Differential effects of atrial and brain natriuretic peptides on human pulmonary artery:An in vitro study
2019-10-31AzarHussainRobertBennettZaheerTahirEmmanuelIsaacMubarakChaudhrySyedQadriMahmoudLoubaniAlynMorice
Azar Hussain,Robert T Bennett,Zaheer Tahir,Emmanuel Isaac,Mubarak A Chaudhry,Syed S Qadri,Mahmoud Loubani,Alyn H Morice
Azar Hussain,Robert T Bennett,Zaheer Tahir,Emmanuel Isaac,Mubarak A Chaudhry,Syed S Qadri,Mahmoud Loubani,Department of Cardiothoracic Surgery,Castle Hill Hospital,Cottingham HU16 5JQ,United Kingdom
Alyn H Morice,Centre for Cardiovascular and Metabolic Research,Hull York Medical School,Castle Hill Hospital,Cottingham HU16 5JQ,United Kingdom
Abstract
Key words: Heart failure; Atrial natriuretic peptide; Brain natriuretic peptide; In-vitro;Humans
INTRODUCTION
Decompensated heart failure is a worldwide health issue that is associated with considerable morbidity and mortality[1,2].Despite the development of several deviceand medical-based therapies over the past few decades,the rate of rehospitalisation and early death has not significantly improved[3].
The natriuretic peptides (NPs) family consists of three structurally interrelated vasoactive peptides,and was initially discovered by de Boldet al[4]in 1981.The family includes atrial natriuretic peptide (ANP),brain natriuretic peptides (BNPs) and Ctype natriuretic peptide (CNP),which are mainly secreted by cardiac myocytes in response to wall stress[5,6].ANP and BNP actviaguanylyl cyclase-linked natriuretic peptide receptor-A (NPR-A),whereas CNP activates the related cyclase natriuretic peptide receptor-B (NPR-B)[7].ANP and BNP exert their beneficial effects by reducing systemic and pulmonary vascular resistance,and by increasing natriuresis and diuresis[8].In addition to their haemodynamic effects,NPs attenuate vascular smooth muscle proliferation and cardiac hypertrophy[9,10].They also inhibit the synthesis of growth factors,by counteracting the effects of the renin-angiotensin system,which is involved in the development of pulmonary hypertension[11].
In vitrostudies on pulmonary arterial rings and isolated lung models have shown that ANP and BNP infusion induced pulmonary vasodilation by reducing pulmonary vascular resistance[12,13].However,in heart failure,increasing levels of circulating ANP and BNP are associated with a worsening of heart failure and a poor prognosis[14].The aim of this study is to evaluate whether BNP acts as a partial agonist,and inhibits the effects of ANP.
MATERIALS AND METHODS
Study patients
Local research ethics committee and institutional (Hull & East Yorkshire Hospitals NHS Trust) Research and Development Department approval was obtained for the use of lung specimens and surplus lung tissue from patients undergoing elective lobe or lung resection for cancer.Patients gave written consent for the use of surplus tissue for research purposes.
In accordance with the recommendations of the human tissue act (2004) 127 and the conditions of the local ethics committee approval,the donor patient was anonymous to the researcher.
Tissue collection
Excess segments of pulmonary artery were obtained from patients undergoing lobectomy,and the sample was immediately transferred to the lab in Krebs-Henseleit solution after resection.After the removal of connective tissue,the pulmonary artery(PA) sample was divided into 2 mm long rings.The small pulmonary vessels with an internal diameter of 2-4 mm were used for these experiments.
Experimental protocol
A multiwire myograph system was used for the measurement of isometric tension.Under physiological conditions (37oC,21%O2),PA rings were mounted in Krebs Henseleit solution.A resting tension of 1.61 gf was applied,which was calculated from earlier experiments[15],and the vessels were left to equilibrate for 60-90 min.After equilibration,vessels were pre-constricted with 11.21 µmol/L PGF2α (EC80,calculated from earlier experiments[16]),and concentration response curves were constructed to ANP and BNP by cumulative addition to the myograph chambers.
In another set of experiments,once the vessels tension reached a plateau after preconstriction with PGF2α,300 nmol/L of BNP was added and the vessels were left for 30 min.When a stable resting tension was achieved,concentration response curves were constructed to ANP.Vessels were then washed for 30 min,and the whole experiment was repeated again without the addition of BNP.
Active tension was calculated in gram force (gf) as maximum tension at plateau (gf)- resting tension (gf).The maximum efficacy (Emax) for each agent was determined in gf and expressed as gf/mm internal diameter of each vessel (to take into account the variability in PA ring diameter).The integrity of the endothelium was confirmed with 1μM Acetylcholine,and KCl was added to check viability.Vessels that did not constrict with KCl were excluded from the study.Figure 1 shows the schematic representation of myograph setup for measuring isometric tension.
Chemicals
A 5% CO2/balance air was sourced from BOC Limited (Guilford,Surrey,United Kingdom).The agents used were (supplier in parentheses) ANP (Tocris Bioscience,part of Bio-Techne,Abingdon,United Kingdom),BNP (Tocris Bioscience)Acetylcholine (Sigma-Aldrich,St.Louis,MO,United States) and PGF2α (Tocris Bioscience).Stock solutions of drugs were prepared using the solvents recommended by the suppliers,and control responses to solvents were obtained when necessary.Fresh serial dilutions were made using the appropriate solvent for each experiment.All other reagents were obtained from Thermo Fisher Scientific unless otherwise stated.
Statistical analysis
Data are presented as mean ± SD,and n represents the number of individual PA rings used in an experiment.Agonist EC50concentrations (the concentration required to elicit 50% of the maximum response) were determined using nonlinear regression to fit a standard slope model using the statistical analysis function of GraphPad Prism version 7.00 for Windows (GraphPad Software,La Jolla,CA,United States).More details can be found at http://www.graphpad.com/guides/prism/6/curvefitting/index.htm).Agonist potency is presented as pEC50(the negative logarithm of the molar EC50concentration).Significance was taken asP< 0.05.
RESULTS
A total of 35 PA rings were obtained from 15 patients.The internal diameter of PAs ranged from 2.5-3.5 mm.Nine rings were not included,as they did not respond to KCl.
Concentration-dependent effect of ANP and BNP on human PAs
ANP and BNP caused a concentration-dependent relaxation of PAs pre-constricted to PGF2α,with a pEC50of 8.96 ± 0.21 and 7.54 ± 0.18 for ANP and BNP,respectively(Figure 2).The maximum efficacy (Emax) for ANP and BNP was -2.03 gf and -0.24 gf,respectively.
Another set of experiments was conducted to determine whether a high concentration of BNP would inhibit relaxation to ANP.After addition of BNP,the Emaxof ANP was reduced by 30% from -0.96 gf to -0.675 gf (P= 0.28,n= 11) (Figure 3).
Concentration response curve of ANP-induced pulmonary vasodilation
All vessels vasodilate in response to ANP.Increasing the concentration of ANP from 3pmol/L-1 μmol/L were used on 8 PA rings.Maximal relaxation was seen at 100 nmol/L (log -7.0 mol/L),and the EC20,EC50 and EC80 were 0.17 nmol/L,1.105 nmol/L and 7.01 nmol/L,respectively.The hill slope was -0.75 ± 0.5.
Figure 1 Schematic representation of myograph setup for measuring isometric tension.
Concentration response curve of BNP-induced pulmonary vasodilation
In order to evaluate the effect of BNP on pulmonary vessels,7 PA rings and a concentration of BNP from 1 nmol/L-1 µmol/L was used.As the concentration went above 10 nmol/L,vessels start to vasodilate and the maximum vasodilatory response was seen at 300 nmol/L (log -6.5 mol/L).The EC20,EC50 and EC80 were 13.3 nmol/L,28.7 nmol/L and 61.5 nmol/L,respectively.The hill slope was -1.818 ± 2.55.
Cumulative concentration response curve of ANP and BNP-induced pulmonary vasodilation
In another set of experiments,the cumulative vasodilator effect of ANP and BNP on pulmonary vascular tone was investigated.Sixteen PA rings from seven patients,and an increasing concentration of ANP from 1 pmol/L-1 µmol/L,was used.Five rings were excluded,as they did not respond to KCl.When a stable resting tension was achieved,vessels were pre-constricted to 11.21 μmol/L PGF2α(EC80).When a stable plateau relaxation was achieved,the effect of ANP on active tension was determined by cumulative addition to the myograph chambers.
The PA rings were washed for 60 min,and were pre-constricted again with 11.21 μmol/L PGF2α(EC80).A single dose of 300 nm of BNP was added and left for 30 min.Once a plateau was achieved by cumulative addition to the myograph chambers,the concentration response curve of ANP was performed.The addition of BNP reduced the Emaxof ANP by 30% (from -0.96 gf to -0.675 gf).
DISCUSSION
In this study,we demonstrated for the first time that (1) both ANP and BNP vasodilate isolated human PA rings; and (2) that BNP acts as a partial agonist and inhibits the effects of ANP.The finding that the addition of BNP inhibits the effects of ANP suggests that BNP does act as a partial agonist,and could be advancing the progression to decompensated heart failure.
The circulating concentration of ANP,BNP and CNP is low in healthy individuals,but it is elevated in heart failure patients,although to variable degrees (e.g.,CNP elevated to a lower extent than its counterparts)[17,18].In patients with HF,circulating concentration of BNP exceeds that of ANP; this consistency of response and high dynamic range makes bioassays for plasma BNP more useful than ANP[19,20].This might be due to the fact that BNP is also a marker of cardiac remodelling[21].Previous studies have shown that in heart failure (HF) patients,BNP and NT-pro BNP (Nterminal pro b-type NP) are independent predictors of cardiovascular mortality,worsening HF and need for hospitalization[22-24].Although BNP and NT-pro BNP have prognostic value,their therapeutic value is inconclusive in HF patients[25].
In the early 21stcentury,the United States Food and Drug Administration (FDA)approved the use of nesiritide (recombinant endogenous BNP) for heart failure patients[26].However,several subsequent studies demonstrated that Nesiritide is associated with worsening renal function and increased risk of death[27].A randomized,double blind,placebo-controlled,ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) trial concluded that nesiritide showed no substantial improvement in dyspnoea or clinical outcomes[28].Another double-blinded,multicentre,randomized clinical trial,ROSE-AHF (Renal Optimization Strategies Evaluation - Acute Heart Failure) enrolled 360 patients.The study was designed to evaluate the use of low dose nesiritide,with the view that there would be less side effects and substantial therapeutic effects.However,the study failed to provide significant evidence in support of the routine use of nesiritide in heart failure patients[29].
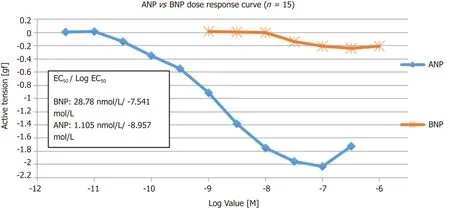
Figure 2 Cumulative concentration response curve to ANP and BNP (n = 15).
Although NPs are always attractive therapeutic targets for heart failure treatment,their use is limited by inadequate clinical efficacy.It is thought that the activity of neprilysin,a protease produced by the kidney that cleaves various vasoactive compounds including BNP,is increased in heart failure[30].In heart failure,increasing levels of circulating ANP and BNP are associated with worsening heart failure and a poor prognosis.This raised the suspicion that BNP might act as a partial agonist and inhibit the effects of ANP,as shown in this study.These findings could also explain the disappointing results seen in clinical trials of ANP and BNP analogues for the treatment of heart failure.Further studies are needed to confirm the findings of this study,which raises the possibility that selective BNP antagonists could be of greater clinical benefit than BNP agonists for the treatment of heart failure.
Limitations
Our study had several limitations.It was a laboratory-based project that was carried out in a control setting,which may not truly reflect thein vivoenvironment.The therapeutic dose and the dose provided in the experiments may differ.We also used the pre-constrictor PGF2α,and since the potency of the agent depends on the preconstrictor,other pre-constrictors need to be analysed and compared.The full potential of the study needs to be backed by a double-blinded randomized control trial.
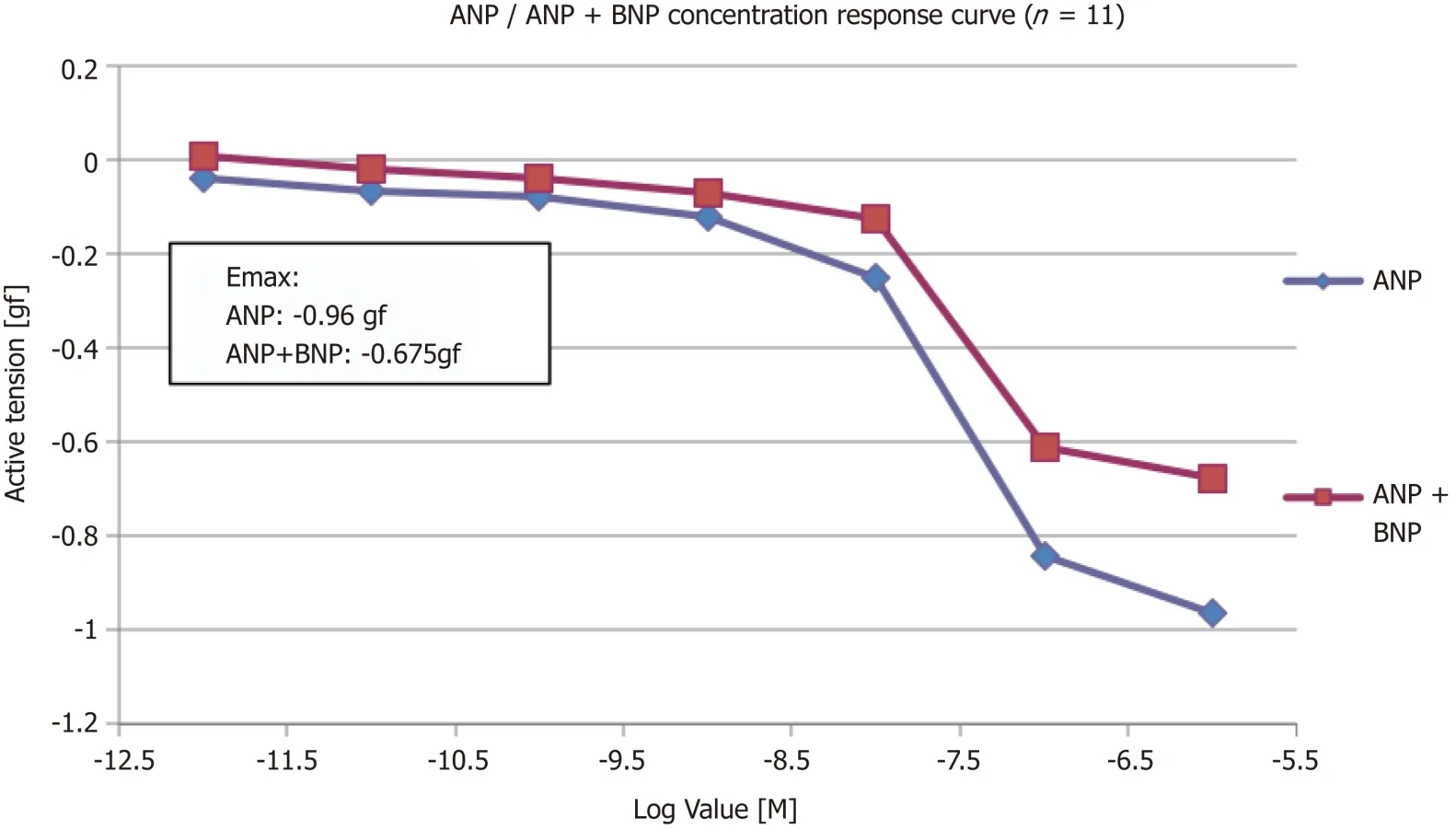
Figure 3 Concentration response curve to ANP alone and ANP + BNP (n = 11).
ARTICLE HIGHLIGHTS
Research background
The prevalence of cardiovascular diseases,especially heart failure,continues to rise worldwide.In heart failure,increasing levels of circulating atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are associated with worsening heart failure and poor prognosis.
Research motivation
ANP and BNP play an important role in homeostasis,but trials with BNP and ANP infusion showed disappointing results for unknown reasons.
Research objectives
The aim of this study was to evaluate whether BNP acts as a partial agonist and inhibits the effect of ANP.
Research methods
In this study,the effect of natriuretic peptides (ANP and BNP) on human pulmonary arteries was evaluated by cumulative addition to the myograph.
Research results
Both ANP and BNP act as pulmonary vasodilators,although ANP was found to be more potent and efficacious than BNP.Also,the addition of BNP reduced the efficacy of ANP.
Research conclusions
The study confirms that BNP inhibits the effects of ANP,and acts as a partial agonist.These findings also explained the disappointing results associated with the ANP and BNP infusion trials.
Research perspectives
Further studies are needed to validate the results of this study,and to evaluate the possibility of the clinical beneficial role of BNP antagonists for heart failure treatment.
