin attenuates intestinal injury, oxidative and endoplasmic reticulum stress in mice with L-arginine-induced acute pancreatitis
- Toxoplasma ROP16I/III ameliorated inflammatory bowel diseases via inducing M2 phenotype of macrophages
- MicroRNA-760 acts as a tumor suppressor in gastric cancer development via inhibiting G-protein-coupled receptor kinase interacting protein-1 transcription
- LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway
