勘误:二甲基亚砜中Er-Ni-Co合金膜的电化学制备。2002, 18 (8), 764. doi: 10.3866/PKU.WHXB20020819
2019-11-04李高仁童叶翔刘冠昆徐常威
李高仁,童叶翔,刘冠昆,徐常威
中山大学化学学院,广州 510275
作者希望对2002年发表的论文1进行一下更正。原论文中的图1和图2使用有误,正确的图1和图2如下图所示。
原论文中结果与讨论2.1部分中的第一段修改如下:
铂(Pt)电极在0.1 mol·L-1LiClO4-DMSO溶液中的循环伏安曲线如图1 (1)所示,该图显示其电化学窗口为4.77 V,阳极极限电势为1.37 V,阴极极限电势为-3.40 V。在扫描速度60 mV·s-1时,Pt电极在0.01 mol·L-1ErCl3+ 0.1 mol·L-1LiClO4-DMSO溶液中的循环伏安曲线如图1 (2)所示,该图中阴极还原峰的峰电势为2.11 V。该阴极还原峰相应于Er(III)的还原。Pt电极在不同扫描速率υ下在0.01 mol·L-1ErCl3+ 0.1 mol·L-1LiClO4-DMSO溶液中的循环伏安曲线如图2所示,根据其峰电势Ep–lnυ线性关系,可显示Er(III)在Pt电极上的电还原为一步完全不可逆反应。
The authors wish to make the following correction to our published paper1. Figures 1 and 2 in the original paper are used incorrectly. The correct Figures 1 and 2 are shown in the following figure.
In the results of the original paper the first paragraph in Part 2.1 are revised as follows:
The cyclic voltammetry curve of platinum (Pt) electrode in 0.1 mol·L-1LiClO4-DMSO solution is shown in Fig.1 (1). The figure shows that the electrochemical window is 4.77 V, the anode limit potential is 1.37 V and the cathode limit potential is -3.40 V. The cyclic voltammetric curves of Pt electrodes in 0.01 mol·L-1ErCl3+ 0.1 mol·L-1LiClO4-DMSO solution at the scan rate of 60 mV·S-1are shown in Fig. 1 (2). The peak potential of the cathodic reduction peak in this figure is 2.11 V. The cathodic reduction peak corresponds to the reduction of Er(III).The cyclic voltammetric curves of Pt electrodes in 0.01 mol·L-1ErCl3+ 0.1 mol·L-1LiClO4-DMSO solution at the different scan rates are shown in Fig. 2. According to the linear relationship of the peak potentialEp–lnυ, the electroreduction of Er(III) on Pt electrodes is a completely irreversible step.
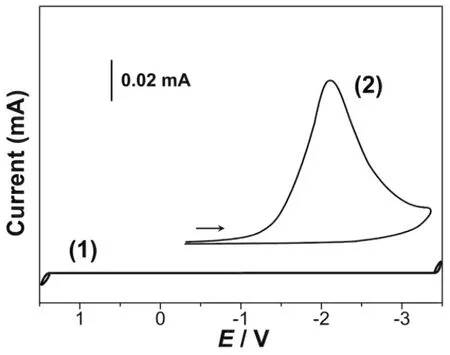
图1 在铂电极上的循环伏安曲线:1) 0.1 mol·L-1 LiClO4-DMSO,2) 0.01 mol·L-1 ErCl3 + 0.1 mol·L-1 LiClO4-DMSO,s = 0.05 cm2,T = 303 K,v = 60 mV·s-1Fig.1 Cyclic voltammograms on Pt electrode: 1) 0.1 mol·L-1 LiClO4-DMSO, 2) 0.01 mol·L-1 ErCl3 + 0.1 mol·L-1 LiClO4-DMSO, s = 0.05 cm2, T = 303 K, v = 60 mV·s-1.
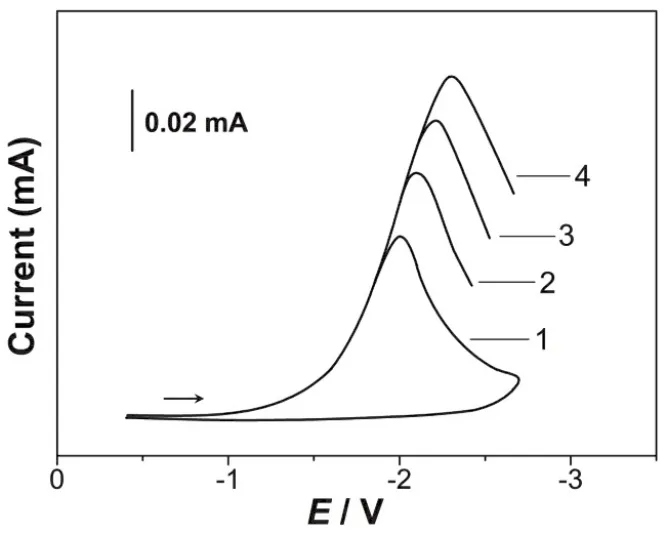
图 2 0.01 mol·L-1 ErCl3 + 0.1 mol·L-1 LiClO4-DMSO在铂电极上的循环伏安曲线,T = 303 K,v/mV·s-1:1) 40,2) 60,3) 80,4) 100Fig. 2 Cyclic voltammograms on Pt electrode in 0.01 mol·L-1 ErCl3 + 0.1 mol·L-1 LiClO4-DMSO, T = 303 K,v/mV·s-1: 1) 40, 2) 60, 3) 80, 4) 100.
