Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: Beyond the natural history
2019-10-19SalvatoreMagrDaniloPaduanoFabioChiccoAriannaCingolaniCristianaFarrisGiovannaDeloguFrancescaTumbarelloMariantoniaLaiAlessandroMelisLauraCasulaMassimoFantiniPaoloUsai
Salvatore Magrì, Danilo Paduano, Fabio Chicco, Arianna Cingolani, Cristiana Farris, Giovanna Delogu,Francesca Tumbarello, Mariantonia Lai, Alessandro Melis, Laura Casula, Massimo C Fantini, Paolo Usai
Abstract BACKGROUND Nonalcoholic fatty liver disease (NAFLD) is a frequently reported condition in patients with inflammatory bowel disease (IBD). Both intestinal inflammation and metabolic factors are believed to contribute to the pathogenesis of IBDassociated NAFLD.AIM To evaluate the prevalence of steatosis and liver fibrosis (LF) in a cohort of IBD patients and the identification of metabolic- and IBD-related risk factors for NAFLD and LF.METHODS IBD patients were consecutively enrolled from December 2016 to January 2018.Demographic, anthropometric and biochemical data were collected so as eating habits. Abdominal ultrasound and transient elastography were performed to evaluate the presence of NAFLD and LF respectively.RESULTS A total of 178 consecutive patients were enrolled and included in the analysis (95 Ulcerative colitis, 83 Crohn’s disease). NAFLD was detected by imaging in 72(40.4%) patients. Comparison between patients with and without NAFLD showed no significant differences in terms of IBD severity, disease duration,location/extension, use of IBD-related medications (i.e., steroids, anti-TNFs, and immunomodulators) and surgery. NAFLD was significantly associated with the presence of metabolic syndrome [MetS; odds ratio (OR): 4.13, P = 0.001] and obesity defined by body mass index (OR: 9.21, P = 0.0002). IBD patients with NAFLD showed higher caloric intake and lipid consumption than those without NAFLD, regardless disease activity. At the multivariate analysis, male sex,advanced age and high lipid consumption were independent risk factors for the development of NAFLD. An increased liver stiffness was detected in 21 patients(16%) and the presence of MetS was the only relevant factor associated to LF (OR:3.40, P = 0.01).CONCLUSION In this study, we demonstrate that risk factors for NAFLD and LF in the IBD population do not differ from those in the general population.
Key words: Nonalcoholic fatty liver disease; Liver fibrosis; Inflammatory bowel disease;Risk factors; Dietary habits
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease,characterized by the presence of steatosis in > 5% of the hepatocytes[1], and whose prevalence is steeply increasing in parallel with obesity. The prevalence of NAFLD among adults is estimated to be 17%-46% in western countries, common risk factors are obesity-related metabolic disorders such as metabolic syndrome (MetS) and insulin resistance. The diagnosis of NAFLD requires the exclusion of both secondary causes of liver disease and daily alcohol consumption ≥ 30 g for men and ≥ 20 g for women[2]. NAFLD can progress from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH), liver fibrosis (LF), cirrhosis and hepatocellular carcinoma(HCC)[3].
Crohn’s disease (CD) and ulcerative colitis (UC), the two major forms of inflammatory bowel disease (IBD), are immune-mediated, chronic-relapsing inflammatory disorders affecting the gut and clinically characterized by abdominal pain, diarrhoea, bleeding, and weight loss, with a severely compromised quality of life[4]. Despite IBD is commonly considered a wasting disease characterized in some cases by malabsorption and severe weight loss, recent data indicate that the prevalence of NAFLD among IBD patients is increased as compared to the general population[5]. Though, the underlining causes and predisposing factors to NAFLD among IBD patients remain poorly investigated. In IBD patients, the increased risk to develop NAFLD might be related to intestinal disease-related factors such as inflammatory activity, disease duration, previous intestinal surgery, prolonged use of steroids, immunosuppressants and biologics[6]. On the other hand, the introduction of new therapies and the better control of the intestinal inflammation and symptoms have increased obesity and the MetS in IBD patients to rates comparable to those of the general population[7].
LF is the fearsome consequence of NAFLD. Although liver biopsy used to be the gold standard to stage LF, this procedure is invasive, expensive, not suitable as a screening tool and prone to sampling errors related to the limited amount of tissue analyzed. Transient elastography (TE) is a non-invasive and reproducible test, proved to be accurate in detecting significant fibrosis, advanced fibrosis and cirrhosis in patients with NAFLD[8]. Barberoet al[9]reported a frequency of LF between 6.4% and 10% in IBD patients, although few studies investigated the prevalence of LF in patients with IBD and NAFLD. The main aim of this observational study was to evaluate the prevalence of NAFLD and LF in a cohort of IBD patients. Furthermore,we investigated the association of IBD- and metabolic-related risk factors for NAFLD and LF in this population.
MATERIALS AND METHODS
Study design and population
Between December 2016 and January 2018, consecutive IBD patients in regular followup at the Gastroenterology Clinic of the Department of Internal Medicine, Polyclinic University Hospital of Monserrato were evaluated through medical examination and complete blood tests. Endoscopy was performed according to the international guidelines for routinely follow-up[10]. At the time of enrollment, each patient underwent abdominal ultrasound to establish the presence and severity of NAFLD and a TE to evaluate LF. The research project has been approved by the Ethics Board(Prot. PG/2018/23). The study was conducted according to the Helsinki Declaration.
Inclusion and exclusion criteria
Patients aged 18 years or older with a diagnosis of IBD for at least 6 mo in active follow-up were enrolled. Exclusion criteria were inability to provide a valid consent,pregnancy, significant alcohol consumption (≥ 20 and 30 g per day in females and males, respectively) and any other known cause of chronic liver disease.
Diagnostic criteria
The diagnosis of IBD was based on a combination of imaging, clinical examination,endoscopy, chemical and histologic analyses according to international guidelines[11,12].The disease activity was calculated according to the Crohn’s disease activity index and clinical MAYO score for CD and UC, respectively. Disease extension was evaluated by endoscopy and/or radiologic [computed tomography (CT) or magnetic resonance imaging] methods. The disease was conventionally referred as “extended”when inflammation involved beyond the rectum for UC, and when inflammation affected one bowel segment longer than 15 cm or more than one segment for CD.
Insuline resistance was detected by Homeostatic Model Assessment (HOMA) Index(fasting blood glucose x fasting serum insulin/22.5). MetS was defined by the presence of at least three of the following criteria: Waist circumference higher than 102 cm in men and 88 cm in women, hypertriglyceridemia, low HDL cholesterole,high blood pressure, and elevated fasting glucose[13]. An expert gastrointestinal radiologist performed an abdominal ultrasound to estimate the presence of NAFLD.Typical findings such as bright liver echo patterns, more echogenic respect to the renal cortex, and a loss of resolution of intrahepatic structures were evaluated.Steatosis was grated form 0 to 3 as previously described[14]. TE was performed on the same day of the abdominal ultrasound. Ten valid measurements were obtained for each patient, and the examination was considered successful only when valid measurements were > 75% and interquartile range was < 30%. The optimal cut-offs values for estimating the different stages of LF were: 7 kPa for fibrosis stage ≥ F2, 10 kPa for fibrosis stage ≥ F3, and 14 kPa for fibrosis stage F4[15]. The presence of other causes of chronic liver disease was excluded through laboratory tests(aminotransferases, colestatic indices, hepatotropic viruses markers, autoimmune markers, serum ferritin, transferrin saturation and ceruloplasmin) and, when necessary, through radiological exams (CT, magnetic resonance cholangiopancreatography).
Dietary assessments
All 178 patients underwent nutritional evaluation. Anthropometric parameters [body mass index (BMI), waist circumference, visceral fat, body fat, lean body mass] were measured using Body Composition Monitor (Omron Healthcare co., ltd.) to evaluate the presence and grade of obesity. Subsequently, a 24-h dietary recall was collected to estimate the usual dietary intake: patients were asked to describe the foods and drinks consumed in the previous 24 h. The nutritionist entered the anthropometric measurements and each 24-h dietary recall into the Dieto System Terapia Alimentare software for nutrient analysis.
Statistical analysis
Continuous variables were reported as mean (± SD) and categorical variables as number of cases and percentage. The non-parametric Mann-WithneyU-test was used to compare continuous variables, the test of proportions or Chi-square test for categorical variables;Pvalue level < 0.05 was considered significant. Univariate and,for variables that showedPvalue levels < 0.1, also a multivariate binary logistic regression analysis was performed using the absence/presence of NAFLD as dependent variable and considering IBD- and metabolic-related variables as predictors. APvalue level < 0.05 was considered significant for all tests. Analyses were performed using STATA/SE, version 14, software (StataCorp LP, College Station, TX, United States) and SPSS v. 20 (IBM, Armonk, NY, United States).
RESULTS
Characteristics of the included population
Two hundred and 23 patients have been evaluated for inclusion in the study. Of these, 45 were excluded because they did not meet the inclusion and exclusion criteria(Figure 1). Of the remaining 178 patients, 95 (53.4%) were identified as having UC and 83 patients (46.6%) had CD. 48 patients (27%) had active disease. When assessed separately, no significant differences were found between UC and CD patients. For this reason, both groups were considered together. The prevalence of NAFLD was determined in 72 patients (40.4%): grade 1, 2 and 3 steatosis was detected in 20(11.2%), 30 (16.8%) and 22 (12.3%) patients respectively. Liver stiffness was evaluated in 162 (91%) patients and LF (F ≥ 2) was found in 21 patients (16%). MetS was diagnosed in 34 (19.1%) patients; 59 (33.1%) and 39 (21.9%) were classified as overweight according to BMI and waist circumference respectively, while, based on the same measurements, 22 (12.3%) and 39 (21.9%) were obese.
Comparison between IBD patients with and without NAFLD
IBD patients with NAFLD were significantly older than patients without NAFLD (54 yearsvs47 years,P= 0.001). No differences between IBD diagnosis (CD or UC) and disease duration were found but the duration of disease remission was significantly longer in NAFLD patients as compared to IBD patients without NAFLD (33.9 movs21.14,P< 0.05, Figure 2). Laboratory tests of patients with and without NAFLD were compared. NAFLD patients showed increased levels of basal glucose (98.07 mg/dL NAFLDvs89.79 mg/dL no-NAFLD,P= 0.03), basal insulin (11.47 mU/L NAFLDvs6.99 mU/L no-NAFLD,P= 0.007) and higher HOMA index (2.49 NAFLDvs1.59 no-NAFLD,P= 0.005). Aspartate aminotransferase, alanine aminotransferase (ALT),glutamyltransferase, triglycerides and cholesterol values were not statistically different between the two groups.
Next, we examined anthropometric measurements in both groups. As expected,IBD patients with NAFLD had higher mean BMI than the those without NAFLD;26.54 kg/m²vs23.88 kg/m² respectively (P< 0.0001). NAFLD patients also had higher mean body weight (73.53 kg NAFLDvs64.24 kg no-NAFLD,P< 0.0001), waist circumference (93.09 cm NAFLDvs85.52 cm no-NAFLD,P< 0.0001), arm circumference (28.31 cm NAFLDvs26.83 cm no-NAFLD,P= 0.003) and visceral fat(10.52 NAFLDvs6.87 no-NAFLD,P< 0.0001). No significant difference in lean body mass and body fat percentage was found between the two groups. When analyzing dietary habits, patients with NAFLD had higher caloric intake and lipid consumption than patients without NAFLD (1970.27 kcalvs1752.35 kcal,P= 0.07 and 75.18 g/dayvs64.88 g/day,P= 0.005). This difference did not change when dietary habits of patients were analyzed in patients with active disease. Finally, although numerically different, the mean values of liver stiffness between IBD patients with and without NAFLD were not statistically different (5.5 kPavs4.6 kPa,P= 0.09, Table 1).
Risk factors for NAFLD
At the univariate analysis, male sex was associated to an increased risk to develop NAFLD (odds ratio (OR): 3.17, 95% confidence interval (CI) 1.67-5.99,P= 0.0005). As shown in Table 2, no significant associations were found between NAFLD and IBDrelated factors. Indeed, NAFLD was not associated to disease activity, evaluated separately in both UC (OR: 0.62,P= 0.20) and CD (OR: 1.70,P= 0.31), presence of extra-intestinal manifestations (OR :0.97,P= 0.94), long disease duration (OR: 1.17,P= 0.35), disease extension (OR: 1.14,P= 0.68), use of steroids (OR: 0.49,P= 0.57),thiopurines (OR: 1.11,P= 0.80), biologic therapy (OR: 0.78,P= 0.41), prior surgery(OR: 0.63,P= 0.24) and inflammatory markers (i.e., fecal calprotectin, OR: 1.53, 95%CI 0.75-3.12,P= 0.24; c-reactive protein, OR: 0.72, 95%CI 0.39-1.33,P= 0.18). Even by stratyfing patients according to their steatosis grade, no significant association between disease activity and NAFLD was found. As for the metabolic profile, MetS(OR: 4.13, 95%CI 1.85-9.24,P= 0.001) and obesity, measured either by BMI (OR: 9.21,95%CI 3.06-27.70,P= 0.0002), waist circumference (OR: 2.69, 95%CI 1.22-5.90,P=0.001) or visceral fat (OR: 3.82, 95%CI 1.91-7.64,P= 0.001) but not diabetes were associated to NAFLD (Table 3). At the multivariate analysis, only advanced age (OR:1.04, 95%CI 1.01-1.07,P= 0.006), male sex (OR: 4.25, 95%CI 1.95-9.26,P< 0.0001),obesity by BMI (OR: 1.17, 95%CI 1.06-1.28,P= 0.001) and high lipid consumption were associated to NAFLD (OR: 1.02, 95%CI 1.01-1.03,P= 0.004, Table 4).
Risk factors for liver fibrosis
No IBD-related risk factors were found to be associated to LF at the univariate analysis. As for the metabolic factors, the presence of MetS was the only factor significantly associated with LF (OR: 3.40, 95%CI 1.26-9.20,P= 0.01).
DISCUSSION
NAFLD is the most common hepatic disease in Western countries[16]and it is recognized as an increasingly relevant health issue. Indeed, NAFLD is candidate to become the first cause of liver transplant in the next years. In our IBD cohort, 46.6% of the patients were diagnosed with NAFLD. Obesity, evaluated by BMI, waist circumference and visceral fat, was significantly correlated with the presence of hepatic steatosis. Accordingly, we observed a significant correlation between highcaloric, high-fat diet and NAFLD. Patients with NAFLD tend to follow a Western rather than a Mediterranean diet[17]. Indeed, we observed a reduced intake of complex carbohydrates, fruit and vegetables and an increased intake of lipids and proteins among IBD patients. This dietary habit might be due to the fact that IBD patients associate symptoms such as abdominal distention and diarrhea to fiber and complex carbohydrates intake, thus limiting their daily consumption, preferring proteic and fat foods instead[18]. Furthermore, a long period of well-being, favored by the therapyinduced improvement of the general conditions and of the intestinal symptoms, in particular diarrhea, may be the reasons for the increased food intake. We also observed a positive correlation between high basal glucose and serum insulin levels,as indicated by the high HOMA index value, and NAFLD, thus suggesting a common pathogenic mechanism. Indeed, insulin resistance in the target organ, further exacerbates hepatic steatosis.
Older age was also independently associated with NAFLD and this finding could be related to the progressive increase of the metabolic risk factors with aging.Conflicting data have been reported on the relationship between natural history of IBD and the presence and grade of steatosis. Bessissowet al[6]showed the association between duration of IBD, disease activity and prior surgery with NAFLD development, and these findings have been replicated by others[19,20]. In contrast with these data, but in line with our findings, Saroliet al[5]failed to demonstrate the association between IBD-related factors including medications (steroids, thiopurine,and TNF inhibitors) and the risk to develop NALFD.
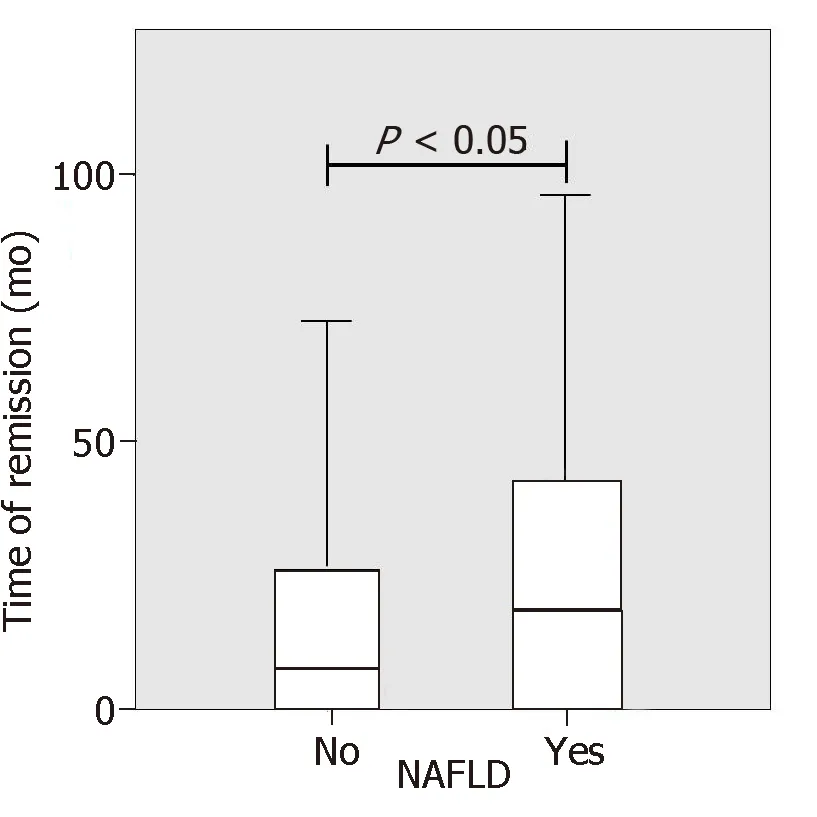
Figure 2 Nonalcoholic fatty liver disease patients showed a significantly longer disease remission compared to inflammatory bowel disease patients without nonalcoholic fatty liver disease.
In our view, the lack of association between IBD-related risk factors and NAFLD and the presence of an unbalanced diet in both active and inactive disease,emphasizes the importance of the dietary habits and the metabolic profile rather than the intestinal inflammation in the stratification risk for liver steatosis. Accordingly, we found that the duration of remission was strongly associated to NAFLD, suggesting that patients in clinical remission tend to follow a more unbalanced diet and to develop more NAFLD.
NAFLD confers increased risk of cardiovascular-related mortality and HCC,therefore therapeutic options for NAFLD targeting either obesity or hepatic inflammation and fibrosis is advisable. The first approach includes weight loss through caloric restriction and increased physical activity[1]. There are currently no recommended routine screening strategies for NAFLD in patients with IBD. However,we believe that the assessment of hepatic steatosis is always necessary, in particular in patients with high-risk features, including obesity, overweight, MetS and advanced age, regardless of the presence of elevated serum transaminases or clinical signs of liver disease. Indeed, NAFLD has been shown to develop in the absence of serum transaminase elevation. Accordingly, only 3.3% of patients in our cohort of IBD patients had elevated serum transaminases.
NAFLD can progress to NASH and LF. TE test performed in our cohort to evaluate liver stiffness, showed LF in 16% of patients. The only significant fibrosis-related factor was MetS, and no significant association between hepatic transaminases and LF was found. Since elevated ALT was not predictive of NAFLD or LF in our cohort, it would be necessary to implement the management of IBD patients with a metabolic and liver profile evaluation in order to prevent the onset of liver complications.
Our results suggest that IBD patients should be screened for NAFLD-associated risk factors in order to prevent the development of liver disease. Moreover, the early detection of fatty liver disease and fibrosis by ultrasonography and TE might have a relevant impact on liver disease evolution though the correction of the dietary habits and the improvement of patients’ metabolic profile. A practical approach might be to encourage the use of complex carbohydrates together with fruit and vegetables and to reduce the intake of fats and proteins thus decreasing the state of hypeinsulinism and insulin resistance.
In conclusion, our study confirms the epidemiological burden of NAFLD in IBD. In addition, it demonstrates the absence of intestinal disease-specific risk factors associated to NAFLD, while confirming as risk factors those already identified in the general population. Moreover, these data suggest that IBD care should not be limited to intestinal disease but should also include metabolic interventions by promoting healthy lifestyle and a correct dietary regimen in order to reduce the occurrence of chronic liver disease.
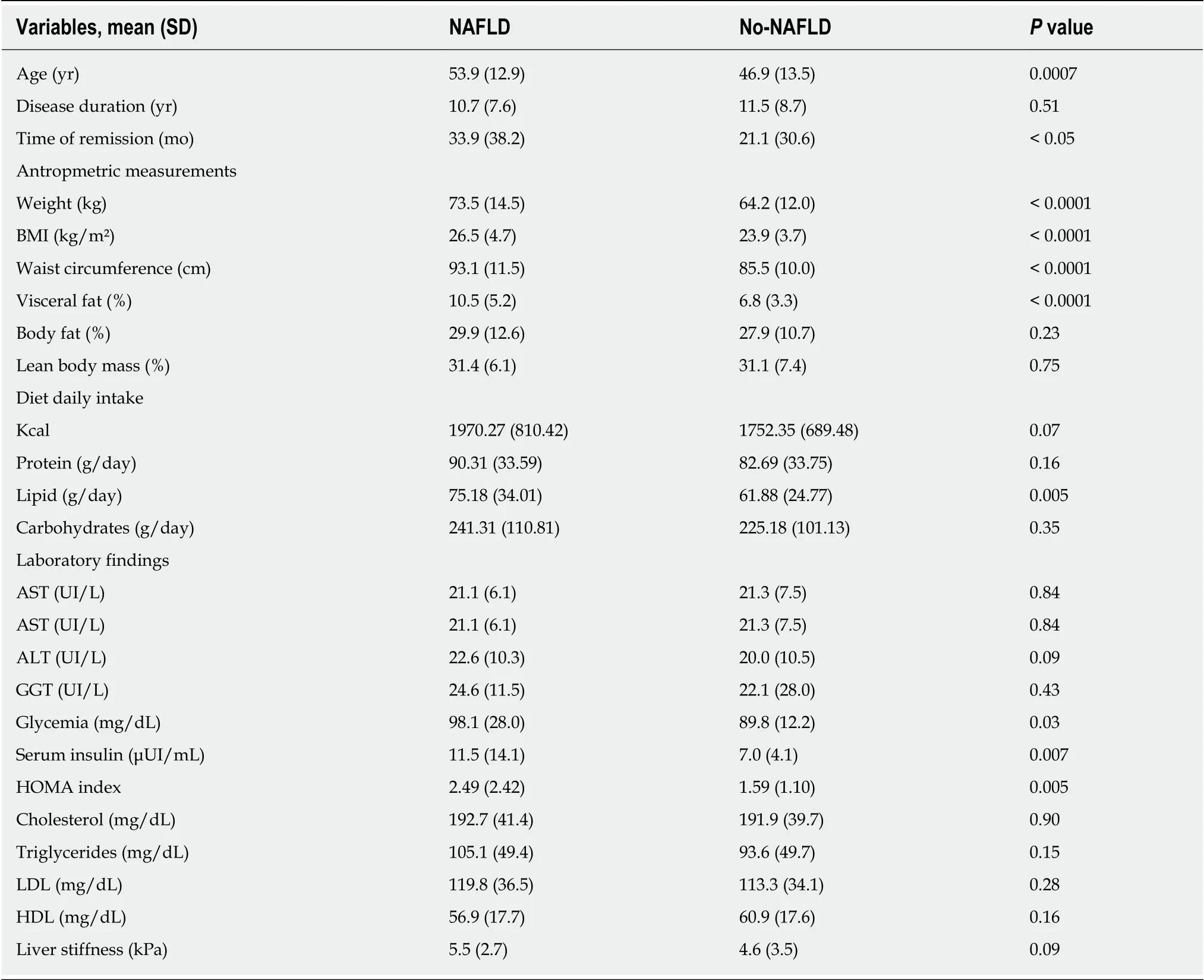
Table 1 Comparison of patient characteristics, metabolic profile and laboratory findings among inflammatory bowel disease patients with and without nonalcoholic fatty liver disease
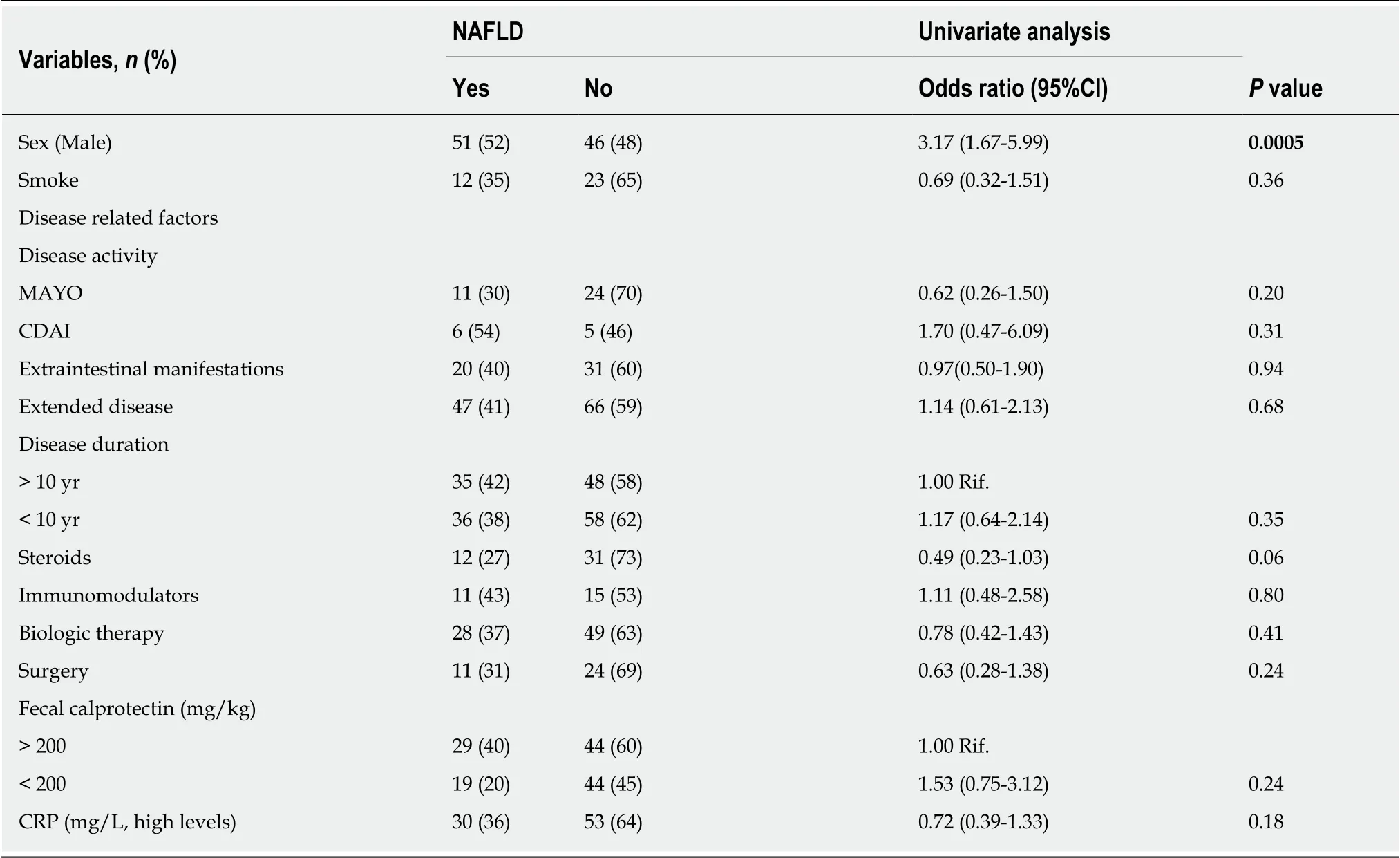
Table 2 Correlation between disease-related factors and nonalcoholic fatty liver disease on univariate analysis
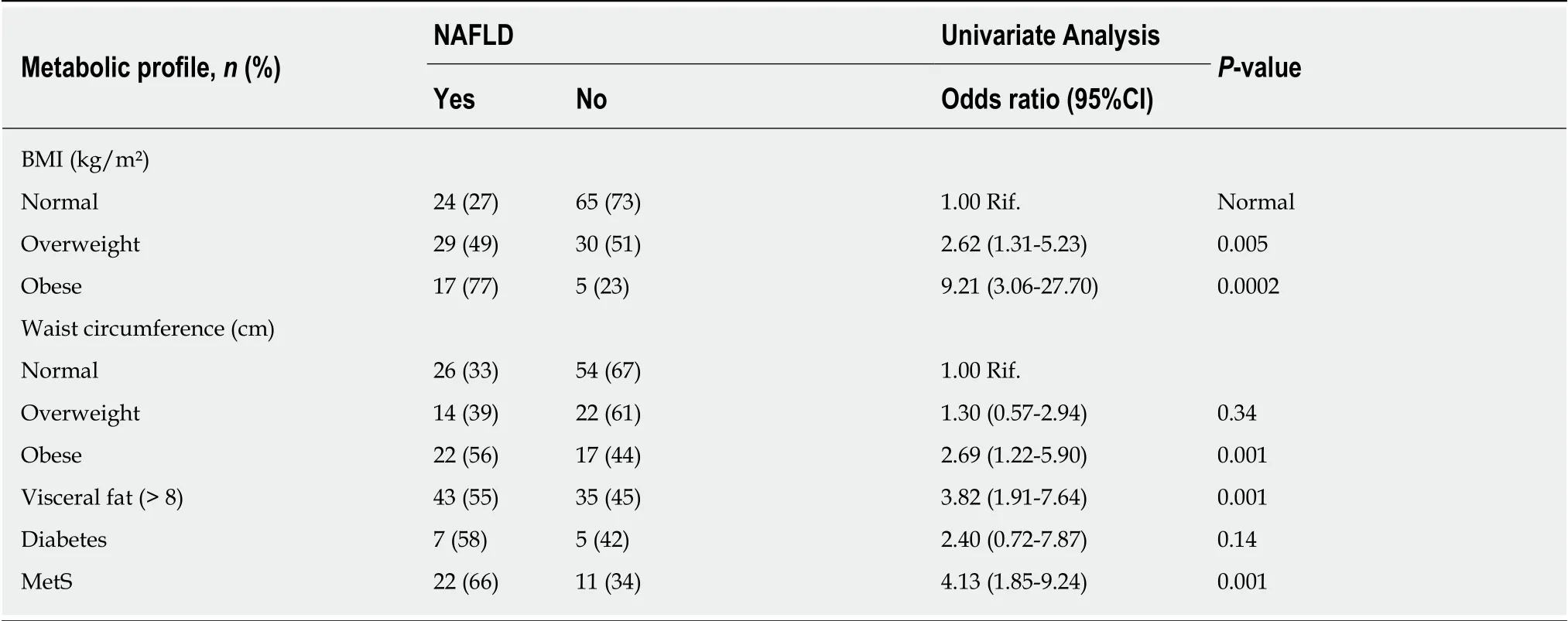
Table 3 Correlation between metabolic factors or alimentary habits and nonalcoholic fatty liver disease on univariate analysis
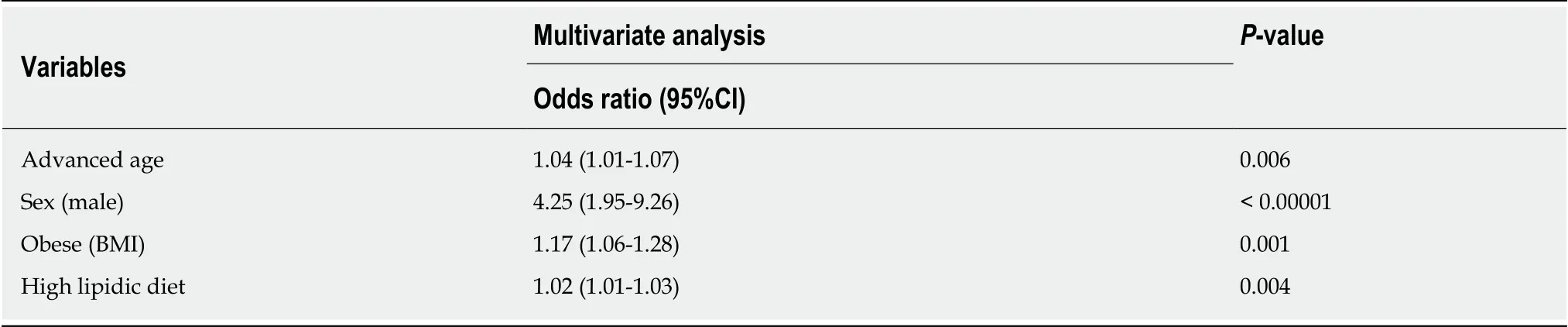
Table 4 Risk factors for nonalcoholic fatty liver disease among patients on multivariate analysis
ARTICLE HIGHLIGHTS

杂志排行
World Journal of Gastroenterology的其它文章
- Pathogenesis and clinical management of Helicobacter pylori gastric infection
- Oncogenic ADAM28 induces gemcitabine resistance and predicts a poor prognosis in pancreatic cancer
- Correlation of plasma miR-21 and miR-93 with radiotherapy and chemotherapy efficacy and prognosis in patients with esophageal squamous cell carcinoma
- Accuracy of an administrative database for pancreatic cancer by international classification of disease 10th codes: A retrospective large-cohort study
- Post-transplant infection improves outcome of hepatocellular carcinoma patients after orthotopic liver transplantation
- Short-term efficacy of robotic and laparoscopic spleen-preserving splenic hilar lymphadenectomy via Huang's three-step maneuver for advanced upper gastric cancer: Results from a propensity score-matched study
