Pathogenesis and clinical management of Helicobacter pylori gastric infection
2019-10-19BrenoBittencourtdeBritoFilipeAntnioFrandaSilvaAlineSilvaSoaresVinciusAfonsoPereiraMariaLusaCordeiroSantosMarianaMirandaSampaioPedroHenriqueMoreiraNevesFabrcioFreiredeMelo
Breno Bittencourt de Brito, Filipe Antônio França da Silva, Aline Silva Soares, Vinícius Afonso Pereira,Maria Luísa Cordeiro Santos, Mariana Miranda Sampaio, Pedro Henrique Moreira Neves,Fabrício Freire de Melo
Abstract Helicobacter pylori (H. pylori) is a gram-negative bacterium that infects approximately 4.4 billion individuals worldwide. However, its prevalence varies among different geographic areas, and is influenced by several factors. The infection can be acquired by means of oral-oral or fecal-oral transmission, and the pathogen possesses various mechanisms that improve its capacity of mobility,adherence and manipulation of the gastric microenvironment, making possible the colonization of an organ with a highly acidic lumen. In addition, H. pylori presents a large variety of virulence factors that improve its pathogenicity, of which we highlight cytotoxin associated antigen A, vacuolating cytotoxin,duodenal ulcer promoting gene A protein, outer inflammatory protein and gamma-glutamyl transpeptidase. The host immune system, mainly by means of a Th1-polarized response, also plays a crucial role in the infection course. Although most H. pylori-positive individuals remain asymptomatic, the infection predisposes the development of various clinical conditions as peptic ulcers,gastric adenocarcinomas and mucosa-associated lymphoid tissue lymphomas.Invasive and non-invasive diagnostic methods, each of them with their related advantages and limitations, have been applied in H. pylori detection. Moreover,bacterial resistance to antimicrobial therapy is a major challenge in the treatment of this infection, and new therapy alternatives are being tested to improve H.pylori eradication. Last but not least, the development of effective vaccines against H. pylori infection have been the aim of several research studies.
Key words:Helicobacter pylori; Virulence factors; Immune response; Antibiotics;Vaccines
INTRODUCTION
Helicobacter pylori(H. pylori) is a gram-negative bacterium that inhabits the gastric environment of more than half of the world population[1]. Studies have demonstrated that the prevalence ofH. pylori-positive status varies according to different factors such as age, geographical area, living condition and socioeconomic status[2]. Oral-oral transmission seems to be the main route ofH. pyloritransmission. This explains the common occurrence of the infection among members of the same family, such as parents and children. In this way, the sharing of utensils during feeding seems to be important for infection establishment[3]. Fecal-oral transmission is another form of infection that occurs through ingestion of contaminated water mainly due to unsatisfactory basic sanitation conditions[4]. Therefore, it is important to highlight that increasing socioeconomic status and the improvement of living conditions are factors that greatly influence the reduction inH. pyloriinfection prevalence[5].
Until Warren and Marshall’s discovery ofH. pyloriinfection in gastric mucosa, it was believed that the gastric environment was sterile because of its high acidity[6,7].Aiming for successful colonization under such hostile conditions, the bacterium uses a wide range of mechanisms that provide improved mobility, robust adherence to epithelial cells and an enzymatic apparatus that allows the establishment of an appropriate microenvironment for infection perpetuation[8-10]. In addition, the potential of pathogenicity of this infection is provided by certain virulence factors such as cytotoxin associated antigen A (CagA), vacuolating cytotoxin (VacA),duodenal ulcer promoting gene A protein (DupA), outer inflammatory protein(OipA) and gamma-glutamyl transpeptidase (GGT)[11-15]. Moreover, the host immune system plays a crucial role in the course of the infection, likely by means of a Th1-polarized response against the pathogen (Figure 1)[16].
Although mostH. pylori-positive individuals are asymptomatic, such infections predispose the development of diseases like peptic ulcers and gastric adenocarcinomas[17]. In this way, proper clinical management with a well-made diagnosis followed by effective treatment are important steps in the improvement of a patient’s clinical outcome[18]. A variety of invasive and non-invasive diagnostic methods have been used forH. pyloridetection and, regarding treatment, bacterial resistance represents a major challenge in infection eradication[19,20]. In this sense, new therapy regimens as well as probiotic implementation have been tried in order to improve treatment results[21,22]. Moreover, the efforts of several researchers have been directed towards the development of vaccines againstH. pyloriinfection.
PATHOGENESIS
Colonization
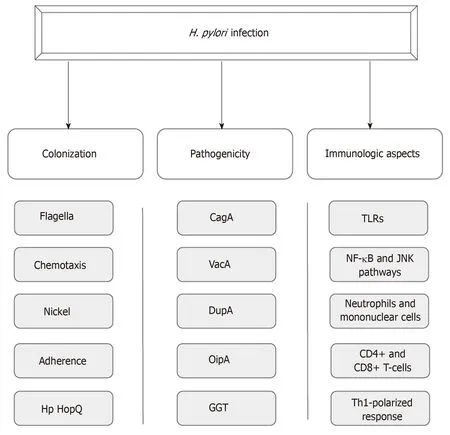
Figure 1 Aspects of Helicobacter pylor infection. CagA: Cytotoxin associated antigen A; VacA: Vacuolating cytotoxin; DupA: Duodenal ulcer promoting gene A protein; OipA: Outer inflammatory protein; GGT: Gamma-glutamyl transpeptidase; TLRs: Toll-like receptors.
H. pylorisuccessful colonization of the hostile gastric environment requires special mechanisms. Firstly, after reaching the gastric environment,H. pyloriuses its crucial flagellar motility for swimming in gastric content, what allows the bacterium to get in the gastric mucus layer[8]. Four to eight sheathed flagella compose the flagellar group situated on a single or on both poles of the bacterium[23-25]. H. pylori flagella can also provide different movements according to the media in which the bacterium is located. In liquid media, it presents a “swimming motility”, whereas in soft agar and on the surface of solid media, “spreading” and “swarming” movements can be observed, respectively[25]. Various studies have shown that several mutations in genes that encode specific flagellar proteins such as fliD, FlaA and FlaB impair the proper motility of H. pylori, which can reduce or even cease its capacity to colonize the gastric mucosal layer[26-28].
Besides flagella, H. pylori mobility also depends on chemotaxic action in response to different molecules, such as mucin, sodium bicarbonate, urea, sodium chloride and some specific amino acids[29,30]. At least ten H. pylori genes are related to reception,signal transduction, and processing of chemotactic stimuli[31]. Different H. pylori chemoreceptors have been described: T1pA, B, C, and D, CheA kinase and various coupling proteins. These proteins are all crucial for bacterium colonization, as demonstrated by various studies over recent years[32].
In addition, some transition metals are essential for living organisms, as they serve as cofactors for enzymatic reactions and some physiological processes, especially for enzymes that carry out the genetic material replication and transcription, attenuation of oxidative stress, and cellular energy production. In bacteria, these metals are crucial for survival and successful infection[33]. Nickel is an indispensable metal for H. pylori,since it is the cofactor for two important enzymes: urease and hydrogenase. These enzymes have a strong role in the infection process[10]. The activity of H. pylori urease contributes to the colonization of the microorganism, once this enzyme catalyzes the hydrolysis of urea to carbon dioxide and ammonia, which are buffer substances that attenuate the acidity of the stomach environment[34]. In turn, hydrogenase is part of a signaling cascade that induces an alternative airway, allowing H. pylori to use molecular hydrogen as a source of energy for its metabolism[35].
Adhesion molecules (Table 1) and surface receptors of gastric cells are also important in the interaction between bacteria and host[9,36]. One of the most wellcharacterized molecules is the blood group antigen binding adhesin A (BabA), which carries out specific binding to Lewis H-1 antigens[37,38]. Bacteria with high BabA expression are more virulent, and cause duodenal ulcer and gastric adenocarcinoma pathogenesis[39]. Recently, another bacterial-host interaction was identified through the adhesion of the outer membrane Hp HopQ. These adhesins bind to the CEACAMs (cell adhesion molecules related to the carcinoembryonic antigen) 1, 3, 5 and 6. That binding gives rise to cell signaling mediated by the HopQ-CEACAM interaction, which allows the translocation of CagA, the main virulence factor of H.pylori, thus increasing proinflammatory mediators in the host cell[40-42].
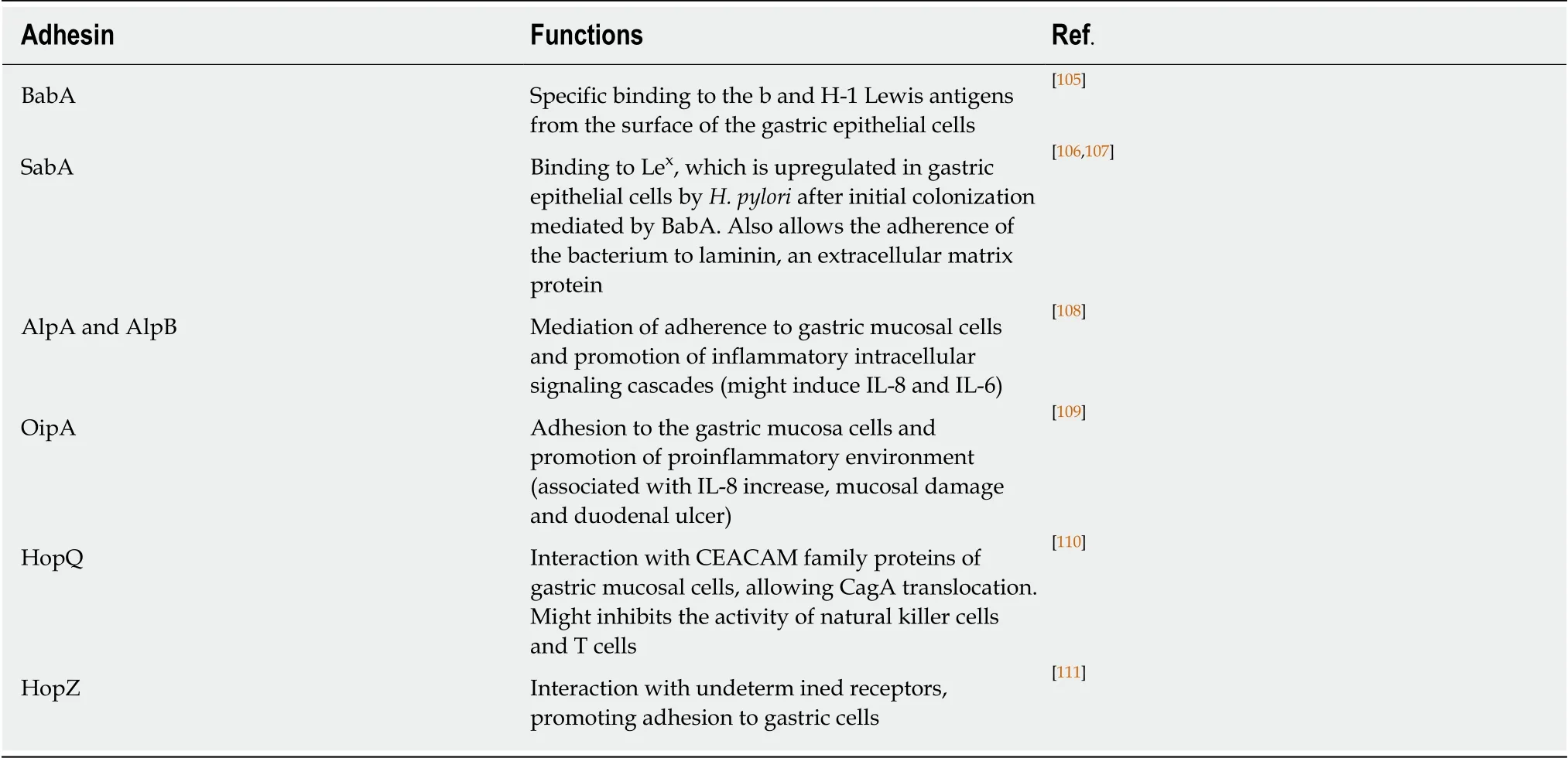
Table 1 H. pylori adhesion molecules
CagA
CagA is a bacterial protein that induces specific modifications in the morphology of epithelial cells while altering cell polarity, leading to a “hummingbird” phenotype.Changes in cytoskeleton associated with the development of gastric adenocarcinoma can also be triggered by this virulence factor[43]. TheCagA gene is contained in acagpathogenicity island, a region that also possesses the coding sequence of a type IV secretion system (T4SS)[11]. This bacterial structure is responsible for performing the translocation of CagA, as well as peptidoglycans, into host cells[44]. Within the host cell, CagA undergoes tyrosine phosphorylation at a Glu-Pro-Ile-Tyr-Ala (EPIYA)motif, a variable C-terminal CagA region that can be composed by different EPIYA segments (EPIYA-A, EPIYA-B, EPIYA-C and EPIYA-D)[45]. EPIYA-A and EPIYA-B segments have been found in mostcagA-positiveH. pyloristrains, while EPIYA-C and EPIYA-D segments are related to Western and Eastern strains, respectively[46].H. pyloristrains containing EPIYA-D or at least two EPIYA-C segments in itscagA gene are associated with a higher risk of cancer development[47]. In addition, Queirozet al[48]demonstrated in a Brazilian population that first-degree relatives of patients with gastric cancer tend to be infected byH. pyloristrains containing two or more EPIYA-C segments. After phosphorylation, CagA activates SHP-2 (SH2-containing proteintyrosine phosphatase), which promotes the cell changes mentioned above[49].
Non-CagA virulence factors
Other various virulence factors have been related to an increasedH. pyloricapacity to impair gastric homeostasis. Among them, VacA is a determinant protein forH. pyloripathogenicity, and its gene is present in almost all bacterial strains. VacA promotes the formation of acidic vacuoles in the cytoplasm of gastric epithelial cells.Consequently, the integrity of mitochondria, cytoplasmic membrane, and endomembranous structures is destabilized, leading cells to collapse[50]. Moreover, this protein might also promote the activation and suppression of the immune response,inducing immune tolerance and persistentH. pyloriinfection through its activities on T-cells and antigen-presenting cells[51]. The set of changes performed by this virulence factor adds to enhanced gastritis, as well as to ulcer and cancer development[12].
Another bacterial protein, DupA, seems to provide a higher acid resistance to the bacterium, and also might promote an increase in the production of IL-8 in the antral gastric mucosa. Enhanced IL-8 levels lead to mucosal inflammation and polymorphonuclear leukocyte infiltration, which contributes to the emergence of gastritis and duodenal ulcers[13]. Interestingly, the relation betweendupA-positiveH.pyloristrains and duodenal ulcers has been observed in Asian countries, but not in the Western population[52]. Furthermore, our group demonstrated that the presence of functionaldupA inH. pyloristrains has been considered as a protective factor for gastric carcinoma development[53]. The gene products ofdupA are homologues of the VirB4 ATPase, which is related to the mounting of the secretion apparatus, however,the probable association ofdupA withH. pyloriT4SS still needs to be better elucidated[13].
OipA, an outer membrane protein, contributes to both adhesion and increased inflammation by inducing enhanced IL-8 production[54,55]. The discovery of the relationship between OipA and the increased development of peptic ulcers and gastric cancer resulted in a larger number of studies on thisH. pylorivirulence factor[14]. The functional status of OipA has been described as an important factor in the outcome of the infection, since the expression of theoipA gene is regulated by a repair process called “slipped strand mispairing”, which depends on the quantity of CT dinucleotide repeats in theoipA 5’ region. Such a process determines whetheroipA is nonfunctional or functional in a given bacterial strain, and the latter condition is related to increased gastric pathogenicity[56,57]. In addition, OipA might be related to changes in β-catenin signaling, cell proliferation and reduction of cell-cell junctions[58].
The enzyme GGT is a N-terminal nucleophile hydrolase also produced byH. pylorithat catalyzes the conversion of glutamine into glutamate and ammonia, as well as the hydrolysis of glutathione into glutamate and cysteinylglycine[15]. Its activity leads to the production of reactive oxygen species (ROS), which, like ammonia, induce cellcycle arrest, apoptosis and necrosis[59,60]. In addition, studies have demonstrated that this enzyme inhibits T cell proliferation and dendritic cell differentiation[61,62]. Higher GGT activity has been observed in peptic ulcer patients when compared to individuals with other gastroduodenal diseases[63].
Immunologic aspects
Complex host immune responses, embracing innate and adaptive mechanisms, are induced byH. pyloriinfection[64,65]. Given the initial contact with the pathogen, variousH. pyloriantigens such as lipoteichoic acid, lipoproteins, lipopolysaccharide, HSP-60,NapA, DNA, and RNA bind to gastric cell receptors, including toll-like receptor (TLR)1, TLR2, TLR4, TLR5, TLR6, and TLR10 located on epithelial cell membranes, and TLR9, found in intracellular vesicles[66,67]. Such interaction promotes, among other signaling pathways, NF-κB and c-jun N-terminal kinase activation, followed by proinflammatory cytokine release[68]. Besides receptor activation by pathogenassociated molecular patterns, injection of CagA through T4SS also leads to the production of cytokines, in another NF-κB-dependent process[69].
Subsequently, gastric mucosa is infiltrated by neutrophils and mononuclear cells,resulting in the production of nitric oxide and ROS[70]. Moreover, CD4+ and CD8+ T cells, components of adaptive immunity, are also recruited. A preferential activation of CD4+ cells to the detriment of CD8+ cells might occur, and a specific response is directed to the bacterium[71]. Regarding general cytokine profiles inH. pylori-positive patients, studies have suggested a Th1-polarized response, characterized by scarce IL-4 (a Th2 cytokine) and enhanced levels of gamma interferon, tumor necrosis factor, IL-1β, IL-6, IL-7, IL-8, IL-10, and IL-18[72,73]. With the exception of IL-10, which seems to play a role in limiting the inflammatory response, other increased cytokines might promote proinflammatory effects duringH. pyloriinfection. Furthermore, we demonstrated that an increase in IL-17 is also associated withH. pyloriinfection,especially in adults[74]. In regard to immunoglobulin production,H. pylori-specific serum IgM antibodies can be detected in patient serum 4 wk after infection[75]. In chronic infection, serum IgA and IgG immunoglobulins are directed toward several bacterial antigens[76,77]. Such inflammation is asymptomatic in mostH. pylori-positive patients, however it increases the risk of duodenal and gastric ulcer disease, as well as gastric malignancy development[78].
CLINICAL MANAGEMENT
Clinical manifestations and diagnosis
As a consequence of the mechanisms explained above,H. pylori-positive individuals are under increased risk of presenting various clinical manifestations[17]. The course of infection is variable and strongly dependent on host factors. Besides this, the pattern of gastric mucosal involvement is correlated with the risk of initiation and progression of different gastric disorders. Development of antral-predominant gastritis is associated with duodenal ulcers, while a corpus-predominant gastritis and multifocal atrophy tend to turn into gastric ulcers, gastric atrophy, intestinal metaplasia and gastric carcinoma[79]. Among gastrointestinal conditions, dyspepsia and peptic ulcer disease are frequently observed in clinical practice, and bacterial detection, when it is present, followed by infection eradication are crucial steps in the management of such disorders[80]. In addition, recent studies have associatedH. pyloriinfection with a wide range of diseases. The infection was linked with the pathophysiology of neurological, dermatological, hematologic, cardiovascular, ocular,metabolic, hepatobiliary and allergic diseases[81].
Various diagnostic tests, with their specific advantages and disadvantages, are offered forH. pyloridetection. Histology is the precursor method forH. pyloriinfection diagnosis, which, in such a technique, consists in the observation of typical bacteria associated with inflammatory reactions in the tissue slides. This method includes the use of several stains, such as Giemsa staining, and immunostaining to allow pathogen detection[19]. Another importantH. pyloridiagnostic method, the rapid urease test (RUT), detects an increase in reagent pH after the addition of a biopsy specimen containingH. pylorito the reagent. Such pH variation is caused by the conversion of the urea test reagent into ammonia. RUT is a relatively cheap, quick,easy, specific and widely available test[82]. Polymerase chain reaction (PCR) has also been applied forH. pyloridetection. Al-Moayadet al[83]concluded that standardized PCR allows an accuracy superior to that observed in RUT, with improved detection in specimens with lower bacterial charge[84]. However, the necessity of endoscopy is an important limitation of the three methods mentioned above, and the advances in noninvasive diagnostic techniques have strengthened the idea of prioritizing the use of diagnostic alternatives for which endoscopy is dispensable.
The urea breath test (UBT) is now the main non-invasive method for such a diagnosis, gradually taking the place of RUT as the most suitable method forH. pyloridetection. This test is based on the mechanism of bacterium degradation of 13C or 14C-labeled urea into CO2, which can be measured in the exhaled air using a mass or infrared spectrometer[85]. A Brazilian study[86]evaluated the use of a locally manufactured isotope in UBT, trying to reduce the importation costs of this substance,which is considered a limitation for the performance of this method in many countries. The assay concluded that the substrate manufactured in Brazil with reduced costs had similar performance when compared to the one imported from foreign countries. Such a reduction in the costs of this substrate can contribute to the dissemination of UBT use around the world.
A less expensive option for UBT, stool antigen tests (SATs), are good alternatives forH. pyloridiagnosis. SATs can be made by means of enzyme immunoassay or immuno-chromatography[87]. In addition, a new promising non-invasive method, the urine test for the diagnosis ofH. pyloriinfection, has been largely studied as an alternative. A meta-analysis from 2017[88], which included 23 studies, showed that testing for antibodies in urine samples might be a good diagnostic option. However,further studies are necessary to confirm the accuracy of this method. Finally, new strategies for serologic diagnosis ofH. pyloriinfection have been developed through the discovery of specific serological markers. A recent study evaluated the accuracy of the “hook-associated protein 2 homologue”, FliD, as a marker of this infection. The use of the Flid ELISA method in the detection ofH. pyloriinfection provided high specificity (99%) and sensibility (97%). Moreover, this method presents a simple technique at low cost[89].
Treatment
There is not a universally accepted regimen for the treatment ofH. pyloriinfection.However, all of them target the regressing symptomatology and healing of the mucosa damaged by the infection process[20]. Since the 1997 Maarstricht consensus, the standard triple therapy with proton pump inhibitors (PPI) in standard dose,clarithromycin (500 mg), and amoxicillin (1 g) twice daily for 7 d have been employed in most countries as a first-line regimen to eradicateH. pylori. The quadruple therapy,with addition of bismuth (120 mg) to the regimen, has also been used as a first-line regimen[90]. However, the increase in microbial resistance to clarithromycin, whose prevalence varies with time and geographic region, is leading to changes in the therapeutic regimen. The indiscriminate use of azithromycin and erythromycin in the treatment of respiratory infections and cross-resistance among macrolide antibiotics may be responsible for the increased microbial resistance to clarithromycin[91]. As a consequence, longer therapeutic regimens have been used forH. pylorieradication[92].In areas with high clarithromycin resistance, the addition of metronidazole (500 mg)concomitantly with PPI, clarithromycin and amoxicillin twice daily for 5 d,characterizing a quadruple therapy, improves the efficacy of the treatment, with an intention-to-treat higher than 90%[21]. Moreover, in regions with clarithromycin resistance above 15%-20%, and quinolone resistance below 10%, clarithromycin could be substituted by levofloxacin (250/500 mg) in triple therapy. Such exchange increases the per-protocol and intention-to-treat eradication rates of the treatment[21,93].In addition, the use of hybrid therapy has been suggested as an alternative to the standard approaches in some countries. This therapeutic scheme consists of administering PPI and amoxicillin for 14 d, and then adding both clarithromycin and nitroimidazole as a quadruple therapy for the final 7 d[94]. Finally, faced with such a situation, studies have proposed the use of tailored therapy as a possible new firstline treatment. Conducting tests for identifying the susceptibility of the bacterial strains to the different regimens appears to be a great alternative for bacterial eradication[95].
Probiotics are being used in the prevention and treatment of many gastrointestinal infections, so it is strongly believed that they might be useful for the treatment ofH.pyloriinfection[20]. Research about the use of probiotics for this purpose are typically divided into treatments with and without antibiotics, and data available in the literature are still controversial[96]. Zagariet al[97]showed that probiotic supplementation did not improve either the efficacy or tolerability of the treatment,regardless of the species of microorganism used. On the other hand, some studies suggest that probiotics help in the restoration of the intestinal microbiota disturbed by antibiotics, leading to a decrease in side effects and, consequently, increased adherence to treatment, corroborating successful therapy[98]. However, no effect has been observed againstH. pyloriinfection using treatment with probiotics alone. Other studies claim that the use of probiotics in combination with antimicrobial therapy has a potentiating effect by increasing eradication rates; however, the relationship with adverse effects is still uncertain[99]. The beneficial effects of probiotics on this infection may be associated with immunological and non-immunological mechanisms, such as substance production, gastric mucosal strengthening, and regulation of immune function[100]. As seen, the role of probiotics in this infection eradication is not wellestablished and consolidated, and the use of different species of microorganisms,doses and research methods contribute to such uncertainties.
Vaccines
The development of vaccines is a promising alternative that targets the prophylaxis and/or the treatment of the infection (Table 2)[101]. Recently, studies have focused on the development of reverse vaccines with the help of bioinformatics, and five antigenic epitopes have been prioritized as potential vaccine candidates: babA, sabA,fecA, vacA and omp16[20]. However, their development has been a major challenge in theH. pylorifield, since many studies have not been successful in experimental models. In contrast, a randomized phase 3 study with children has been conducted in China, which was efficacious and safe in providing oral vaccines with recombinant B urease againstH. pylori[102]. However, a more accurate evaluation of its long-term effect is required. In another study by Wanget al[103], intramuscular administration was compared with oral administration of the multi-epitope vaccine, evidencing a better protection rate by oral administration. The development of nanovaccines is also being explored, and presents a nice potential to become an excellent alternative in triggering an effective immunological response againstH. pyloriinfection[90,104].
CONCLUSION
Although the knowledge about the differentH. pyloriinfection characteristics have been expanded since its discovery, much still needs to be done for a broader understanding of its underlying mechanisms. Furthermore, the new diagnostic methods should be better explored in order to reduce health expenditure and to provide less invasive diagnostic alternatives to patients. Finally, the growing resistance ofH. pylorito antimicrobial therapy alerts to the necessity of developing satisfactory strategies for bacterial eradication, as well as vaccine implementation aiming at reducing infection prevalence.
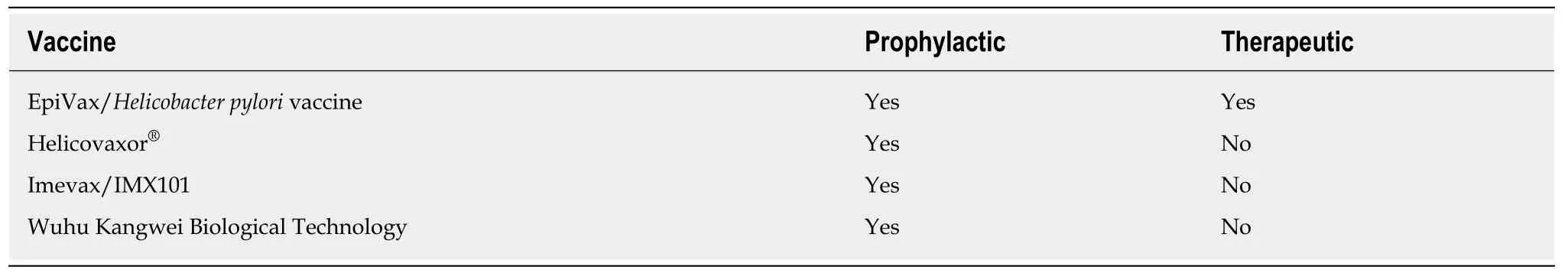
Table 2 Preliminary effects of developing vaccines against Helicobacter pylori infection
杂志排行
World Journal of Gastroenterology的其它文章
- Oncogenic ADAM28 induces gemcitabine resistance and predicts a poor prognosis in pancreatic cancer
- Correlation of plasma miR-21 and miR-93 with radiotherapy and chemotherapy efficacy and prognosis in patients with esophageal squamous cell carcinoma
- Accuracy of an administrative database for pancreatic cancer by international classification of disease 10th codes: A retrospective large-cohort study
- Post-transplant infection improves outcome of hepatocellular carcinoma patients after orthotopic liver transplantation
- Short-term efficacy of robotic and laparoscopic spleen-preserving splenic hilar lymphadenectomy via Huang's three-step maneuver for advanced upper gastric cancer: Results from a propensity score-matched study
- Estimating survival benefit of adjuvant therapy based on a Bayesian network prediction model in curatively resected advanced gallbladder adenocarcinoma
