Precision medicine in gastric cancer
2019-10-23PatriziaBonelliAntonellaBorrelliFrancaMariaTuccilloLucreziaSilvestroRaffaelePalaiaFrancoMariaBuonaguro
Patrizia Bonelli,Antonella Borrelli,Franca Maria Tuccillo,Lucrezia Silvestro,Raffaele Palaia,Franco Maria Buonaguro
Patrizia Bonelli,Antonella Borrelli,Franca Maria Tuccillo,Franco Maria Buonaguro,Molecular Biology and Viral Oncology,Istituto Nazionale Tumori - IRCCS - Fondazione G Pascale,Napoli 80131,Italy
Lucrezia Silvestro,Abdominal Medical Oncology,Istituto Nazionale Tumori - IRCCS -Fondazione G Pascale,Napoli 80131,Italy
Raffaele Palaia,Gastro-pancreatic Surgery Division,Istituto Nazionale Tumori - IRCCS -Fondazione G Pascale,Napoli 80131,Italy
Abstract
Key words: Gastric cancer; Molecular characterization; Biomarkers; Precision medicine;Targeted therapy
INTRODUCTION
Gastric cancer (GC) is a complex disease whose onset is linked to a series of genetic and environmental factors such as smoking and a high salt diet.Helicobacter pylori(H.pylori) is considered one of the most significant risk factors of GC.It is present in more than 70% of non-cardia GC cases and 90% of chronic gastritis cases[1],and its presence increases the risk of cancer (as compared to uninfected individuals)[2,3].Recent research has shown that there is a correlation between the risk of GC and the characteristics of specific strains ofH.pylori[4].Moreover,H.pyloriinfection has been demonstrated to be essential for promoting chronic inflammation of the gastric epithelium and histological changes that sequentially lead to GC[5].In this process,genetic and epigenetic alterations occur such as hypermethylation of DNA or mutations in genes including APC,WNT signaling pathway regulator (APC),tumor protein p53 (TP53),and KRAS proto-oncogene,GTPase (KRAS)[6].
Regarding treatment options,surgical resection with adjuvant or neoadjuvant radiotherapy and chemotherapy with cisplatin,5-fluorouracil (5-FU),taxane,or irinotecan are the most effective treatments for GC.However,despite the increasing knowledge and progress in drug development,this disease has a very poor prognosis due to late diagnosis and extreme intra- and inter-tumor heterogeneity.The heterogeneity makes the choice of therapy difficult,emphasizing the need for both new indicators for patient classification and novel therapies capable of addressing genetic,molecular,and cellular heterogeneity within tumors.This review highlights the progress achieved in the molecular characterization of GC and how it has impacted diagnosis,prognosis,and therapy in clinical practice.
EPIDEMIOLOGY OF GC
GC is the fifth most malignant tumor in worldwide and the third leading cause of cancer-related deaths[7].Unfortunately,the disease becomes symptomatic in the advanced stage; thus,the 5-year survival rate is only high (90%) in Japan where diagnosis and early tumor resection are done[8].In European countries,however,the survival rate is low,varying between 10% and 30%[9].The incidence of GC has geographical variation,with more than 50% of new cases occurring in developing countries.The areas most at risk are represented by China and Japan,Eastern Europe,Central,and South America,while the areas with lowest risk are South Asia,North America,New Zealand,Australia,and North and East Africa[10].In recent decades,a decrease in the incidence rate has been observed,especially in young patients with non-cardia,sporadic,and intestinal GC[11,12].The decreased incidence of GC can be attributed to the better preservation of foods,higher hygienic standards,higher intake of fruits and vegetables,and the eradication ofH.pylori[13].Figure1 summarizes the epidemiology of GC.
PATHOLOGICAL CLASSIFICATION OF GC
According to World Health Organization (commonly known as WHO) guidelines,GC can be classified as adenocarcinoma,ring-cell carcinoma,and undifferentiated carcinoma[14].The Lauren's classification,which is widely used,classifies GC into intestinal,diffuse,and mixed/unclassified types based on macroscopic and microscopic differences[15].It has been hypothesized that intestinal GC is associated with chronic atrophic gastritis and intestinal metaplasia,whereas the diffuse type originates from normal gastric mucosa.In European countries,the intestinal type is currently the most common GC[16-20].It tends to occur more often in the distal part of the stomach,in high-risk areas and is often preceded by long-standing precancerous lesions[17].On the other hand,the diffuse type is predominant among young patients.However,Lauren’s classification has a couple of key flaws.First,a large group of carcinomas do not fall into the two main types of carcinomas,intestinal or diffuse.This group of “unclassified” or “undetermined” gastric carcinomas include undifferentiated carcinomas and carcinomas that have dual differentiation (mixed intestinal and diffuse carcinomas).Second,there has been confusion regarding the“intestinal” term.Therefore,a change to Lauren’s classification has been proposed in which GCs are classified into four subtypes:Glandular,solid,isolated cell type,and mixed carcinoma[21].
MOLECULAR CHARACTERIZATION OF GC
Advances in next-generation sequencing (NGS) and microarray technologies and a better understanding of cancer biology have provided opportunities to characterize the genome of tumors including GC.The molecular profile of the GC has enabled The Cancer Genome Atlas (TCGA) and the Asian Cancer Research Group (ACRG) to classify GC into subtypes.The new molecular classification of GC is complementary to the subtyping classification based on histopathological characteristics.It is important to note that the molecular classification of GC helps to identify the molecular alterations that may be targeted by therapy.Furthermore,the molecular profiles of GCs obtained from individual patients have offered new opportunities to identify biomarkers that can be predictive of the tumor response to treatment[22-24]and to guide the selection of cytotoxic drugs and targeted therapies.TCGA and ACRG classifications of GC should facilitate the development of personalized prognosis and treatment,as well as better patient stratification for the design of clinical trials.The molecular characterization of GC from TCGA has used different platforms,including exome sequencing,DNA copy number analysis,DNA methylation,mRNA and microRNA (miRNA) expression.It divides GC into four subtypes:Epstein-Barr virus(EBV)-positive,microsatellite instable (MSI),chromosomal instability (CIN),and genomically stable (GS) (Figure2).Each of these GC subtypes is characterized by distinct features that provide prognostic information and suggest the potential benefits of targeted therapy.
EBV-positive tumors have mainly been found in the fundus and gastric body[25,26],and represent about 9% of GC cases.High DNA hypermethylation has been demonstrated in EBV-positive tumors,particularly of the cyclin-dependent kinase inhibitor 2A (commonly known asCDKN2A) promoter[27].An estimated 80% of EBV-positive subtype tumors contain mutations in phosphatidylinositol 3-kinase CA(PIK3CA)[28]and amplification of Janus Kinase 2 (JAK2),CD274 molecule,and programmed cell death 1 ligand 2 (PDCD1LG2),which encode for respectively tyrosine kinase receptors,PD-L1 and PD-L2[29].Based on these results,JAK2 inhibitors and PD-L1/2 antagonists should be explored as treatment options for EBV-positive tumors.Promising initial results have been reported with pembrolizumab,a humanized monoclonal antibody against programmed cell death 1 (PD-1)[30,31].In addition toPIK3CAmutations,EBV-positive tumors have more recurrent AT-rich interaction domain 1A (ARID1A) (55%) and BCL6 corepressor (commonly known asBCOR) (23%) mutations[29,32],whereas only rareTP53mutations have been observed.
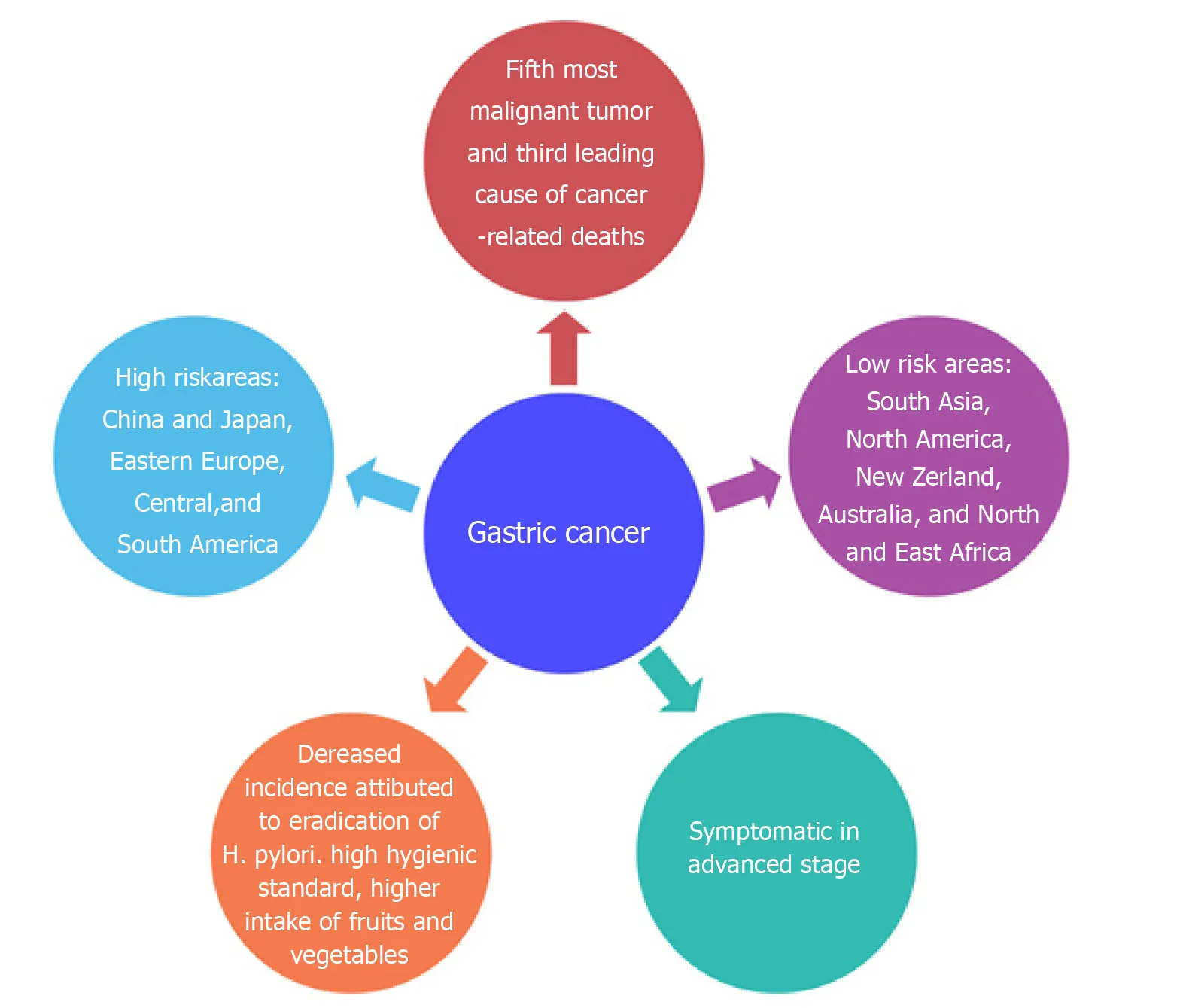
Figure1 Epidemiology of gastric cancer.Frequency of diagnosis,leading cause of cancer death,and risk areas worldwide of gastric cancer.
Patients with MSI subtype generally have intestinal tumors,which are diagnosed in old age.MSI tumors (21.7% of GC cases) are characterized by genomic instability due to methylation of DNA mismatch repair genes including MutL homolog 1 (MHL1)and to a high incidence of mutations inPIK3CA[33],Erb-B2 receptor tyrosine kinase 3(ERBB3)[34],ring finger protein 43 (RNF43)[35],phosphatase and tensin homolog(PTEN)[36],TP53[37],KRAS[38],andARID1A[32]genes.Increased expression of components of the mitotic pathway such as the E2F transcription factor (E2F),aurora kinase A (AURKA),polo-like kinase 1 (PLK1),and forkhead box M1 (FOXM1) has been described in MSI tumors[29].
GS tumors (19.7%) are mainly diffuse and are diagnosed in younger patients[39].GS tumors,which lack chromosomal alteration or MSI,exhibit the high expression of molecules involved in cell adhesion and pathways related to angiogenesis.They also have low mutation rates andARID1A,ras homolog family member A (RHOA),and cadherin 1 (CDH1) are the most frequently mutated genes[40].Previous studies have shown the loss ofCDH1,which encodes the E-cadherin cell adhesion molecule,in hereditary diffuse GC[41].TCGA data have also revealed the fusion of claudin 18-Rho GTPase activating protein 6 (CLDN18-ARHGAP6) or claudin 18-Rho GTPase activating protein 26 (CLDN18-ARHGAP26) and recurrent mutations inRHOA.CLDN18 and ARHGAP6 are respectively involved in the intercellular structure of the tight junction and the activation of Rho signaling (a signaling pathway in which intracellular and extracellular stimuli activate GTPase Rho),whereas RHOA modulates programmed cell death and contractility and motility of actomyosindependent cells[42-44].Therefore,alterations in RHOA or CLDN18-ARHGAP6 could contribute to the lack of cell cohesion,dispersed growth,and programmed cell death resistance.
CIN tumors represent almost half of GC cases (49.8%),are mainly intestinal,and are most frequent in the cardia-gastro-esophageal junction.Chromosomal deletions affectingCDH1,catenin alpha 1 (CTNNA1) and RB transcriptional corepressor 1 (RB1)and mutations inTP53(71%) are frequent in these tumors.CIN tumors present with amplification of genes encoding tyrosine kinase receptors such as epidermal growth factor receptor (EGFR),ERBB2,ERBB3,fibroblast growth factor receptor 2 (FGFR2),and MET proto-oncogene,receptor tyrosine kinase (MET); some transcription factors including the MYC proto-oncogene,basic helix-loop-helix transcription factor (MYC)and GATA binding protein 4 (GATA4); cell cycle regulators such as cyclin-dependent kinase 6 (CDK6),cyclin E1 (CCNE1),and cyclin D1 (CCND1) and other genes such asPDCD1LG2andPIK3CA[29].Alterations of these genes have been observed in advanced/metastatic GC[24].By contrast,the ACRG analyzed samples from 300 Korean patients,classifying GC based on particular genetic signatures such as the activation status ofTP53and the MSI condition[45].Four molecular subtypes have been identified:MSI,microsatellite stable (MSS) with activeTP53(MSS/TP53+),MSS with inactiveTP53(MSS/TP53-),and MSS with epithelial-mesenchymal transition (EMT)signature (MSS/EMT) (Figure3).

Figure2 The Cancer Genome Atlas gastric tumor classification.TCGA study divides GC into four molecular subtypes:CIN (chromosomal instability); EBV (Epstein-Barr virus); GS (genomically stable); and MSI (microsatellite instable).GC:Gastric cancer; TCGA:The Cancer Genome Atlas.
These subtypes are associated with survival and recurrence.The MSI subtype has a better prognosis and a lower tendency to relapse.The MSS/TP53+ and MSS/TP53-subtypes have an intermediate prognosis,whereas the MSS/EMT subtype is associated with a high rate of recurrence and a lower survival rate.Moreover,MSI tumors are diagnosed at an early stage (I/II),and about 60% are intestinal and show a high frequency of mutations ofPIK3CA,KRAS,ARID1A,and ALK receptor tyrosine kinase (ALK) genes; they also show loss ofMLH1.Tumors of the MSS/TP53+ subtype include many EBV-positive cases compared to the other subtypes,and have a high prevalence of mutations in theAPC,KRAS,PIK3CA,ARID1A,and SMAD family member 4 (SMAD4) genes[46]compared to the MSS/TP53- subtype.They also present amplification of theCCNE1gene.The MSS/TP53- subtype is mainly Lauren intestinal and hasTP53mutations,with a low frequency of mutations affecting the other genes.This subtype also has amplification ofEGFR,MYC,ERBB2,andCCNE1genes.The MSS/EMT subtype predominantly consists of Lauren diffuse tumors,and tend to be diagnosed at a younger age.This subtype has low cell adhesion due to loss ofCDH1and has the least number of mutations.ARID1Ais among the most frequently mutated gene.The ACRG classification is also applicable to other large independent cohorts[45].The differences between the two classifications (TGCA and ACRG) reflect the different approaches and platforms used,and the ethnicity of the samples.In the ACRG cohort,GCs of the diffuse type are more represented.However,both identified the MSI subtype with hypermethylation ofMHL1,high mutation frequency and a better prognosis.The EBV and MSS/TP53 + subtypes are similar in that many cases belonging to the MSS/TP53+ subtype is EBV+ and present mutations inPIK3CAandARID1A.The GS and MSS/EMT subtypes,which include younger patients,are mostly diffuse and show low intercellular adhesion.The CIN and MSS/TP53-subtypes present with mutations inTP53and amplification of members of theEGFRfamily,and are mostly intestinal.
APPLICATION OF THE GC MOLECULAR PROFILE IN CLINICAL PRACTICE:PRECISION MEDICINE
Due to new technologies,such as NGS and microarray,recent discoveries have made possible to integrate diagnostic and therapeutic method,based on genotype and phenotype,and to apply them to individual patients with GC in the age of precision medicine.

Figure3 Asian Cancer Research Group gastric tumor classification.Gastric cancer was classified into foursubtypes:MSI (microsatellite instable); MSS (stable microsatellite); MSS/TP53+ (MSS with active TP53); MSS/TP53-(MSS with inactive TP53); MSS/EMT (MSS with epithelial-mesenchymal transition).ACRG:Asian Cancer Research Group.
Biomarkers for diagnosis and prediction
Tumor markers are used to determine the clinical stage,assess the treatment response,and predict the risk of recurrence after treatment.Currently,markers such as αfetoprotein (AFP),carcinoembryonic antigen (CEA),carbohydrate antigen 125 (CA-125),and carbohydrate antigen 19-9 (CA19-9) are frequently used in clinical practice.CEA is a risk factor for liver metastases[47],and increased CEA levels have been observed in all advanced GCs.The sensitivity and specificity of CEA for predicting GC recurrence is < 60% and < 80%,respectively[48].CA19-9 is a marker commonly used in GC,although it is also present in other neoplastic pathologies.In combination with other tumor markers,CA19-9 can provide more information to predict GC recurrence[49].Other markers such as AFP and CA-125 are widely used in the diagnosis of GC.AFP is an indicator of a high stage and presence of hepatic metastases[50],and CA-125 is associated with peritoneal diffusion[47].
Among the new biomarkers,human epidermal growth factor receptor 2 (encoded byERBB2commonly referred to asHER2) represents the first biomarker available in clinical practice for patients with GC.HER2 belongs to the EGFR family and has tyrosine kinase activity[51].An estimated 6%-23% of GCs have overexpression and/or amplification ofHER2[52,53],and it is mainly found in intestinal tumors[54,55].HER2 is used in clinical practice for targeted therapy.EGFR or ERBB1 is expressed in about 30% of GCs[56].The overexpression of EGFR in the pathogenesis of GC is associated with a poorly differentiated histology,vascular invasion,and shorter survival[57].Tyrosine kinase inhibitors,particularly gefitinib and erlotinib,have shown efficacy inEGFR-amplified tumors.Mutations ofEGFRconfer resistance to these drugs[58,59].In addition to anti-HER2 monoclonal antibodies,anti-EGFR therapy also includes gefitinib and erlotinib,tyrosine kinase inhibitors,as well as monoclonal antibodies such as cetuximab and panitumumab.
Other markers have attracted substantial attention as useful therapeutic candidates for targeted anti-cancer agents.For example,a high frequency (30%) of FGFR2 overexpression has been observed in GC.The amplification ofFGFR2is related to poor overall survival (OS)[60].Furthermore,a study found that FGFR2 could be a biomarker for predicting the long-term failure of adjuvant chemotherapy for advanced GC[61].Thus,FGFR2 may be a candidate for targeted anticancer agents.E-cadherin,a molecule involved in calcium-mediated cell adhesion,is a tumor suppressor whose deactivation is correlated with invasion and metastasis[62].The deactivation ofCDH1may occur due to mutations,hypermethylation,loss of heterozygosity,andH.pyloriinfection[62].Patients withCDH1changes have generally worse survival than negative patients.It could be a useful marker for the diagnosis of preoperative biopsies[63].Genetic deregulation of the PI3K/AKT/mTOR pathway has been frequently identified in GC[64,65],and mechanistic target of rapamycin kinase(mTOR) is activated in 60% of GCs[66].
Mutations ofPI3KCA,which encodes the p110α catalytic isoform of PI3K,have been identified in up to 25% of patients with GC[67].These mutations are involved in resistance to antitumor drugs and the acquisition of a metastatic phenotype;moreover,they are found mainly in the EBV-positive subtype of GC[68].It has been reported that the amplification and/or overexpression ofMETis involved in carcinogenesis,the efficacy of therapy,and the outcome of GC[69].MET expression is associated with invasion and overall poor survival[70].Vascular endothelial growth factor (VEGF) encodes for a growth factor that promotes the formation of new blood vessels.VEGF and vascular endothelial growth factor receptor (VEGFR) are upregulated in about 40% of GC cases[71],and their inhibition results in decreased cell proliferation and invasion.VEGF andsVEGFR2 are also used in clinical practice in targeted therapy[72,73].TP53is a tumor suppressor whose incidence of mutation in GC is about 3%-65%[74].In the EBV-positive subtype,the incidence ofTP53mutation is lower[28]; moreover,an increased incidence has been observed in the intestinal type[75].In many human tumors,TP53mutations are associated with a poor prognosis[76].For GC,there is no well-established clinical significance between theTP53status and the outcome of patients.Recent studies,however,have integrated the mutational status ofTP53and other genetic alterations to define subpopulations of GCs in order to define the clinical relevance[77].TP53mutations appear to be a cofactor that supports the expression of genes involved in various signaling pathways; and whose aberrant activation leads to high proliferation,increased metastatic potential,and resistance to treatment.AURKAand MDM2 proto-oncogene (MDM2) encode negative regulators ofTP53.AURKAis amplified and overexpressed in GC[78].By regulating the ubiquitination of TP53 through MDM2,AURKA promotes tumor growth and cell survival[79].
DNA damage is repaired by a series of mechanisms,including basic excision repair,mismatch repair,nucleotide excision repair,single-strand annealing,homologous recombination,and non-homologous end joining.The poly (ADP-ribose)polymerases,known as PARPs,are proteins involved in the basic excision repair pathway and catalyze the transfer of ADP-ribose to target proteins[80,81].PARP1 and PARP2 are the best known of these proteins.Numerous studies have highlighted an upregulation of PARPs in different tumors,including GC[82,83].A high expression of PARP1 in GC is associated with tumor invasion and a poor prognosis[84].
Proteins in the matrix metalloproteinase (MMP) family are involved in breakdown of the extracellular matrix in normal physiologic processes and can promote cancer cell invasion and metastasis by degrading the extracellular matrix.Increased matrix metallopeptidase 15 (MMP15) expression is associated with poor prognosis in GC[85].In addition,overexpression of matrix metallopeptidase 9 (MMP9)is a poor prognostic factor in patients with GC[86].
Fibrinogen C domain containing 1 (FIBCD1) is an acetyl group-binding receptor,which shows high affinity and calcium-dependent binding to acetylated structures such as chitin,some N-acetylated carbohydrates,and amino acids but not to their non-acetylated counterparts.The expression ofFIBCD1is significantly increased in GC tissues compared with normal tissues,and its overexpression is related to a poor prognosis[87].FIBCD1 may be a novel prognostic marker in gastric GC; however,the mechanisms underlying its function require further studies.
PD-1 and PD-2 are the immune checkpoint receptors expressed on T and B lymphocytes,natural killer T cells,and monocytes[88].After binding with PD-L1 and PD-L2 on activated T cells,they downregulate the activity of cytotoxic T cells and thus induce immunotolerance to the tumor.In 15%-70% of patients with GC,PD-L1 expression has been observed and this expression correlates with poor outcome[89].Upregulation ofPD-L1/PD-L2expression in the EBV-positive subtype has been observed[29].
Circulating tumor cells (CTCs),single or in clusters,originate from primary tumor or metastases[90].Clinically,they are related to the progression and metastatic processes,and therefore can be used as surveillance markers.CTCs can identify early stages of metastasis and thus identify patients who may benefit from treatment after primary tumor surgery[90,91].The presence of CTCs,which have the characteristics of stem cell-like or EMT cells,allows evaluation of the tumor stage and the prediction of recurrence.Circulating cell-free DNA is more sensitive than CTCs,originates from normal and cancerous cells,and is present in the blood[92].Circulating tumor DNA(ctDNA) originates from the primary tumor or metastases and can be used for the specificity of the diagnosis,even if the sensitivity is lower than the common markers used[93].The ctDNA shows the presence of EBV DNA,and is useful for identifying EBV-positive subtypes[94].The response to therapy can also be assessed with ctDNA.
MiRNAs are small,non-coding RNAs that,by regulating gene expression,play a role in the processes of proliferation,differentiation,and cell invasion[95].They can increase the expression of oncogenes or reduce the expression of oncosuppressor genes[96].Numerous miRNAs have been identified and play a role in GC[97,98].Circulating cell-free miRNAs can be used as non-invasive biomarkers for the diagnosis and relapse of GC[99-101].Approximately 135 long non-coding RNAs(lncRNAs),non-transcribed RNA sequences longer than 200 nucleotides,are dysregulated and strongly correlated with tumorigenesis,metastasis,and prognosis of GC[102-103].Some lncRNAs are overexpressed in GC compared to healthy control tissue and may be prognostic markers[104,105].However,further studies are needed to determine their possible clinical use.
Biomarkers for targeted therapy
Surgery is the elective treatment for many stages of GC.In a patient with GC at stage 0,I,II,or III,surgery (often together with other treatments) is currently the only treatment.Depending on the type and stage of GC,it is possible with surgery to remove all or part of the stomach including the nearby lymph nodes (the principles).Even when the tumor is too widespread to be removed entirely,patients can be helped by surgery because it can help prevent bleeding from the tumor or remove stomach obstruction due to tumor growth.This is termed palliative surgery because it allows the reduction or prevention of symptoms,but is not indicated for the treatment of GC[106].
Minimally invasive surgery,including laparoscopic gastrectomy and robotic gastrectomy,is receiving much attention in GC management[107,108].The laparoscopic gastrectomy has the advantage of leading to a faster recovery with shorter hospital stays compared to the traditional surgery[109].However,it has the disadvantage of limited movements.The robotic gastrectomy has overcome these limitations and its use is spreading rapidly[110-112].Some studies have been carried out in order to compare both the advantages and disadvantages of two technologies[113-115].The disadvantages of robotic gastrectomy concern its cost,duration of the procedure,and training needs[116].Unfortunately,the lack of controlled and randomized studies has precluded the ability to establish a clear indication of robotic gastrectomy in the treatment of GC[116].
Surgical resection with pre- and post-operative chemotherapy and/or radiotherapy is the primary curative treatment of early-stage GC with a 5-year survival of about 30%[117-119].Systemic chemotherapy is used to treat patients with localized and advanced GC.Palliative systemic therapy and chemo/radiotherapy are standard treatment options for patients with unresectable or metastatic advanced GC.Neoadjuvant chemotherapy with surgery is associated with the improved survival of patients with metastatic disease[120].Perioperative chemotherapy with docetaxel,oxaliplatin,fluorouracil,and leucovorin (FLOT) significantly improves progressionfree survival (referred to herein as PFS) and OS among patients with resectable GC compared with epirubicin,cisplatin,and fluorouracil or capecitabine (ECF/ECX)[121].A Bayesian network meta-analysis obtained an estimate of the efficacy of perioperative FLOT and neoadjuvant treatments for resectable GC.Compared with surgery alone,perioperative cisplatin with fluorouracil (CF),perioperative ECF/ECX,and perioperative FLOT significantly improved survival.The most effective neoadjuvant treatment for the disease is likely to be perioperative FLOT[122].Targeted therapy,a new therapeutic strategy,may improve the survival of patients with advanced GC.Clinical trials with targeted therapies have been performed in patients with GC.Table1 shows some clinical trials,completed or ongoing,classified by specific molecular target.
EGFR signaling pathway
The EGFR signaling pathway is activated in the GC[56,123].Overexpression of EGFR has been associated with reduced OS[56,71].This behavior may depend on the observation that EGFR targeting molecules may be potential agents for target therapy.Trastuzumab is the first molecular targeted agent approved as standard therapy for GC.It is a monoclonal antibody against HER2,which binds to the extracellular domain of the receptor.A phase III clinical trial (ToGA) (NCT01041404) enrolled 594 patients with GC who had high HER2 expression.These patients were randomized to chemotherapy alone or combined with trastuzumab.Treatment with trastuzumab led to an increase in OS of 2.7 mo and the PFS was heightened compared to that of patients treated with chemotherapy alone[124].The benefits observed in patients treated with the combination of trastuzumab and chemotherapy were even more evident in patients who expressed high levels of HER2 compared to those with low HER2 expression.The 2015 National Comprehensive Cancer Network guidelines recommended the first-line treatment of trastuzumab combined with chemotherapy in patients overexpressing HER2.To date,trastuzumab is the only targeted therapy allowed for the treatment of advanced GC[124].A clinical trial (NCT01736410),which evaluated the efficacy of trastuzumab with tegafur,gimeracil,oteracil (TS-1) and cisplatin as first-line treatment for advanced HER2-positive GC,has been completed.The combination of trastuzumab with TS-1 and cisplatin demonstrated good activity,was well tolerated,and is a first-line treatment that can be used for advanced HER2-positive GC[125].In the GATSBY multicenter phase II/III study (NCT01641939),the efficacy of trastuzumab emtansine was evaluated in patients with advanced HER2-positive GC who had already received previous treatment.The results obtained werenot encouraging since treatment with trastuzumab emtansine did not yield increases in OS compared to standard treatment with a taxane (docetaxel,paclitaxel)[126].

Table1 Clinical trials classified on molecular targets

PI3KCA Alpelisib +AUY922 NCT01613950 Multicenter,open-label Second/third Ib Advanced/m etastatic gastric cancer,PIK3CA mutations and/or HER2 amplification Completed [145]AKT Ipatasertib ±5FU/oxaliplat in/leucovorin NCT01896531 Randomized,double Second II Advanced/m etastatic gastric cancer Active [146]HGF/MET HGF Rilotumumab vs rilotumumab±epirubicin/cis platin/capecit abine NCT00719550 Multicenter,randomized First Ib/II Locally advanced/me tastatic gastric cancer Completed [149]HGF Rilotumumab±epirubicin/cis platin/capecit abine RILOMET-1(NCT0169707 2)Multicenter,randomized,triple First III Locally advanced/me tastatic gastric cancer,MET-positive Terminated [150]HGF Rilotumumab±/cisplatin/ca pecitabine RILOMET-2(NCT0213734 3)Multicenter,randomized,triple First III Advanced gastric cancer Terminated [151]MET Onartuzumab± 5-FU/leucovori n/oxaliplatin NCT01662869 Multicenter,randomized,double First III Metastatic gastric cancer,HER2 negative,MET-positive Completed [152]VEGF/VEG FR VEGFR2 Ramucirumab+ BSC vs placebo +BSC REGARD(NCT0091738 4)Randomized,quadruple First III Metastatic/lo cally recurrent gastric cancer Completed [72]VEGFR2 Ramucirumab± paclitaxel RAINBOW(NCT0117066 3)Multicenter,randomized,double Second III Metastatic,refractory gastric cancer Completed [73]VEGFR2 Apatinib vs placebo NCT01512745 Randomized,quadruple Third III Advanced/m etastatic refractory gastric cancer Completed [156]TP53 TP53 Polymorphis ms of xenobiotic metabolism,DNA repair,and TP53 genes NCT01470404 Gastric cancer treated with adjuvant chemotherap y Completed [161]TP53 APR-246 + 5-FU/cisplatin NCT02999893 Open-label Second I/II Advanced/m etastatic platinum resistant gastroesopha geal cancer,TP53 mutated Recruiting [167]TP53 AZD1775(WEE inhibitor) +paclitaxel NCT02448329 Single center Second II Advanced gastric cancer,TP53 mutated Recruiting [169]TP53 HDM201(inhibitor TP53/MDM2 interaction)NCT02143635 Multicenter,nonrandomized,open-label Second I Advanced/m etastatic gastric cancer,TP53 wildtype Active [170]PARP PARP Olaparib +paclitaxel vs paclitaxel Study 39(NCT0106351 7)Multicenter,randomized,double Second II Metastatic/re current gastric cancer,low ATM expression Active [174]PARP Olaparib +paclitaxel vs placebo +paclitaxel GOLD(NCT0192453 3)Multicenter,randomized,double Second III Advanced gastric cancer Active [175]
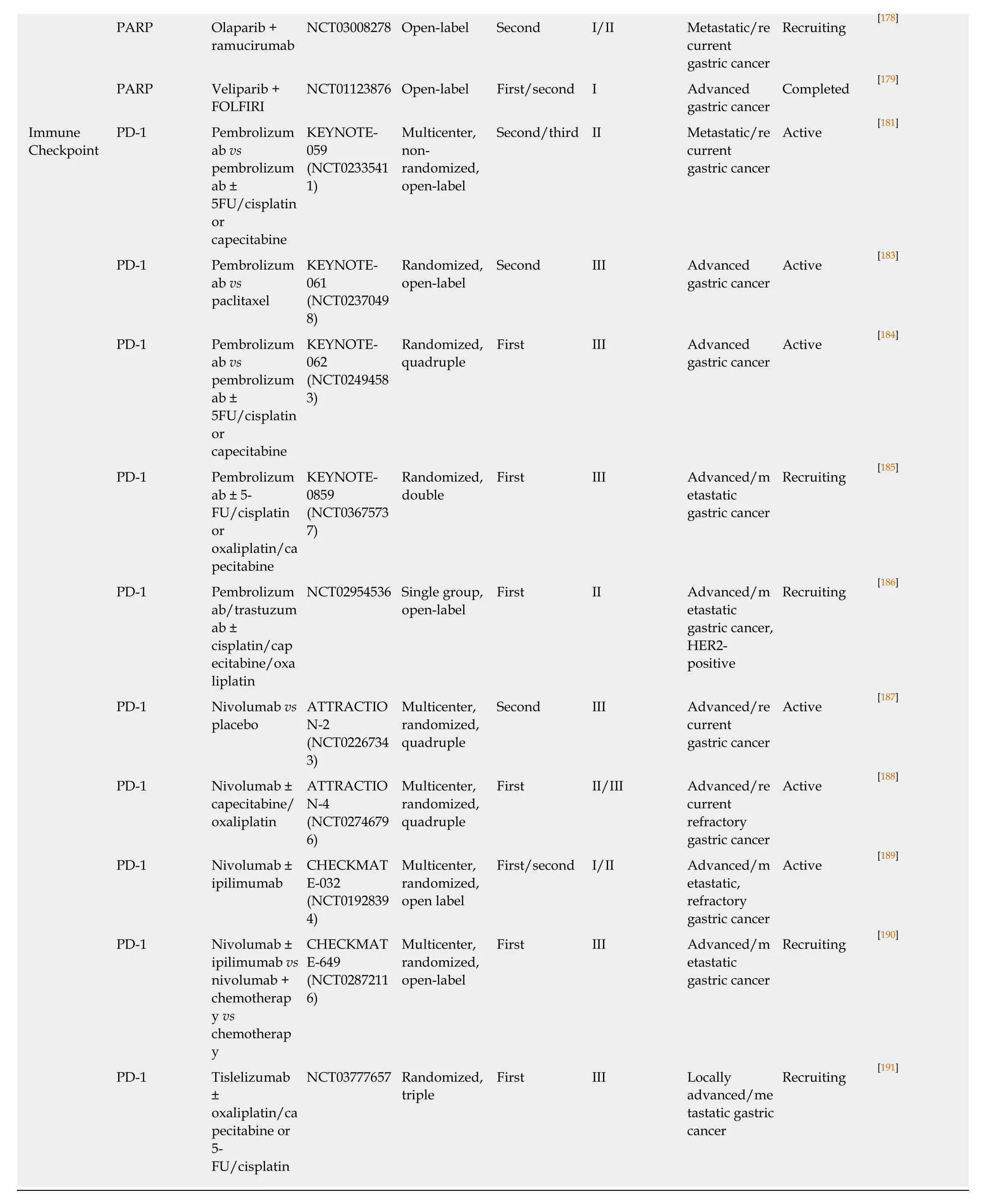
EGFR:Epidermal growth factor receptor; mTOR:Mechanistic target of rapamycin kinase; HGF:Hepatocyte growth factor; VEGF:Vascular endothelial growth factor; TP53:Tumor protein p53; PARP:Poly (ADP-ribose) polymerase; PD-1:Programmed cell death 1.
Other agents that target HER2,such as pertuzumab and lapatinib,have been used in clinical trials in patients with advanced GC and HER2 overexpression.One study(JACOB,NCT01774786) was performed with pertuzumab,trastuzumab,and chemotherapy in patients with untreated HER2-positive metastatic GC.This trial was the first to investigate the dual antibody blockade of HER2.Unfortunately,no significant improvement in OS was observed in the dual blockade group[127].The clinical trial (TRIO-013/LOGiC,NCT00680901) performed with lapatinib in combination with oxaliplatin and capecitabine did not produce significant results in terms of OS[128].A parallel study of biomarkers was conducted using immunohistochemistry and NGS.The most common alteration found in HER2-positive patients was amplification ofCCNE1,which correlated with a lack of response to therapy.Patients with high levels ofERBB2amplification were more responsive to therapy.The analysis of cell-free DNA showed that the amplification ofERBB2,detectable in the plasma of patients,was a predictive response.During disease progression,genetic changes were detected such as amplification ofMYC,EGFR,FGFR2,andMET[129].A phase I clinical trial (NCT02795988) evaluated the safety,tolerability,and immunogenicity of IMU-131,a peptide composed of three epitopes selected from the protein structure of HER2.In the phase II portion of the same trial,IMU-131 was used in combination with chemotherapy in patients overexpressing HER2.The study is ongoing,as only phase I has been completed,and no conclusions have been drawn[130].Pyrotinib is an irreversible inhibitor of both HER2 and EGFR.The phase I studies (NCT02500199,NCT02378389) with pyrotinib and pyrotinib plus docetaxel in patients with HER2-positive GC are recruiting[131,132].
Studies have also been performed to identify markers to be used in monitoring the efficacy of trastuzumab alone or in combination with chemotherapy.Resistance has occurred in patients treated with trastuzumab.One of the main mechanisms that lead to this resistance are mutations inPI3KCAandPTEN[64,65,67].The combination of trastuzumab with PI3K inhibitors may bring substantial benefits to patients with HER2-positive GC.One of the markers of resistance to trastuzumab isCCNE1,whose amplification is negatively correlated with the response to therapy directed against HER2[133].Other monoclonal antibodies used to target EGFR include cetuximab and panitumumab.The results showed that anti-EGFR antibodies did not provide further benefits for patients with advanced GC receiving chemotherapy as first-line treatment(EXPAND) (NCT00678535)[134].Panitumumab was used as first-line treatment in a clinical phase I/II trial (NCT01716546) in association with 5-FU,cisplatin,and docetaxel for locally advanced or metastatic GC.However,this study did not reach its primary endpoint because in an intermediate analysis,the number of responses obtained was lower than the prefixed limit[135].Nimotuzumab is the first EGFR humanized monoclonal antibody that binds with high specificity to the extracellular region of EGFR.Two clinical trials have been concluded.The phase III study(NCT01813253) was performed to evaluate the OS in advanced GC patients with EGFR overexpression who were treated with nimotuzumab in combination with irinotecan and compared to a group of patients who received only irinotecan.This study,completed in 2018,has not yet had its results reported[136].The second study(NCT02370849) evaluated the efficacy of cisplatin and S-1 with and without nimotuzumab in patients with advanced GC who were not previously treated[137].The combination of nimotuzumab and S-1-cisplatin provided no additional benefit compared to chemotherapy alone in the first-line treatment of unresectable or metastatic GC[138]
mTOR/PI3K/AKT signaling pathway
Everolimus (RAD001) is an mTOR inhibitor with antitumor activity.In a phase I clinical trial,RAD001 was used in combination with capecitabine in patients with refractory GC; the clinical benefits were modest[139].In phase I clinical trials(NCT01049620) and (NCT01042782),RAD001 was used in combination with capecitabine and oxaliplatin and with mitomycin C,respectively,in patients with advanced GC; the results of these trials are unknown[140,141].In a multicenter phase II study (NCT00519324),RAD001 was used in patients with metastatic GC with previous chemotherapy failure; particularly,10.1 mo was the median OS for which the monotherapy with everolimus,in patients in which the previous chemotherapy had failed,showed a satisfying disease control rate[142].Another clinical trial(NCT00729482) evaluated the efficacy of RAD001 as a monotherapy in patients with advanced GC in whom standard first-line treatment had failed.In addition to the efficacy of RAD001,the expression of markers was evaluated in order to identify biomarkers of response to therapy.Tumors that did not have mTOR pathway activation did not benefit from treatment with RAD001[143].The median OS was lower than that reported in the study conducted by Doiet al[142].The results of this study showed that the efficacy of RAD001 was unsatisfactory compared to conventional treatment for advanced GC[143].In the phase III GRANITE-1 study (NCT00879333),the median OS in patients treated with RAD001vsplacebo was 5.4vs4.3 mo.Compared to best supportive therapy (referred to as BSC in Table1),RAD001 did not significantly improve OS in patients with advanced GC who were previously administered one or two lines of systemic chemotherapy[144].A clinical trial(NCT01613950) was performed to investigate the efficacy of the combination of alpelisib (BYL719),a potent and selective inhibitor of mutatedPI3KCAand AUY922,an inhibitor of heat shock protein 90 (HSP90),in patients with advanced GC withPIK3CAmutations and/or amplification ofHER2,respectively.The results are not yet known[145].Ipatasertib (GDC-0068),an inhibitor of serine/threonine kinase (AKT),has been used in combination with 5-FU,folinic acid,and oxaliplatin (mFOLFOX6) in advanced or metastatic GC in a multicenter placebo-controlled clinical trial(NCT01896531).The trial is ongoing[146].
Hepatocyte growth factor/MET signaling pathway
High MET expression has been observed in intestinal GC rather than in the diffuse type and in advanced stage disease[147].MET positivity is a prognostic factor for OS in GC[148].Patients with GC and MET expression can benefit from anti-MET drugs.Rilotumumab is a hepatocyte growth factor (HGF) monoclonal antibody that blocks binding between HGF and its receptor MET.The efficacy of first-line rilotumumab in patients with GC in combination with ECX was demonstrated in a phase Ib/II clinical study (NCT00719550).The group of patients who received ECX plus rilotumumab showed a better prognosis than placebo[149].The RILOMET-1 clinical trial(NCT01697072) evaluated the efficacy of rilotumumab in combination with epirubicin,cisplatin,and capecitabine.Regarding OS,the addition of rilotumumab to chemotherapy did not bring about benefits compared to chemotherapy alone in MET-positive patients[150],unlike the phase II study in which OS was 10.6vs5.7 mo in MET-positive patients who received rilotumumab compared to the placebo group[149].The multicenter phase III clinical trial,RILOMET-2 (NCT02137343),in which patients with advanced GC were treated first-line with rilotumumab plus cisplatin and capecitabine,was closed for a review of the safety of the study[151].The randomized,multicenter study (NCT01662869) evaluated the efficacy of onartuzumab (monoclonal anti-MET antibody) in combination with mFOLFOX6 in patients with metastatic HER2-negative and MET-positive GC.Onartuzumab did not yield satisfactory results in combination with FOLFOX[152].
VEGF/VEGFR signaling pathway
Antibodies against VEGF and VEGFR have shown anti-tumor effects in combination with chemotherapy as first and second-line treatments for GC.Bevacizumab,a humanized monoclonal antibody against VEGF,inhibits the VEGF/VEGFR signaling pathway[153].A phase II study (NCT00447330) was performed in patients with metastatic GC in combination with capecitabine and oxaliplatin; an OS of 7.2 and 10.8 mo was demonstrated in the two groups of patients treated with chemotherapy alone and with the combination with bevacizumab,respectively[154].Ramucirumab is a humanized monoclonal antibody specific for VEGFR2.By blocking downstream VEGFR2 signaling,ramucirumab provides antitumor effects both as a single agent(REGARD trial,NCT00917384)[72]and in combination with paclitaxel (RAINBOW trial,NCT01170663)[73]in patients with metastatic refractory GC.The median OS was significantly longer in the group of patients treated with ramucirumab plus paclitaxel(9.6 mo) compared to those treated with paclitaxel plus placebo (7.4 mo).Thus,ramucirumab may be a new second-line treatment for patients with metastatic GC.The addition of ramucirumab to FOLFOX (leucovorin,5-FU,oxaliplatin) did not improve OS in patients with advanced GC[155].In a phase III study (NCT01512745),apatinibvsplacebo was used in patients with advanced/metastatic GC who failed two lines of chemotherapy.The median OS was 6.5vs4.7 mo[156].Other studies with apatinib have been started but have not yet been completed regarding the use of apatinib alone as first-line maintenance treatment in patients with advanced GC(NCT03255811)[157]and as maintenance treatment with capecitabine (NCT03598348)after first-line chemotherapy[158].The clinical trial (NCT03104283) assessed the efficacy and safety of apatinib as monotherapy in elderly advanced GC patients,and determined the relationship between VEGFR2 expression and efficacy of apatinib treatment[159].In a retrospective study,the efficacy of the association of apatinib with docetaxelvsapatinib as monotherapy as a second- or third-line treatment in advanced GC was evaluated.The median OS was 3.3vs6.0 mo in patients with apatinib monotherapy and those with apatinib and docetaxel combination,respectively.Patients with advanced GC benefited more with the combination of apatinib and docetaxel than with apatinib monotherapy[160].
TP53 signaling pathway
A pharmacogenomic study (NCT01470404) was performed to evaluate the effects of germline polymorphisms in xenobiotic metabolism genes on the toxicity profile,and the role of germline polymorphisms of genes involved in DNA repair and theTP53tumor suppressor to predict disease recurrence and survival in GC patients treated with adjuvant chemotherapy[161].TP53mutations represent a very attractive target for cancer therapy.One of the objectives being pursued is to identify molecules that can restore the function of wild-type TP53.Among these,APR-246 was identified[162],and has already been tested in mouse models of cancer[163-165]and in phase I/II clinical trials on hematological and prostate malignancies[166].A phase I/II study(NCT02999893) was prepared for the treatment of gastroesophageal tumors with mutatedTP53[167].BecauseTP53mutations are still somewhat difficult to address adequately,identifying TP53-dependent targets may provide new opportunities for alternative targeted therapies.For example,targeting WEE1 G2 checkpoint kinase(WEE1),a protein kinase that plays a role in the G2-M cell cycle checkpoint,prevents cells from entering mitosis in response to DNA damage[168].AZD1775,an inhibitor of WEE1,was used in the clinical trial (NCT02448329) as second-line therapy in combination with paclitaxel in GC harboringTP53mutations[169].Overexpression of AURKA improves the stabilization of MDM2 and promotes the degradation of TP53,inhibiting its proapoptotic function in response to chemotherapy[79].This result justifies the use of AURKA inhibitors in the treatment of GC.The TP53/MDM2 interaction inhibitor (HDM201) was used in the clinical trial (NCT02143635) in patients with advanced GC characterized by wild-type TP53; this study is ongoing[170].No clinical trial has been performed on the use of MMP inhibitors in GC.
PARP signaling pathway
In response to DNA damage,sensors and effectors are activated that induce cell cycle arrest,damage repair,and eventually cell apoptosis.PARP inhibitors act by preventing breakage of the single DNA strand and induce tumor cell death[171].In vitro,gastric carcinoma cell lines,particularly those in which the ATM serine/threonine kinase expression levels are low,were sensitive to the action of olaparib (PARP inhibitor)[172].In a phase II study,the efficacy of olaparib (AZD-221)plus paclitaxel was evaluatedvspaclitaxel in patients with recurrent or metastatic GC whose ATM expression levels were low or undetectable (Study 39; NCT01063517)[173].The combination of olaparib plus paclitaxel significantly improved OS compared to placebo/paclitaxel,both in the general population and in the population with low ATM levels (13.1vs8.3 mo)[174].A multicenter phase III trial has evaluated the efficacy of olaparib in combination with paclitaxelvsplacebo plus paclitaxel in patients with advanced GC who are progressing after first-line treatment (GOLD,NCT01924533).The OS did not differ between treatment groups in the overall population (median OS 8.8 mo in the olaparib groupvs6.9 mo in the placebo group or the negative ATM population (12.0 movs10.0 mo)[173].
The GOLD study did not achieve its primary objective to show a significant improvement in OS with olaparib in the overall or ATM-negative population of patients with advanced GC.However,the study provided data on efficacy and safety related to the use of olaparib in combination with a chemotherapy drug and the study itself is foundational for other studies in this type of patients[175].The GOLD trial has been negative for its endpoints of improved OS,both in overall patient population and the ATM-negative population.The differences between the GOLD trial and Study 39 are the enriched population of ATM-negative patients in Study 39 (51%vs19%)with respect to the GOLD study.PARP inhibitors are effective in tumors with a definite molecular signature,so it may not be realistic to expect efficacy from olaparib in an unselected marker population[176].
Furthermore,the time of exposure to olaparib was shorter in the GOLD trial than in Study 39[177].A phase I/II pilot study was prepared to analyze the efficacy of olaparib in combination with ramucirumab in patients with metastatic,recurrent or unresectable GC (NCT03008278).The study is currently recruiting[178].Another phase I clinical trial (NCT01123876) studied the combination of veliparib (PARP inhibitor)with FOLFIRI in patients with advanced solid tumors,including GC[179].The antitumor activity of veliparib in combination with FOLFIRI and the acceptable safety profile lay the foundation for further studies.
Immune checkpoint signaling pathway
Pembrolizumab is the first immune checkpoint inhibitor approved by the United States Food and Drug Administration (FDA) for the treatment of advanced or metastatic GC[180].In a multicenter phase II trial (KEYNOTE-059,NCT02335411),the efficacy of pembrolizumab alone was demonstrated in patients with advanced GC who had previously been treated[181].Treatment with pembrolizumab showed a higher overall response rate in PD-L1-positive patients than in PD-L1-negative patients.Furthermore,in MSI-high patients,the response was higher than that in non-MSI high patients.These results suggest that PD-L1 and MSI levels may be predictive biomarkers of pembrolizumab efficacy[182].In the trial KEYNOTE-061 (NCT02370498),which was performed in patients with advanced PD-L1-positive GC,the efficacy of second-line treatment of pembrolizumabvspaclitaxel was compared.Pembrolizumab did not significantly improve OS compared to paclitaxel but had a better safety profile than paclitaxel[183].Pembrolizumab was used as first-line monotherapy or in combination with cisplatin,5-FU,or capecitabine in patients with advanced PD-L1-positive GC (KEYNOTE-062,NCT02494583); the results of this study are not yet known[184].In the clinical trial KEYNOTE-859 (NCT03675737),the efficacy of pembrolizumab in association with chemotherapy with cisplatin and 5-FU or oxaliplatin and capecitabine,in advanced/metastatic HER2-negative GC expressing PD-L1,will be evaluated[185].Another clinical trial (NCT02954536) is evaluating the first-line efficacy of the combination of pembrolizumab and trastuzumab in combination with chemotherapy in patients with HER2-positive metastatic GC.Preliminary results have been obtained on the safety and efficacy of the treatment.Resistance phenomena have occurred because mutations ofTP53(63%) andKRAS(16%) and loss ofERBB2amplification in disease progression have been observed[186].Nivolumab is a monoclonal antibody that targets PD-1 and has received FDA approval for neoplastic pathologies.In a clinical trial (NCT02267343) performed in patients with locally advanced or metastatic GC refractory to chemotherapy,nivolumab was effective with improvement of the median OS[187].In another clinical trial (NCT02746796),the efficacy of nivolumab in combination with chemotherapy as first-line treatment was tested in patients with advanced or recurrent non-resectable GC[188].In the clinical trial CheckMate-032 (NCT01928394),performed on solid tumors including GC,the efficacy of nivolumab in combination with ipilimumab,an anticytotoxic T-lymphocyte associated protein 4 antibody (CTLA4),was evaluated.Nivolumab with ipilimumab demonstrated encouraging long-term OS in patients with GC refractory to chemotherapy[189].In the clinical trial CheckMate-649(NCT02872116),the efficacy of nivolumab as first-line treatment in combination with ipilimumabvsnivolumab plus chemotherapyvschemotherapy alone,is being evaluated in patients with advanced or metastatic GC[190].The clinical trial(NCT03777657) is evaluating the efficacy of tislelizumab,a humanized anti-PD1,in combination with oxaliplatin and capecitabine or 5-FU and cisplatin[191].
Other targets
Clinical trials have been performed to study the significance of CTCs in advanced/metastatic GC.Some trials (NCT03156777[192],NCT01625702)[193]have been designed to evaluate CTCs as markers of prognosis and response to chemotherapy.In a clinical trial (NCT01625702) in HER2-positive patients,an increased HER2 extracellular domain was a predictor of a better prognosis.The elevated levels of HER2 after therapy were correlated with a negative therapeutic response[194].Other trials have been designed to evaluate CTCs and cell-free DNA as clinical prognosis markers (NCT01299688)[195]and response to HER2 (NCT02610218)[196]or VEGFR(NCT02048540)[197]targeting.Only one clinical trial (NCT01848015) was designed to establish the predictive value of CTCs in the recurrence of advanced GC after radical resection[198].Some of these trials have been completed,but the results are not yet known.A study conducted in patients with HER2-positive metastatic GC revealed that the ctDNA of these patients provided useful information for monitoring the response to trastuzumab,for the purpose of developing therapeutic strategies for HER2-positive but trastuzumab-resistant patients[199].A phase III clinical study(NCT01178944) was performed to determine if miR-215-5p levels could be predictive of the response to pralatrexate (a folate analog metabolic inhibitor) in association with oxaliplatin in patients with non-resectable GC[200].Another study (NCT03253107) was conducted to determine if miRNAs levels may be predictive biological markers for the response to chemotherapy[201].The results of these trials are not yet known.A study(NCT03057171) is ongoing on the control ofH.pylorion the expression of lncRNAs in gastrointestinal diseases including GC[202].
CONCLUSION
GC is the fifth most malignant tumor worldwide and the third leading cause of cancer-related deaths[7].Unfortunately,the disease becomes symptomatic in the advanced stage.GC is a complex disease whose onset is linked to a series of environmental and genetic factors[1-6].Despite the increasing knowledge and progress in drug development,due to late diagnosis and extreme intra- and inter-tumor heterogeneity,the prognosis of GC patients is poor.The heterogeneity of GC is mainly linked to genetic and epigenetic alterations,but also interactions with the microenvironment and the presence of intratumoral cellular clones.Hence,there are variations between patients and within the same tumor.The new classifications,TCGA and ACRG,based on molecular profiles and complementary to those based on pathological characteristics[15],have highlighted four GC subtypes,each characterized by specific genetic alterations[29].The molecular classification of GC has helped to identify molecular alterations that may be targeted by the therapy.Furthermore,the molecular profiles of GCs obtained from individual patients has provided new opportunities to identify biomarkers that may predict the tumor response to treatment[22-24].Unfortunately,even today,the molecular characteristics of tumors are not taken into significant consideration in the management of patients.
H.pyloriis responsible for the onset of peptic ulcers and 80% of GC cases.Eradication of theH.pyloriinfection treats gastritis and peptic ulcers and is a mean to prevent GC.Obviously,for the treatment of the eradication of theH.pylori,guidelines have been issued by three separate authoritative groups[203-205]but none overcome the problem of resistance.Falloneet al[206]recently revised the guidelines to arrive at the best treatment options; however,GC still develops after the eradication.Many Japanese investigators have reported that the presence of severe atrophy after eradication represents a risk factor for the development of GC[207].Hence,there is a need for specific endoscopic surveillance programs for this type of patients.
Endoscopy plays an important role in the diagnosis of GC[208].More than 90% of GC cases are reportedly revealed by biopsy-associated endoscopy.The increased use of endoscopy,thanks also to the revolutionary developments that have occurred recently and that have produced new,more sophisticated systems,has allowed highlighting of the “early” GC[209,210].Ultrasonographic endoscopy is useful for TNM staging of GC patients,having a high diagnostic value.This technique allows the patient to be managed for the most appropriate treatment,limiting the occurrence of unnecessary exploratory surgical procedures[211].Endoscopy can also be curative for early GC or used as palliative care for more advanced cases.In early GC,the endoscopic mucosal resection provides similar effects as traditional surgical resection[212,213].
Surgical resection with adjuvant or neoadjuvant radiotherapy and chemotherapy with cisplatin,5-FU,taxane,or irinotecan,remains the most effective treatment for advanced GC.The recent MRC MAGIC/UK study (ISRCTN93793971) showed that perioperative ECF/ECX chemotherapy led to an improvement in OS and PFS in patients with resectable GC[214].Perioperative chemotherapy is the standard of care in most of Europe for localized GC with accepted ECF or ECX regimens[215].However,objective response rates to chemotherapy range from 20% to 40%,indicating variable clinical responses that are mostly likely caused by the biologic heterogeneity of the tumor.As with chemotherapy,therapeutic regimens based on targeted therapy have recently been introduced,which makes use of small molecules or monoclonal antibodies that can act on specific molecules capable of modifying molecular pathways involved in proliferation,differentiation,and cell invasion.
Based on phase III clinical trials in patients with advanced/metastatic GC,trastuzumab (anti-HER2) and ramucirumab (anti-VEGFR2) have been approved as first- and second-line therapies in these patients[72-74,124].However,the survival of patients receiving these therapies is not very high,and with the exception of HER2,there are no markers that can be used to evaluate the response to therapy.Data from preclinical studies have shown a relationship between HER2 overexpression and activation of angiogenesis in breast cancer cells[216].A retrospective study showed significant efficacy of the combination of a biological therapy with ramucirumab with a chemotherapeutic (paclitaxel) in patients in whom trastuzumab therapy had failed[217].This study has demonstrated the crosstalk between HER2 signaling and angiogenesis in GC,which can explain tumor survival.Therefore,trastuzumab resistance could be overcome by inhibiting the angiogenic pathway.An analysis of subgroups extrapolated from the RAINBOW study showed that patients who had already been treated with trastuzumab benefited from treatment with ramucirumab in combination with paclitaxel[218].New studies will be needed to evaluate the efficacy of sequential blockades of both pathways to improve the survival of patients with GC.
Other monoclonal antibodies,such as cetuximab and panitumumab (anti-EGFR),have also been tested in advanced/metastatic GC but the results on survival rates have not been encouraging[134-135].Unacceptable results were obtained with RAD001,an mTOR inhibitor.The efficacy of RAD001 is unsatisfactory compared to conventional treatment for advanced GCs[139,143].Anti-MET monoclonal antibodies,such as rilotumumab and onartuzumab,in combination with chemotherapy,did not bring benefits compared to chemotherapy alone[149-152].A study was prepared to more effectively target MET with a mixture of two humanized monoclonal antibodies that target two non-overlapping MET epitopes.The results represent efficacy data demonstrated on preclinical models and are part of a clinical trial (NCT02648724)[219]carried out on patients with NSLC and MET amplification[220].The advantage of antibody mixtures is their ability to orchestrate the internalization of the receptor and its degradation more effectively than a single monoclonal antibody,as previously shown for the EGFR family[221].
PARP inhibitors are very effective in the treatment of ovarian and breast tumors in which DNA repair systems are altered and BRCA1/2 mutations are present,which makes them more sensitive to these inhibitors[222].New biomarkers are being explored,which go beyond BRCA1/2 mutations and DNA repair mechanism deficits to stratify sensitive patients,new combinations of PARP inhibitors,and/or combinations with checkpoint inhibitors to determine who will be eligible for this treatment for other solid tumors,including GC[223].It has been hypothesized that the inhibition of PARP may trigger mechanisms based on the recognition of new tumor cell antigens by the immune system,making the PARP inhibitors potential partners for combination with immune checkpoint inhibitors.
Recently,patients with GC have also been studied from an immunotherapy viewpoint.Pembrolizumab and nivolumab received FDA approval for GC[180].Anti-PD1 antibodies have been used in phase II and phase III clinical trials and appear to be promising,especially in patients overexpressing PD-L1.Further clinical trials are underway to evaluate the efficacy of these antibodies in association with chemotherapy.At the same time,other pathways such as the TP53 signaling pathway,are being studied to identify inhibitory molecules[162-166].Strategic opportunities can also be provided by studying the potential of biomarkers such as CTCs,ctDNA,miRNAs,and lncRNAs to predict response to therapy and resistance phenomena.
There is no doubt that targeted therapies allow patients to live longer,whether they are administered alone or in combination with chemotherapy.Today the probability of observing patients who survive several years after the diagnosis of cancer is much higher,thanks to the targeted therapies.The targeted therapies must be provided to groups of patients who can benefit from them,screened on the molecular profiles to which the therapy is effective.Molecular profiling regarding the overexpression and/or mutation of the targets must be carried out on tissue biopsies,both in resectable and unresectable patients,to establish the correct targeted therapy to be used alone or associated with chemotherapy.It is necessary to continue to study the heterogeneity of GC.The fact that GC has genetic variations between different patients and/or in the same patient during its progression and/or during or after therapy (conventional or targeted) should drive investigations into the molecular characteristics present in tumor tissue,and the use of circulating biomarkers to predict and monitor disease progression and response to therapy.Furthermore,the association of several markers should be considered in order to appropriately classify the tumor and to establish therapeutic strategies that increase survival rates.
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Cancer-specific metabolism:Promising approaches for colorectal cancer treatment
- Prognostic and pathological impact of tumor budding in gastric cancer:A systematic review and meta-analysis
- Race,the microbiome and colorectal cancer
- Endoscopic management of esophageal cancer
- Retrospective review of total neoadjuvant therapy
- Retrospective Cohort Study Fat clearance and conventional fixation identified ypN0 rectal cancers following intermediate neoadjuvant radiotherapy have similar long-term outcomes
