Microfluidic three-dimensional cell culture of stem cells for highthroughput analysis
2019-10-31JeongAhKimSoohyunHongWonJongRhee
Jeong Ah Kim, Soohyun Hong, Won Jong Rhee
Jeong Ah Kim, Soohyun Hong, Research Center for Bioconvergence Analysis, Korea Basic Science Institute, Cheongju 28119, South Korea
Jeong Ah Kim, Department of Bio-Analytical Science, University of Science and Technology,Daejeon 34113, South Korea
Soohyun Hong, Program in Biomicro System Technology, Korea University, Seoul 02841,South Korea
Won Jong Rhee, Division of Bioengineering, Incheon National University, Incheon 22012,South Korea
Won Jong Rhee, Department of Bioengineering and Nano-Bioengineering, Incheon National University, Incheon 22012, South Korea
Abstract
Key words: Stem cell; Microfluidic technology; Three-dimensional cell culture; Highthroughput screening
INTRODUCTION
Stem cell engineering, the interface of engineering with the world of stem cells, has emerged over the last decade and covers fields from the basic science to engineered approaches[1].With the significant advances in the development of stem cells technologies, many approaches have been introduced for modeling genetic diseases,and these models have been made available for applications, such asin vitrodrug tests[2-5].Usually, immortalized cell lines lack the differentiated functions of specific organs, and they may not display the disease-specific or patient-specific phenotypes.Also, these cell lines may include oncogenic factors, such as SV40, during the transformation[6].Stem cells self-renew extensively and have pluripotency in that they can differentiate into all types of cells in an organism.Thus, stem cells have gained significant attention in providing a variety of specialized cells that are relevant for modeling human development and disease as well as applications in regenerative medicine[7-9].However, stem cells tend to be very sensitive to various biochemical and physiological cures, and their fate is altered easily by their microenvironment.Also,stem cells themselves cannot recapitulate the microenvironment that is physiologically relevant to the complex structure of human organs.
Recently, emphasis has been placed on the roles of the three-dimensional (3D) cell culture techniques that can precisely control multiple cues in the biological microenvironment of stem cells.The 3D cell culture systems are comprised of organspecific cells and their microenvironments, so they were able to mimic human physiology more accurately.Indeed, organ-on-a-chip platforms consist of tissuespecific cells and their extracellular matrixes (ECMs) that can remodel 3D tissue architectures and also mimic the physiological conditions, such as shear stress and fluidic flow[10,11].In this regard, microfluidic devices are ideally suited for stem cell cultures and their maintenance by providing a way to recreate a microenvironmentin vivo.Also, this system has flexibility and feasibility that can be coupled to robust hardware systems that are capable of high-throughput analysis, rapid sampling, and liquid handling, allowing them to process hundreds of samples[12,13].Such advantages have led to the innovative development of organ-on-a-chip or organoid-on-a chip systems based on stem cells and their applications in high-throughput drug screening[9,14-16].
In this review, we discuss the most recent advances in 3D microfluidic technology in the field of stem cell research and their applications for high-throughput screening(HTS).Also, we review the progress that has been made to generate organ-on-a-chip platforms and, more recently, organoid-on-a-chip, particularly with an emphasis on important innovations of different microfluidic aspects to improve stem cell research for high-throughput analysis.Then, we discuss how these technologies combined with high-throughput analysis might be enhanced in the future.
3D MICROFLUIDIC CELL CULTURE
It is difficult to maintain the cellular functions in conventional two-dimensional (2D)cell cultures for prolonged periods of time because these cultures lack the physiological microenvironment ofin vivotissue.Such cell systems may not be able to prove the real cellular response to drugs due to their inability to control and mimic the microenvironment of complicated organs.Also, drug diffusion kinetics is not modeled accurately in a 2D cell culture.Therefore, 2D cell cultures increase the chances of providing misleading and non-predictive preclinical results forin vivotest[17,18].On the other hand,in vivoanimal tests have traditionally been the gold standard models for preclinical efficacy tests in the drug discovery process, but various issues still exist, such as ethical issues and genetic differences between species.In addition, animal models have many drawbacks, such as high cost and uncertainties in the interpretation of the results in many pathological studies.Due to these weaknesses of the traditional models, an alternative cell culture model that corresponds to anin vivosystem is required in order to obtain better predictions of the preclinical response to drugs.
In recent years, advances in microfluidic technology in 3D cell cultures have resulted in promising alternative methods to the conventionalin vivoandin vitromodels in the field of drug development[4,15,19-23].In nature, the fate ofin vivocells is affected largely by external physical and chemical factors, and cell-cell and cell-ECM interact actively with each other.The 3D microfluidic cell culture platform is considered to precisely control these external cuesin vitro, thereby producing more reliable and predictive preclinical data than either animal models or conventional 2D cell-based models[5,20].This is consistent with the trend toward more physiologicallyrelevant models, such as 3D organs or organoid-on-a-chips, for use in the early phase of drug discovery and development.
Over the past few decades, advances in microfluidic technologies have accelerated the development rate of the 3D cell culture or tissue model by virtue of the following significant features[19,24,25].First, the microscale dimensions of microfluidic platforms are suitable for creating the biological microenvironment ofin vivotissues that have high complexity and spatial heterogeneity.Also, the physical structure of microfluidic channels can provide a well-controlled hydrodynamic environment, such as a chemical gradient or fluidic flow[26,27].Second, the small scale of the systems requires only a small amount of cells and reagents in the experiments, which lowers the cost as the research progress from bio-analysis to drug development.Third, microfluidic technology can integrate the multiple and subsequent steps of bioanalysis, from culture and liquid handling to detection and analysis[28,29].In addition, this technology is amenable to high-resolution, real-time monitoring, as well as the analysis of biochemical, genetic, and metabolic processes under conditions that closely resemblein vivoconditions.With these advantages, various approaches using microfluidic technology have been suggested in association with the study of stem cells, such as the cell culture, identification, and screening of cells as well as modeling diseases.We discuss the 3D microfluidic technologies in more detail because they provide potential solutions for problems in stem cell engineering.
3D MICROFLUIDICS IN STEM CELL ENGINEERING
Microfluidic chips provide a new platform with unique advantages to mimic complex physiological microenvironmentsin vivo.Since some groups started to use microfluidic technology for patterning or capturing stem cells in the early 2000s[30-32],the use of this technology in stem cell research has increased significantly.The emerging and rapid development of microfluidic technologies has presented an ideal solution in stem cell engineering, as summarized in Table 1.Many studies have been reported that focused on the application of microfluidic devices for stem cell research,such as culture, differentiation, patterning, tissue engineering, recreating organs, drug discovery, and therapeutics.In stem cell culture, it is important to control the biochemical microenvironment of cells to regulate the basic cell functions and biological processes, such as differentiation, development, and immune response.The temporal and spatial control over defined gradients of soluble factors or immobilized factors[33]provided by microchannel-based microfluidic devices can be an important advantage in stem cell research.For patterning cells or ECMs in desired locations, the patterned channel or the polydimethylsiloxane (PDMS) microwell generated using the soft lithography technique are simple and traditional method, while stably interfacing with other supporting cells[34,35].The benefits of combining biomaterial engineering and microfluidics for stem cell applications are clear.Microfluidic technology could be used to mimic the spatial heterogeneity of stem cell microenvironment[36].In particular, the chemical gradient in a microfluidic channel is one of the unique features that allows for this heterogeneous microenvironment.Some groups have used microfluidic approaches in which cells within hydrogels were exposed to desirable soluble gradients in 3D microenvironments[26,37].Also, chemical gradient generators that use multiple microfluidic channels with flow control have been suggested to investigate the neural stem cell differentiation by the chemokine(CXCL12) gradient generated within a single device[38].To study cell-cell or cell-ECM interactions, the spatially-isolated compartments in a microfluidic device are also useful in investigating the differentiation or migration behavior of stem cells[39], and they help visualize their biological processes within a microscale device.
Despite the high potential impact of stem cell technologies, there are some technical challenges associated with culturing and differentiating stem cells for use in drug discovery and development.With a conventional well plate or dish, it is difficult to mimic the physiological complexity of the stem cell niche because it is a microenvironment that provides a variety of stimuli.Flow is one of the most important stimuli since some organs are affected by the shear flow induced by the blood stream.Microfluidic devices are the only platform capable of supplying flow,thereby inducing the important flow shear stress.This provides a way of observing stem cells by the effect of physical stimuli[40].
A 3D co-culture for niche construction can be achieved with droplet-based technology.By varying the ratio of the flow rates of the two cell streams, the ratio of the concentrations of the two types of cells can be altered within the microgel[41,42].This technique enables the cells to be compartmentalized into a mono-dispersed and physicochemically-defined 3D matrix.Another advantage of this technique is its generation of high-throughput and microscale cell-matrix environments.For example,Sakaiet al[41]reported the enclosed rat-adipose-derived stem cell aggregates in gelatin microbeads using a microfluidic droplet technique in which the stem cells were recoated with additional supporting cells to construct a heterogeneous tissue structure.
Microfluidic devices combined with electrics and physics have been used to separate single cells[43-45].Optical tweezers, electrical impedance, and dielectrophoresis techniques combined with microfluidic technologies can be used to sort or separate cells.For instance, Songet al[45]have developed a method to identify the differentiated state of human embryonic stem cells (ESCs) using electrical impedance in a microfluidic channel.Numerous other approaches have been tried by combining microfluidic technology with different analysis methods and by integrating various structures and functions.Recent advances in microfluidic technology using hydrodynamic trapping have resulted in an array culture method that enables precise and standardized tools that are controllable, constituent and high-throughput.Throughout the drug discovery and development process, a human stem cell-based cell culture system can be important in screening, validating candidate compounds and preclinical studies, such as the toxicity test, efficacy test, and the mechanism studies (e.g., integration and automation[46-50], mechanical and electrical actuator[51,52]).Next, we discuss the microfluidic technologies in more detail for the high-throughput analysis of stem cells.
HIGH-THROUGHPUT ANALYSIS TECHNIQUES FOR STEM CELL ENGINEERING
The development of drugs requires a series of complex procedures that involves preclinical and clinical studies with well-established regulatory compliance.Developing a new drug,i.e., from the discovery stage to approval by the United States Food and Drug Administration, generally takes more than 10 years and costs more than two billion dollars, and only about 10% of the compounds progress successfully through clinical development[6,20].Current standard drug discovery traditionally starts with the 2D cell culture-based screening of compounds, followed by animal model testing and clinical trials.While 2D cell-based assays are used extensively because they have certain advantages, such as lower cost and higher throughput than animal tests, they also have limitations.These limitations include the lack of a cell-cell or a cell- ECM, which results in failure to reconstitute thein vivocellular microenvironments, which means they cannot maintain the differentiated functions of the cells.Animal tests also cause errant pharmacokinetic predictions due to the differences between animal and human species that make it impossible to directly translate the findings in animal models to human biology.Therefore, there is a considerable need for new approach with a more accurate and cost-effective system that is representative of humans to efficiently screen and validate the potential drug candidates in the early stages of drug development[53].
Miniaturized, high-throughput techniques using microfluidics are required to identify efficient and cost-effective compounds using stem cell-based models and to gain insight into the possible underlying mechanism[23,54,55].Microfluidic devices with micro-sized scale, automatic operation, and large-scale integration possibly can offer many unique benefits, including high-throughputs, low cost, and high efficiency in drug development.Also, due to the nature of microfluidic devices, quantitativeanalysis can be a useful tool in combinatorial mixing and processing samples[28,56,57]In drug discovery, HTS is a major instrumental technique.HTS commonly uses well plates ranging from 96 to 1536 plates, and these plates enable parallel and simultaneous testing of multiple factors.This allows rapid analysis of thousands of chemicals and biochemical using genetic or pharmacological tests in parallel, and this allows us to identify specific compounds for specific biological processes.Among these systems, the development of fast and automated microscopes, such as the highcontent screening (HCS) microscope, has been accelerated by hardware advances and innovations in the software for analyzing images[13].This system uses an automated liquid handler to simultaneously process hundreds of biological samples, and it provides the unbiased, multiple-parametric data with the high-spatiotemporal resolution from the acquired images, and it does so at the levels of individual proteins, organelles, whole cells, or even entire organisms.Therefore, this approach has been used to understand the complexity and dynamics of the cell biological processes that occur in cells and to identify a plethora of quantitative phenotypes of varying complexity in numerous different models.

Table 1 Features of microfluidic techniques for stem cell engineering
With such advances in the scientific equipment, different approaches have been suggested for stem cell-based screening platforms using microfluidic devices.Table 2 provides a summary of some examples of microfluidic systems that have highthroughput capability for stem cell research.Miniaturization of the microfluidic platform increases the throughput of assays used to analyze stem cells because the small scale of the samples reduces both the consumption of reagents and the number of cells required[58-60].Leeet al[61]and Duet al[57]suggested the microarray technique(1080 chips) and the microfluidic droplet array technique (342 droplets), respectively,for generating miniaturized cell array systems using cancer cell lines for the highthroughput testing of drugs.These techniques also can be applied to stem cell research since they provide rapid and cost-effective testing for a wide range of applications that involve in high-throughput toxicity tests[60,62].With the advent of robotic spotting technology and microfabrication, it is possible to generate the pattern of cells that are encapsulated in a 3D ECM matrix and that support cell growth at the microscale[59,62].One of the powerful techniques of microfluidics for high-throughput 3D cell generation is the flow focusing technique, which is used for the encapsulation of cells in the ECM or hydrogel beads[42,63,64].To understand the fate of stem cells, it is important to regulate the stem cell niche.Gobaaet al[65]reported microengineered niche spotting that was comprised of a hydrogel array for controlling the stiffness of the gel.As a similar example, Beachleyet al[66]reported a 3D microtissue array when they used the spotting technique to investigate the tissue-specific response based on the composition of the ECM.
Also, soft lithography can be used to fabricate an array of wells with physicallydefined dimensions, allowing for the cellular aggregates in the wells.The defined the sizes of wells can control the size of the cell aggregates and offer an attractive solution for controlling the fate of stem cells.Vrijet al[67]used optically-clear, cyclic olefin polymer (COP) films based on a thermoforming technique to develop a roundbottom, 96 microwell array for the generation of uniform-sized embryoid bodies(EBs).As a combined technique, arrayed microwell fabrication using PDMS soft lithography technique and droplet generation of cell suspension using surface tension due to hydrophobic and hydrophilic difference enable the formation of induced pluripotent stem cell (iPSC) arrays in a 512 well[68].As another example, Occhettaet al[69]suggested that a high-throughput serial dilution generator could be used for making different concentrations and combinations of cytokine to investigate the effect of cytokines on the expansion and differentiation of embryonic stem cells (ESCs).
A major focus of the miniaturized HTS of stem cells is on screening for drugs,compounds and small molecules that could affect the properties of stem cells, such as differentiation, self-renewal, and expansion.3D HTS platform also can be used to recreate the stem cell niche for mimicking thein vivoenvironment.Co-culturing different, interdependent types of cells is an important part of stem cell niche[70].In general, HTS requires the use of robotics due to the multiple pipetting steps, and it consumes large quantities of reagents and valuable cells, resulting in the experiments having high costs.Despite the disadvantages, including the labor and time required,HTS technology using well plates is used extensively for developing various protocols for cell cultures and 2D and 3D screening of cells because the microplate is still a wellestablished platform for HTS applications so many research groups view it as a userfriendly approach.For this reason, Yuet al[71]developed the well plate-based gel unit array for HTS analysis.This platform has a unique feature in that it has hydrogelincorporating compartments integrated in a well to culture 3D tissue with uniform thickness while co-culturing with other neighboring cells in a single well.This can be used as HCS integrated with a co-culture model.
3D TISSUE MODEL FOR STEM CELL ENGINEERING
Stem cells have their unique ability of self-renewal and the potential to differentiate into many specific types of cells.Immortalized cell lines are capable of extended proliferation but exhibit fewer organ-specific activities than primary cells or stem cells.Moreover, primary cells are functional, but have limited cell number and a finite lifespan.Therefore, stem cells that was able to differentiate into specific organs are considered to be more functional, and an ideal source to mimic the architecture and specific activity of human organs, and are more likely to be accurate with respect to human bodies.As a more reliable and sustainable human source that represents phenotypical characteristics of the inherited disease or genetic disorders, patientspecific cells are needed.Recently, iPSCs and their organoid techniques have undergone a rapid increase in popularity.These techniques allow reprogramming of fibroblasts into stem cells that can be differentiated into various tissues, such as neurons, cardiomyocytes (CM), and several types of blood cells.The iPSC technology provides a new and powerful tool for drug-screening for personalized medicines, and it allows the use of cells with the same genetic background as the patients.Furthermore, these sources of cells allow the recapitulation of various inherited diseases in vitro, and allow researchers to study the genotypic differences.For these reasons, iPSCs were have been used extensively in recent 3Din vitroorgan models[22].
There are the two distinct strategies in generatingin vitro3D tissue and organ models,i.e., the bottom-up and the top-town approaches[2].A key example of the use of the top-down approaches is an organ-on-a-chip model, the aim of which is to engineer individual components of tissue environments, such as cells and ECMs in amicrofluidic device, and this work is conducted mostly by bioengineers.Bottom-up approaches rely on biological self-organization, which refers to intrinsic abilities of biological systems, and they are led largely by stem cell biologists.These two approaches both have the same goal,i.e., achieving the generation of high-fidelity 3D tissue.However, both approaches have their own limitations.For instance, organoid systems have low controllability for recreating the biochemical and biophysical microenvironment of 3D organoids, while organ-on-a-chip systems have limitations when reconstituting the biological complexity of tissue development.Thus, by combining the strengths of both two approaches, the organoid-on-a-chip platform has emerged as a synergistic approach to recapitulate both the physiological and biochemical features ofin vivotissue[10,14].In this section, we introduce examples of stem cell-based organ-on-a-chip and organoid-on-a-chip system using microfluidic technologies for high-throughput analysis.
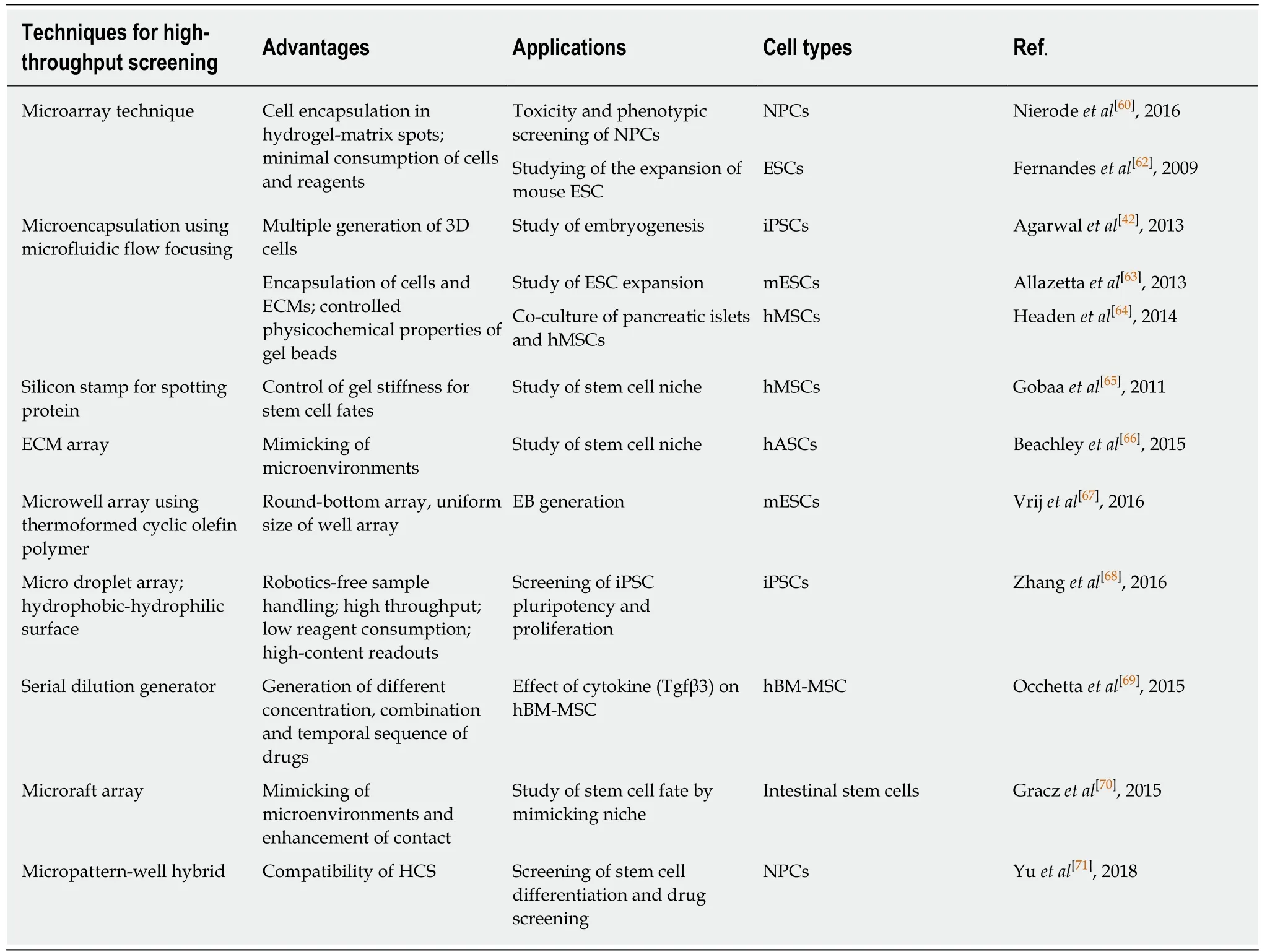
Table 2 High-throughput screening analysis for stem cell engineering
ORGAN-ON-A-CHIP AND ORGANOID-ON-A-CHIP FOR HIGH-THROUGHPUT ANALYSIS
With the development of the generation of iPSCs, tissue models and disease models based on organ-on-a-chip technology have been proposed, and they are expected to serve as a platform for cell-based, high-throughput assays during the drug discovery and development.The organ-on-a-chip, which utilizes the microfluidic approach to mimic the architecture and function of 3D tissue, consists of microengineered biomimetic systems that represent key functional units of living human organs.Also,recent advances in microfabrication, cell engineering, and imaging technologies have led organ-on-a-chip to become an innovative technology that is capable of reproducing physiological cell behaviorsin vitro.These systems include important design considerations for developing systems,i.e., (1) Organizing the spatial distribution of multiple types of tissues; (2) functional tissue-tissue interfaces; and (3)organ-specific mechanical and biochemical microenvironments.
Stem cell-derived organoid systems that are 3D self-organized tissue models provide new biological models for the development of new drugs.Organoids have been generated from both pluripotent stem cells and tissue-resident adult stem cells by mimicking the biochemical and physical cues of tissue development and homeostasis[72,73].Because of these unique features, conventional 3D organoid systems may be more advantageous in some aspects than organ-on-a-chip systems in drug discovery.One of the important applications of organoid cultures is to model pathologies of diseases.Organoid-on-a-chip engineering has been emerged recently based on the integration of the two distinct approaches of organoid and chip technology.
Stem cells, including iPSCs, have the potential to serve as a source of cells that can be engineered to suit specific needs in the development of organ-on-chips[5,6].In recent years, the organ- and organoid-on-a-chip approaches using stem cells have been used extensively to establish the new microengineered models that recapitulate the structure and functional complexity of human organs, such as the liver[74-77], heart[78-86],brain[71,87-94], intestine[95-97], kidney[98-100], and bone[101-103].Recently, organ-on-a chip technology has been able to integrate multiple organ or tissue models to simulate the human body, and multi-organ systems generated using stem cells have been developed for a human body-on-a chip system[16,75,104,105]It is possible for such a system to provide a predictive model for pharmacokinetics of drugs by mimicking the activities of the human body such as absorbing, distributing, metabolizing, and eliminating drugs.
We introduce examples of organ- and organoid-on-a-chip platform using stem cells for high-throughput assay, as summarized in Table 3.In general, the primary focus of organ-on-a-chip has been on the microengineered liver due to the importance of its central role relative to hepatic drug toxicity and metabolism[74-76].Wareet al[74]demonstrated the possibility of a high-throughput hepatotoxicity test on iPSC-derived hepatocytes co-cultured with fibroblasts, which were micropatternd islands using the soft-lithography technique.In another approach, Scheperset al[76]developed a liveron-a-chip using human iPSCs from a patient.This cell was cultured as 3D organoids using a perfusable system, and the organoids that were constructed were integrated in a chip with multiple patterned C-traps, as shown in Figure 1A.The liver organoids were long-term cultured for 28 d during perfusion.Recently, researchers have begun to explore the potential of heart-on-a-chip as a HTS tool for the monitoring of contractile functions and cardiomyopathy using iPSC-derived CMs[83,84,86].For instance,Millset al[86]developed a 96 well-type screening platform for screening functions in hiPSC-derived cardiac organoids to reveal the cardiac metabolic mechanism, as shown in Figure 1B.
Similarly, there have been recent developments in brain-on-a-chip for HTS.The attention for brain models fits in a wider trend towards attention for neural progenitor-cell-derived brain models for diseases, such as Alzheimer’s disease[71,91].This follows the more generic increase in the popularity of iPSC techniques and progress in controlling the stem cell niche of differentiated tissues.Wanget al[93],developed brain-organoids using iPSCs to model neurodevelopment disorders under prenatal nicotine exposure and showed the potential of drug testing.In addition, the 96-well plate-based, HTS-compatible 3D cell culture platform for the brain model was developed for preclinical drug screening applications[71,92].Especially, Yuet al[71]developed a micropattern array platform combined with conventional well-plate for HTS drug screening to show the proof-of-concept for the Alzheimer’s disease model using neural progenitor cells, as shown in Figure 1C.Weverset al[94]showed 3D ECM-embedded neuronal-glial networks in a microfluidic platform using iPSC-derived neural stem cells.The iPSC-derived mature neurons and astrocytes were cultured in the microfluidic channel-based OrganoPlate, which is the integrated microtiter plate that is comprised of 96 tissue chips developed by MIMETAS, lnc., as shown in Figure 1D.An HTS-compatible platform also was developed in kidney-on-a-chip by Czernieckiet al[100].The iPSCs were cultured and differentiated on this platform with fully-automated and HTS-compatible formats for multi-dimensional phenotypic screening.
CHALLENGES AND FUTURE PROSPECTS

Figure 1 Representative examples of high-throughput screening microfluidic systems using stem cells based on organ-on-a-chip or organoid-on-a-chip.
In the field of drug screening, the need for a 3D stem cell platform will become more pressing because it provides a more efficient approach in the early, preclinical stage of drug development.Although 3D microfluidic technology provides significant potential for creating a highly complex, well-controlled 3D dynamic environment as anin vivosystem, there are certain to be technical challenges in both the engineering and biological technologies of this platform.In general, microfabricated devices contain various complex designs within small areas, and this limits biochemical experiments and requires advanced skill and optimization.Under such physical conditions, certain types of stem cells can be very sensitive to the excessively high shear stress induced by flow, which might cause phenotypic changes or adversely affect cell viability in microfluidic devices during long-term cultures[106].In addition,high adsorption of proteins on the PDMS or the plastic walls of microfluidic devices also can hinder the accurate evaluation of the effects of drugs.Currently, several of these problems are being addressed by simpler designs, and stem-cell-specific changes in the design of the devices.Recent approaches that have relatively simpler hybrid systems that combine traditional cell culture plates with microfluidic compartments by decoupling the handling of cells from handling of microfluidic liquids could be alternative approaches[37].Also, the various microfluidic designs, such as low perfusion, deeper chambers, and large input/output reservoirs to avoid handling the tubes, could be solutions.
In the aspects of high-throughput analysis, the nature of microfluidic systems,which require complicated handling and multiple processes for a series of biological processes, present barriers to high-throughput analysis[19,28,50,53].This is especially important in the case of primary patient-derived stem cells with time constraints that could be cultured outside of the organism due to rapid changes in their microenvironments duringin vitroculture.For this reason, miniaturized screening compartments, systemized cell manipulation, and robotic liquid handling must be developed.

Table 3 High-throughput screening-based three-dimensional organ- or organoid-on-a-chip
In addition, many proposed systems, as with many other HTS platforms, are focusing largely relying on biomolecular engineering techniques coupled with microscopy-based imaging.However, practicalin vitrosystems require a system that both observe and analyze a variety of biochemical and physiological responses[54].
Despite of these challenging issues, the high demand for microfluidic devices for HTS of stem cells is uncontroversial.Microfluidic technology is still evolving to overcome these current issues, and the techniques are becoming more sophisticated and acceptable for miniaturization, automation, and versatile testing of all critical parameters for stem cell research.The combination of microfluidic technologies with stem cell analysis may fill the gaps between the present knowledge about stem cells and an in-depth understanding of the underlying mechanisms for their broad applications.By using these techniques in the future,in-vivo-like culture of stem cells and their drug discovery applications can be improved, and the prediction of drug responses will be more reliable.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) (NRF-2017R1C1B2002377, NRF-2016R1A5A1010148, and NRF2019R1A2C1003111) funded by the Ministry of Science and ICT (MSIT) and partly supported by the Technology Innovation Program (No.10067787) funded by the Ministry of Trade, Industry &Energy (MOTE, Korea).
杂志排行
World Journal of Stem Cells的其它文章
- Effect of poly(3-hydroxyalkanoates) as natural polymers on mesenchymal stem cells
- Neural stem cell transplantation therapy for brain ischemic stroke:Review and perspectives
- Unmodified autologous stem cells at point of care for chronic myocardial infarction
- Characterization of inflammatory factor-induced changes in mesenchymal stem cell exosomes and sequencing analysis of exosomal microRNAs
- Stem cell treatment and cerebral palsy: Systemic review and metaanalysis
