STW 5 is effective against nonsteroidal anti-inflammatory drugs induced gastro-duodenal lesions in rats
2019-10-30MohamedKhayyalWalaaWadieEnasAbdElHaleimKawkabAhmedOlafKelberRamyAmmarHebaAbdelAziz
Mohamed T Khayyal, Walaa Wadie, Enas A Abd El-Haleim, Kawkab A Ahmed, Olaf Kelber, Ramy M Ammar,Heba Abdel-Aziz
Abstract BACKGROUND Proton pump inhibitors are often used to prevent gastro-intestinal lesions induced by nonsteroidal anti-inflammatory drugs. However, they are not always effective against both gastric and duodenal lesions and their use is not devoid of side effects.AIM To explore the mechanisms mediating the clinical efficacy of STW 5 in gastroduodenal lesions induced by nonsteroidal anti-inflammatory drugs (NSAIDs),exemplified here by diclofenac, in a comparison to omeprazole.METHODS Gastro-duodenal lesions were induced in rats by oral administration of diclofenac(5 mg/kg) for 6 successive days. One group was given concurrently STW 5 (5 mL/kg) while another was given omeprazole (20 mg/kg). A day later, animals were sacrificed, stomach and duodenum excised and divided into 2 segments:One for histological examination and one for measuring inflammatory mediators(tumor necrosis factor α, interleukins-1β and 10), oxidative stress enzyme (heme oxygenase-1) and apoptosis regulator (B-cell lymphoma 2).RESULTS Diclofenac caused overt histological damage in both tissues, associated with parallel changes in all parameters measured. STW 5 and omeprazole effectively prevented these changes, but STW 5 superseded omeprazole in protecting against histological damage, particularly in the duodenum.CONCLUSION The findings support the therapeutic usefulness of STW 5 and its superiority over omeprazole as adjuvant therapy to NSAIDs to protect against their possible gastro-duodenal side effects.
Key words: Diclofenac; STW 5; Omeprazole; Gastro-intestinal lesions; Inflammation
INTRODUCTION
The long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs), such as diclofenac, has been reported to cause various adverse effects on the gastro-intestinal tract, including lesions in the stomach and duodenum, irritation of the mucosal surface and dyspepsia[1]. Various mechanisms have been involved in the development of these side effects, such as local epithelial irritation, inhibition of prostaglandin synthesis, and interference with barrier function and mucosal blood flow[2]. While proton pump inhibitors (PPIs) have been advocated to protect against the injurious effect of NSAIDs, some authors failed to show that omeprazole was capable of healing mucosal lesions caused by diclofenac[3]. Because of the fact that PPIs and other agents like H2 receptor blockers are not devoid of side effects on their own, attention has been shifted to the use of relatively safe and better tolerated herbal preparations.
A recent review article[4]emphasizes the use of natural products and herbal medicines to counteract the undesirable side effects of NSAIDs. One of the herbal preparations with established clinical efficacy in gastro-intestinal disorders such as functional dyspepsia and irritable bowel syndrome[5]as well as in drug-induced gastro-intestinal disturbances[6]is STW 5. It was shown to have a favorable safety profile[7]and has recently been included in the Rome IV treatment algorithm as a treatment option for functional dyspepsia[8].
The anti-inflammatory and mucosal protective properties of STW 5 were investigated and documented in several animal models of gastrointestinal (GI)disorders, including reflux esophagitis[9], gastric ulcer[10]and ulcerative colitis[11]. The present study aims at shedding more light on mechanisms mediating the clinical efficacy of STW 5 in gastro-duodenal lesions induced by NSAIDs[6], exemplified here by diclofenac, using an experimental model.
MATERIALS AND METHODS
Animals
Adult female Wistar rats, each weighing 150-200, were purchased from the National Research Centre, Giza, Egypt. They were housed in Perspex cages, housing 5 animals per cage, at the Faculty of Pharmacy, Cairo University, at a temperature of 25 ± 2 °C and a relative humidity of 60%-70%, and a 12-h light/12-h dark cycle. Taking in consideration that the diet might influence the outcome of some pharmacological experiments[12], it should be emphasized that the animals were fed a normal chow diet consisting of 66% carbohydrates, 22% protein, 6% fats, 3% fiber and 3% minerals and vitamins mixture and purchased from Alfa Media Trade (Giza, Egypt) and allowed water ad libitum. This diet was previously used in earlier experiments with STW 5 with no influence on the outcome of the results[11]. They were allowed to acclimatize for 1 wk before being subjected to experimentation. The study was approved by the Ethical Committee for experimentation with laboratory animals (Faculty of Pharmacy,Cairo University) following the revised guidelines of the European Economic Community regulations (86/609/EEC) (Permit Number: PT 2292).
Chemicals
Diclofenac was obtained from Novartis Pharma, Egypt; Omeprazole from Eipico,Egypt; STW 5 (Iberogast®) was generously supplied by Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany. STW 5 consists of a combination of standardized fixed proportions of hydro-alcoholic extracts of the following medicinal herbs Iberis amara,
Melissa officinalis, Matricaria recutita, Carum carvi, Mentha piperita, Angelica archangelica,Silybum marianum, Chelidonium majus and Glycyrrhiza glabra. The other chemicals used were all of analytical grade.
Experimental design
Four groups of animals, consisting of 10 rats each, were randomly allocated as follows:
Group 1: Normal untreated control rats; Group 2: Rats treated with diclofenac orally in a daily dose of 5 mg/kg for 6 d; Group 3: Rats treated orally with diclofenac(5 mg/kg) together with omeprazole (20 mg/kg) daily for 6 d. The dose of omeprazole was formerly reported to be effective for this purpose[13]; Group 4: Rats treated orally with diclofenac (5 mg/kg) together with STW 5 (5 mL/kg) daily for 6 d.
The dose of diclofenac and length of period of administration were chosen on the basis of preliminary experiments carried out in our lab to determine an appropriate dose of diclofenac to be given for a suitable number of days in order to induce reproducible gastro-duodenal lesions without causing appreciable animal mortalities under our experimental conditions. Accordingly, treatment in the above groups was given for 6 d. This is in agreement with an earlier study which reported that diclofenac was used in a dose of 5 mg/kg for 7 d in rat for evaluation of its gastric tolerability[14]. Furthermore, the dose of STW 5 was based on earlier studies in our lab showing good anti-inflammatory activity and good tolerability in experimental animals[15].
The animals were sacrificed by decapitation under light ether anesthesia on the seventh day and the stomach and duodenum were isolated and divided into 2 segments. One segment was preserved in 10% formalin for histological examination,while the other was homogenized in ice-cold normal saline to obtain a 10%homogenate that was stored at -20 °C for the assay of different relevant parameters at a later stage. These comprised the cytokines involved in inflammation, namely,interleukin 1β (IL-1β), IL-10, tumor necrosis factor-α (TNF-α) as well as the enzyme,heme oxygenase-1 (HO-1), as a measure of oxidative stress and B-cell lymphoma 2(Bcl2) as an indicator of apoptosis. These parameters were determined using rat specific enzyme-linked immunosorbent assay (ELISA) kits (R and D Systems GmbH,Wiesbaden, Germany) according to manufacturer’s instructions.
Histological examination
The fixed stomach and duodenum segments were processed by paraffin embedded technique and transverse 4-5 µm sections were stained with Hematoxylin and Eosin for light microscopic examination. The histological damage in the gastric sections was assessed by examining them for 5 criteria representing cell damage, namely: (1) Focal mucosal necrosis; (2) Congestion of the sub-mucosal blood vessels; (3) Sub-mucosal edema; (4 and 5) Inflammatory cell infiltration in the mucosa and sub-mucosa. The extent of damage for each of these signs was assessed on a score from 0 (normal) to 3(severe). An overall damage score (ODS) was then computed semi-quantitatively for each examined slide[16].
For the duodenal sections, six criteria were studied, namely, focal necrosis of the
mucosa, infiltration of inflammatory cells in the lamina propria, mucosal edema,congestion of blood vessels, activation of glands and sub-mucosal edema.Accordingly, a maximal ODS for gastric and duodenal sections was computed to be 15 and 18, respectively. The data was then represented as a box plot.
Statistical analysis
The histological scores were represented as a box plot using the medians analyzed by Kuskal-Wallis followed by Dunn’s test. The other data were expressed as means ±standard deviation (SD) and analyzed by one-way-analysis of variance test (ANOVA)followed by Tukey Kramer multiple comparisons test, setting the level of significance at P ≤ 0.05.
RESULTS
Biochemical parameters
Diclofenac administration showed a marked 4-fold increase in the level of the proinflammatory cytokine, IL-1β, in both stomach and duodenum as compared to untreated control. Treatment with omeprazole and STW 5 tended to protect against this increase nearly to the same extent, such that the increase of the cytokine was reduced only to about two-fold (Figure 1A and B). The other pro-inflammatory cytokine, TNF-α, was also markedly increased after diclofenac administration, albeit not as much as IL-1β. Both omeprazole and STW 5 in the doses used tended to prevent this increase in both tissues (Figure 1C and D). In parallel with these findings,the anti-inflammatory cytokine, IL-10, was significantly reduced by diclofenac in both stomach and duodenum, an effect that was largely prevented by both omeprazole and STW 5 mainly in the stomach, albeit to a non-statistically significant extent in the duodenum (Figure 1E and F).
With respect to HO-1, an anti-oxidant as well as anti-inflammatory enzyme,diclofenac was shown to induce a marked drop in its gastric and duodenal level.Omeprazole was effective in preventing the changes in both tissues, whereas STW 5 was effective only in ameliorating the diclofenac-induced reduction in the duodenum(Figure 1G and H). The levels of the apoptotic regulator, Bcl2 were suppressed by more than half by diclofenac in both the stomach and duodenum, an effect which tended to be prevented by treatment with either omeprazole or STW 5 almost to the same extent. (Figure 1I and J).
Histopathological examination
Stomach:The stomach of control untreated rats showed a normal histological structure of gastric mucosa, submucosa and muscularis layers (Figure 2A). On the other hand, stomach of rats treated with diclofenac showed severe histopathological alterations evidenced as multiple focal necrosis of gastric mucosa associated with mononuclear cells infiltration as well as submucosal edema and inflammatory cell infiltration (Figure 2B). Some sections also showed cystic dilatation of the gastric glands. However, the stomach of rats treated with diclofenac/omeprazole showed only mild submucosal edema associated with mononuclear cell infiltration and congested submucosal blood vessel (Figure 2C). A further improvement of the picture was shown in the stomachs of rats treated with diclofenac/STW 5, where only a few sections showed mild submucosal edema with some inflammatory cell infiltration and congested submucosal blood vessels, but most sections showed no histopathological alterations whatsoever (Figure 2D).
Duodenum:The duodenum of control untreated rats showed a normal histological structure of the villi, crypts and muscularis layer (Figure 3A). However, the duodenum of rats treated with diclofenac showed focal mucosal necrosis associated with focal inflammatory cell infiltration and edema in the lamina propria, congested mucosal blood vessel and activation of the glands (Figure 3B). A marked amelioration of the histological picture was seen after combining diclofenac with either omeprazole or STW 5. Thus, sections from rats treated with diclofenac/omeprazole showed only mild changes in the lamina propria exhibited as a few inflammatory cell infiltration,slight activation of the glands and slight congestion of blood vessels (Figure 3C) while sections from rats treated with diclofenac/STW 5 showed hardly any histopathological alterations (Figure 3D). The ODS of the lesions in the stomach and duodenum is represented in Figure 4, where it becomes evident that STW 5 was superior to omeprazole in preventing the histological damage induced by diclofenac in both tissues.
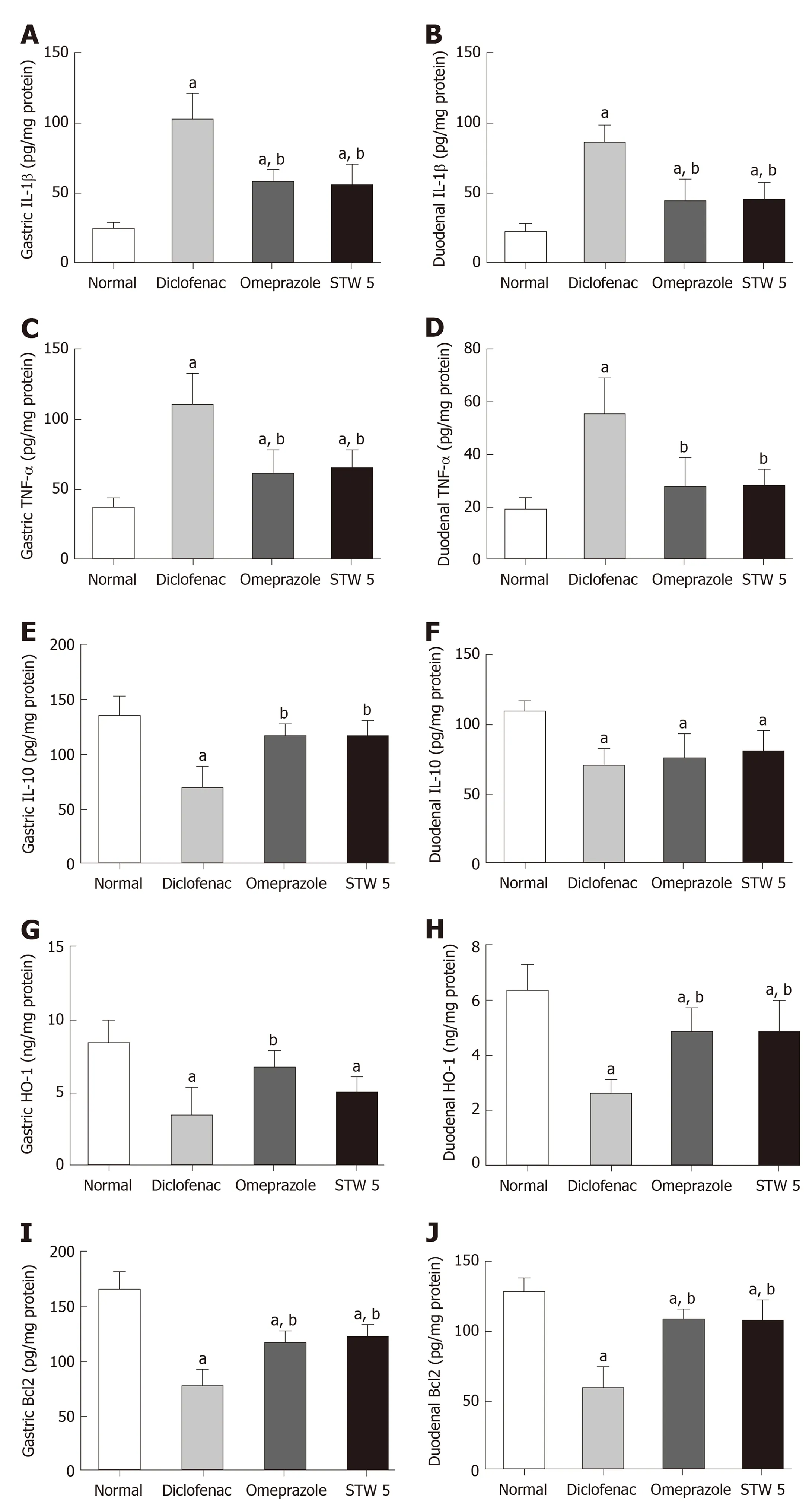
Figure 1 Changes in interleukin 1β, tumor necrosis factor-α, interleukin 10, heme oxygenase-1, and B-cell lymphoma 2 concentration in gastro-intestinal lesions induced by diclofenac in rats after treatment with omeprazole (20 mg/kg) or STW 5 (5 mL/kg). A: Changes in interleukin 1β concentration in gastric lesions; B:Changes in interleukin 1β concentration in duodenal lesions; C: Changes in tumor necrosis factor-α concentration in gastric lesions; D: Changes in tumor necrosis factor-α concentration in duodenal lesions; E: Changes in interleukin 10 concentration in gastric lesions; F: Changes in interleukin 10 concentration in duodenal lesions; G: Changes in heme oxygenase-1 concentration in gastric lesions; H: Changes in heme oxygenase-1 concentration in duodenal lesions; I:Changes in B-cell lymphoma 2 concentration in gastric lesions; J: Changes in B-cell lymphoma 2 concentration in duodenal lesions. Data are expressed as means ± SD. The significance of the difference between means was tested by ANOVA followed by Tukey Kramer multiple comparisons test. aP < 0.05 vs normal; bP < 0.01 vs diclofenac-treated group, n = 7. IL-1β: Interleukin 1β; IL-10: Interleukin 10; TNF-α: Tumor necrosis factor-α; HO-1: Heme oxygenase-1;Bcl2: B-cell lymphoma 2.
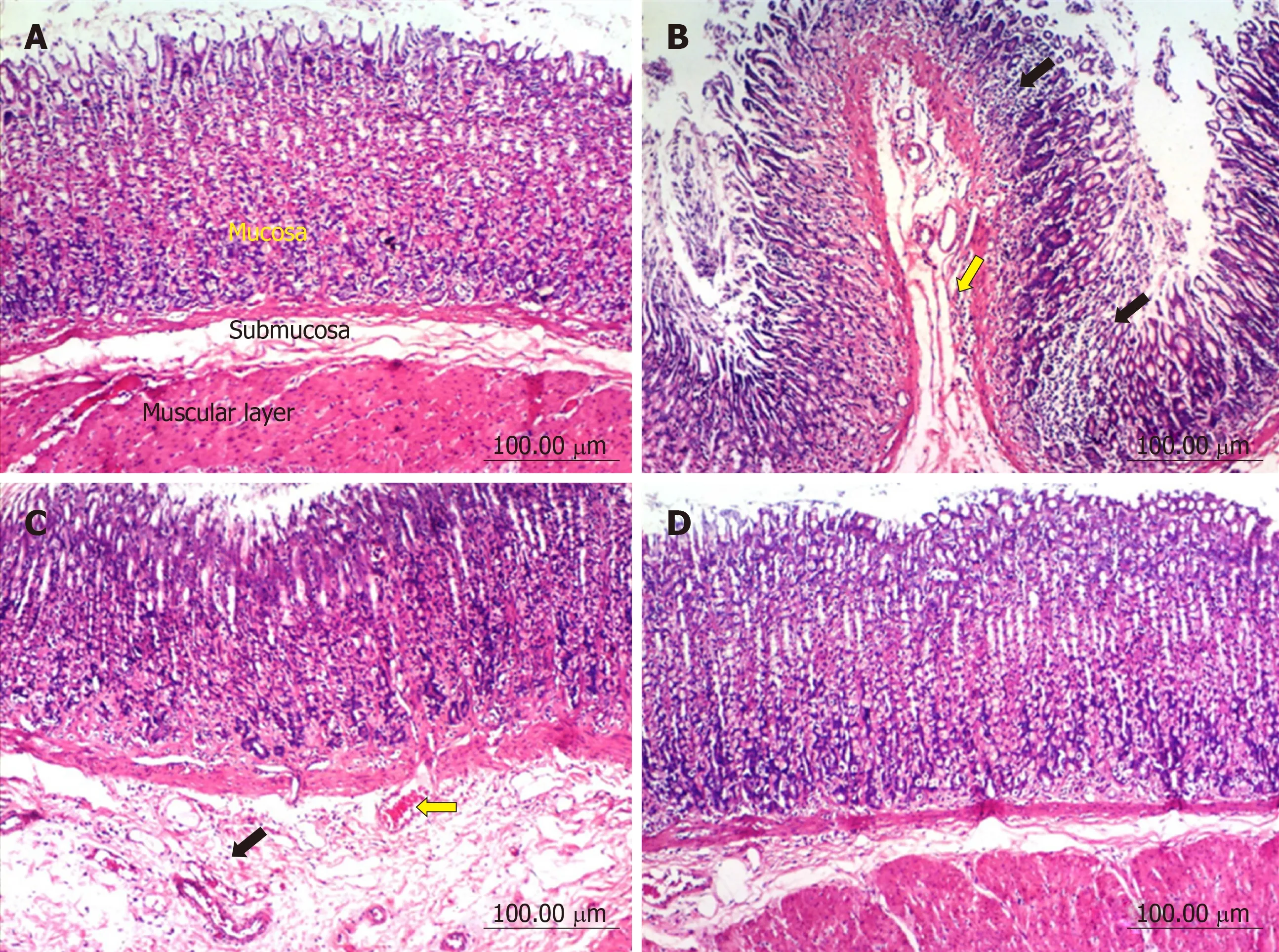
Figure 2 Histopathological changes in rat stomach.
DISCUSSION
The fact that the frequent use of NSAIDs, including diclofenac, is prone to induce GI lesions has been well documented[17]. In alignment with earlier reports, the administration of diclofenac in the given dose over several days resulted in changes in histological as well as associated biochemical parameters in both the stomach and duodenum. The histological changes were evidenced as gastric and duodenal mucosal necrosis, blood vessel congestion and edema as well as inflammatory cells infiltration into lamina propria and submucosa. Similar findings were reported by Devi et al[18].
The GI histological changes were also reflected on the biochemical findings, where diclofenac induced a marked rise in the gastric and duodenal contents of IL-1β and TNF-α, while reducing that of IL-10, indicating a disturbed balance between proinflammatory and anti-inflammatory cytokines. The role of these cytokines in the inflammatory process is well established. Thus, TNF-α besides contributing towards the induction of neutrophils to the inflamed mucosa[19], activates the transcription factor, NF-κB, thereby helping to up-regulate many genes involved in inflammatory and immune responses[20]. The other pro-inflammatory cytokine, IL-1β, enhances the production of other pro-inflammatory cytokines in the gut and contributes towards the expression of adhesion molecules on the endothelium[21]. Among the different cells producing the anti-inflammatory cytokine, IL-10, are the regulatory B and T cells which are also involved in inflammation[22]. HO-1 (also designated as heat shock protein-32) is constitutively found in the GI mucosa and becomes up-regulated during inflammation[23]. It inhibits oxidative damage and reduces inflammatory events induced by inflammatory cytokines[24].
In order to reduce the risk of GI injury induced by NSAIDs, adjuvant therapy has been advocated. Towards this end, PPIs have been reported to be very effective.Omeprazole has been effectively used in patients taking NSAIDs in order to heal and prevent recurrence of gastroduodenal injury[25,26]. In support of this, the present study showed its good protective effect against the diclofenac-induced gastroduodenal lesions with marked improvement in the gastric and duodenal levels of the measured cytokines, antiapoptotic protein as well as HO-1 content. Nevertheless, there is some controversy in the literature in this respect. Some authors could not confirm that the drug is able to heal adequately the diclofenac induced GI lesions[3].
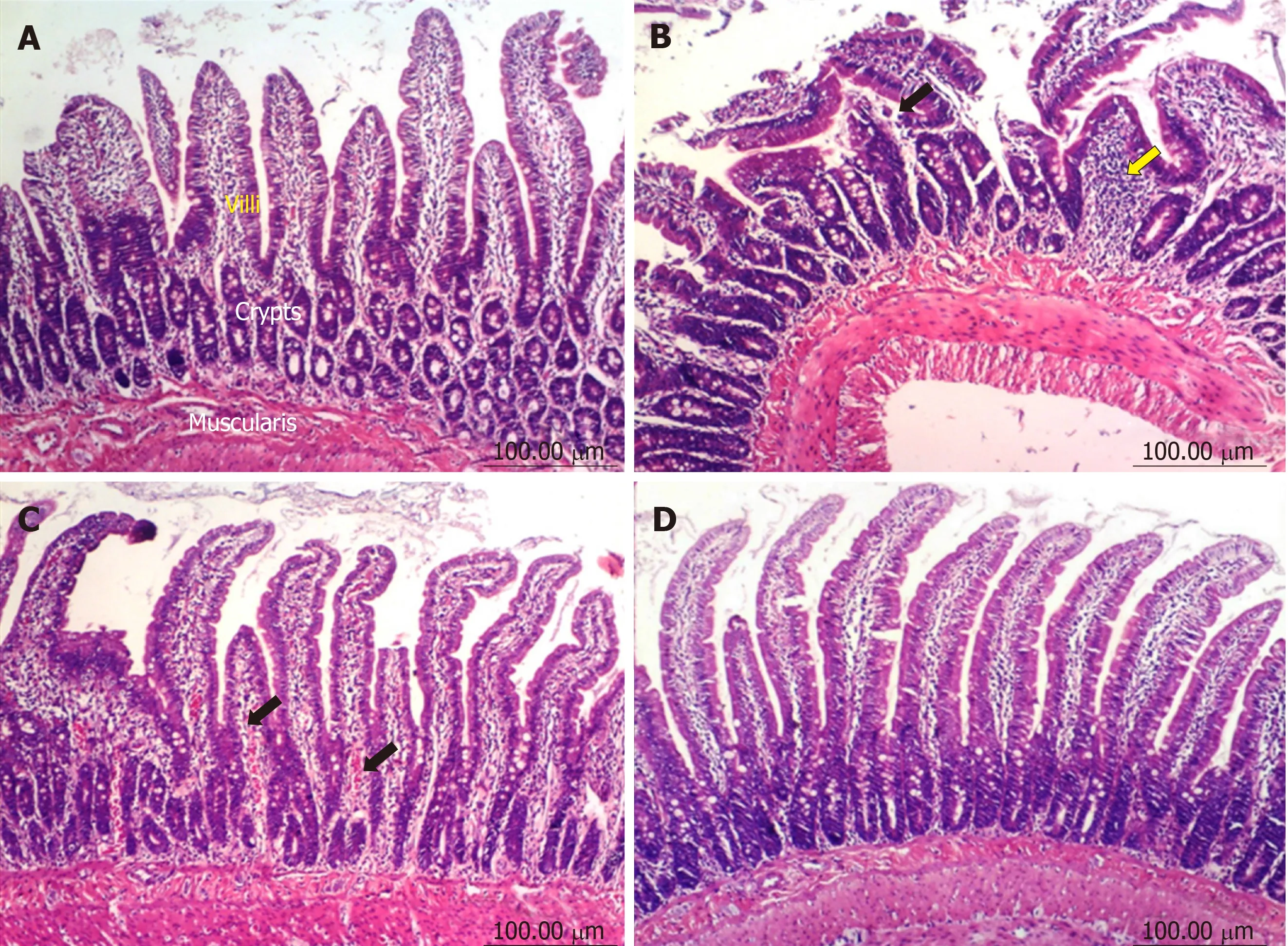
Figure 3 Histopathological changes in rat duodenum.
The present study also showed that STW 5 had a better protective effect than omeprazole against the histological changes induced by diclofenac, as evidenced by a reduction in the diclofenac-induced gastro-duodenal mucosal necrosis, blood vessel congestion, leukocytic infiltration, and edema. The superiority of STW 5 over omeprazole was previously reported in another experimental model of GI disorders,namely reflux esophagitis[9], where the associated changes in the biochemical parameters were also significantly reduced or prevented.
The histological protective effect of STW 5 was associated with a reduction of the level of the pro-inflammatory cytokines, TNF-α and IL-1β, while increasing that of IL-10. The herbal preparation was previously shown to have a dose-dependent inhibitory effect on TNF-α and/or IL-1β both in vitro[27]and in vivo using rat models of reflux esophagitis[9]and colitis11. Furthermore, STW 5 was reported to have antiulcerogenic and mucosal protective effects against indomethacin-induced gastric ulcers in rats[10]. STW 5 has been reported to be clinically effective in functional dyspepsia and irritable bowel syndrome[5], conditions that are often associated with inflammatory processes. The fact that STW 5 reduces the level of pro-inflammatory cytokines may at least partly be explained by its potential to inhibit the translocation of nuclear factor-kappa B, which plays an important role in regulating proinflammatory gene transcription[28]. This reasoning is further supported by the work of Bertalot et al[29]who showed that activation of Wnt pathway by STW 5 in a zebra fish model has a negative impact on NF-κB signaling in enteric epithelial cells and on the enteric nervous system in general.
The relevance of gastric and/or duodenal HO-1 inflammatory lesions of these tissues is controversial in the literature. The intensity of colitis induced by dextran sulfate sodium was found to be associated with a decreased expression of HO-1[30]while an increased expression was shown in many GI inflammatory conditions,including gastric ulcers[31], colitis[32]and radiation enteritis[33]. In the present study,diclofenac led to a marked decline in the gastric and duodenal HO-1 contents, an effect which was largely prevented by omeprazole. STW 5, however, was mainly effective in the duodenum. Induction of HO-1 was previously reported to have a good protective effect against NSAIDs-induced gastric ulcers. It reduced gastric inflammation, tissue neutrophil activation, and pro-inflammatory cytokine expression caused by indomethacin[34].
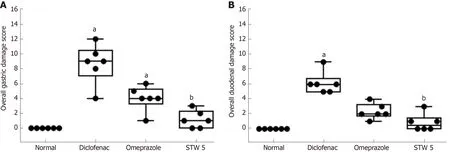
Figure 4 Changes in overall damage score in gastro-intestinal lesions induced by diclofenac in rats after treatment with omeprazole (20 mg/kg) or STW 5 (5 mL/kg).
Since many of the observed histological changes involve apoptotic mechanisms,and since the Bcl2 family of proteins are critical regulators of the mitochondrial pathway of apoptosis, it was important to study the involvement of Bcl2 in our experiments. Diclofenac led to a marked decline in the gastric and duodenal Bcl2 contents, thereby enhancing the apoptotic mechanisms. The effect was largely prevented to the same extent by both STW 5 omeprazole, pointing to their similar anti-apoptotic activity. The anti-apoptotic property of STW 5 was previously reported in detail by Khayyal et al[15].
Collectively, the present findings show that both STW 5 and omeprazole are effective nearly to the same extent in preventing the changes in biochemical parameters associated with the inflammatory response in both the stomach and duodenum of diclofenac treated rats. However, STW 5 was more effective than omeprazole in preventing the histological changes induced by the NSAID,particularly in the duodenum. Furthermore, the study provides pharmacological evidence underlying the clinical usefulness of STW 5 in patients suffering from diclofenac and other NSAID induced gastro-intestinal disorders in placebo-controlled clinical trials[6]. The findings further support more recent clinical and pre-clinical studies showing the efficacy and good tolerability of the herbal preparation[35]. STW 5 may thus represent an effective and safe adjuvant therapy for use in patients taking NSAIDs to guard against the possible occurrence of gastro-duodenal lesions.
ARTICLE HIGHLIGHTS

杂志排行
World Journal of Gastroenterology的其它文章
- Gut-liver axis signaling in portal hypertension
- Role of tristetraprolin phosphorylation in paediatric patients with inflammatory bowel disease
- Superior gallstone dissolubility and safety of tert-amyl ethyl ether over methyl-tertiary butyl ether
- Gender differences in vascular reactivity of mesenteric arterioles in portal hypertensive and non-portal hypertensive rats
- Construction of a replication-competent hepatitis B virus vector carrying secreted luciferase transgene and establishment of new hepatitis B virus replication and expression cell lines
- Differentially expressed long noncoding RNAs and regulatory mechanism of LINC02407 in human gastric adenocarcinoma
