De novo malignancies after liver transplantation: The effect of immunosuppression-personal data and review of literature
2019-10-11TommasoMariaManziaRobertaAngelicoCarloGaziaIlariaLenciMartinaMilana
Tommaso Maria Manzia, Roberta Angelico, Carlo Gazia, Ilaria Lenci, Martina Milana,
Oludamilola T Ademoyero, Domiziana Pedini, Luca Toti, Marco Spada, Giuseppe Tisone, Leonardo Baiocchi
Abstract
Key words: Pediatric liver transplant; Immunosuppression weaning; Clinical operational tolerance; Adult liver transplant; Graft rejection; Immune system; De novo malignancies;Immunosuppression minimization; Cancer
INTRODUCTION
It has been shown that progress in surgical techniques and enhanced standards in patient selection, standard of care, peri-operative management, survival rates and quality of life after orthotopic liver transplant (OLT) has remarkably improved over the last three decades. This has led to OLT being the treatment of choice for end-stage acute and chronic liver failure. However, the life-long immunosuppression (IS)regimens following transplantation still burden OLT recipients. In fact, major risks include infections, oncogenic viruses and renal, cardiovascular and metabolic complications along with a worrisome time-dependent susceptibility to de novo malignancies (DNMs)[1]. The incidence of DNMs among transplant patients is two to four times higher than in the healthy population[2]. These numbers increase to greater than 19 times in the pediatric counterpart[3], and DNM-related mortality is becoming the most prevalent cause of death amongst transplant subjects[4-6]. Beyond the therapeutic strategies for DNMs after OLT, IS drug minimization or withdrawal has been proposed.
Several studies have demonstrated the tolerogenic potential of the liver[7,8]. Because of its unique anatomy, several cell types in the liver have the capacity to act as antigen-presenting cells. In fact, dendritic cells, Kupffer cells and hepatocytes are capable of presenting antigens that activate CD8+T cells[7]. These mechanisms are believed to play a role in allowing IS discontinuation and a permanent IS-free state(IFS) in up to 30%-40% of adult OLT recipients and in up to 60% of the pediatric population[9,10]. The present review aimed to detect the role of IS and its minimization or withdrawal in OLT adult and pediatric recipients on DNMs.
The primary goal of the current review was to assess the incidence and the characteristics of the diagnosed DNMs after an OLT in adult and pediatric populations in comparison with the non-transplanted immunocompetent population.The secondary goals were to determine: the incidence and outcome of those recipients that were successfully weaned off IS; and to address whether the maintenance of an IFS decreases the incidence of DNMs in LT recipients.
MATERIALS AND METHODS
Search strategy
A literature review was conducted in February 2019 through MEDLINE databases (via PubMed) and Google Scholar to find studies pertaining to OLTs, DNMs, IS regimens and the clinical operational tolerance (COT) threshold. Articles published in languages other than English were excluded. All texts were full text accessible.Multiple keywords were used: “de novo tumor”, “adult”, “pediatric”, “liver transplantation”, “malignancy”, “review” and “operational tolerance”. The combination of words was used to maximize the results and achieve the highest possibility of articles related to the field of the present review. A flow chart of the article selection is provided in Figure 1.
Inclusion and exclusion criteria
Studies published in journals describing DNMs and risk factors for their development were searched for both adult and pediatric OLT recipients including experiences from article bibliographies. Records on post-transplant lymphoproliferative diseases(PTLDs), skin, head, neck, breast, lung, prostate, kidney, colorectal and other DNMs were collected and discussed from systematic reviews, randomized clinical trials,observational studies and case-control studies. IS regimens included calcineurin inhibitors (CNIs), corticosteroids, azathioprine, mammalian target of rapamycin inhibitors (mTORi) and antibody-mediated induction therapies. No time limits were applied to provide the closest results to the effective impact of DNMs on OLT patients. Non-English articles and cohorts of patients who underwent allografts other than liver were excluded from this review.
Data extraction
Information extracted from each selected article was first author name, year of publication, number of patients, follow-up period, characteristics of the detected malignancies, number of tolerant patients and study outcomes.
RESULTS
De novo malignancies in the OLT population
Recurrence and DNMs are the most frequent cause of mortality in adult OLT recipients[11]with an incidence up to 26%[12]. Conversely to cardiovascular complications, mortality from DNMs is increasing fast[13]: OLT recipients experience the highest onset rate of lymphomas (57%), and both PTLDs and non-PTLD tumors appear to develop after a shorter time in OLT recipients than other solid organ transplant patients[14]. Moreover, liver-localized PTLDs may originate from the donor and their treatment effect is very different. According to the donor/host origin of PTLDs, the prognostic significance might significantly change: Donor originated PTLDs might have different clinical and pathological features compared with the case of host originated PTLDs[15].
The probability of developing non-skin malignancies is higher in patients who underwent OLT for primary sclerosing cholangitis (PSC) (22% at 10 years) or alcoholic liver disease (ALD) (18% at 10 years)[16]. In particular, alcohol abuse[17]correlates with a three-fold increased risk of developing DNMs, and similar results are encountered in smokers of long duration due to the induced DNA damage[18,19](Table 1). Overall, skin cancers are commonly diagnosed DNMs along with PTLDs after an OLT. The incidence of other malignancies is subject to a large variability due to the majority of epidemiological data coming from registry databases or single-center retrospective studies.
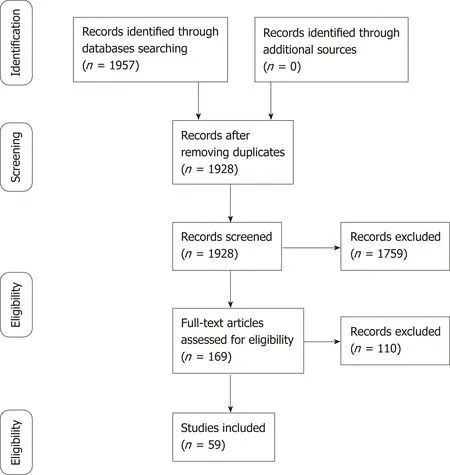
Figure 1 Flow diagram of the article selection procedure.
Major de novo malignancy incidence in adult OLT recipients
PTLDs:PTLDs are the second most diagnosed DNMs after an OLT accounting for around 35% of non-skin malignancies[20]. Most PTLDs are due to Epstein-Barr virus(EBV). Even if a clear cut-off range of EBV-DNA levels has not been well recognized,virus detection may be sufficient to reveal early PTLDs[2,21-24]. Although the mortality still remains high (up to 85% and 69% after 1 and 5 years, respectively), PTLDs are decreasing due to the PTLD type, prognosis and efficacy of the available treatments[23,25,26].
Certain types of IS regimens including anti-thymocyte globulin, cyclosporine (CsA)or muromonab-CD3 are more likely to determine the onset of PTLDs[27,28]. The survival rate was significantly better in patients undergoing tacrolimus regimens compared to CsA (81.2% vs 50% after 5 years from the PTLD diagnosis)[29]. Multidisciplinary approaches that include IS weaning, interferon, surgery, radiotherapy and chemotherapy were attempted to reduce the incidence or recurrence from PTLDs[30].
Non-PTLDs:The most represented malignancies in adult OLT recipients are skin cancers[31-33]despite their lower recurrence after other SOTs[33-35]. Non-melanoma skin cancer are the most represented, and OLT recipients express a much higher risk when compared to the healthy population[36]. The vast majority of non-melanoma skin cancer is represented by squamous cell carcinomas and basal cell carcinomas[18,35].However, a recent report from Rademacher et al[37]described an inverted trend with a decline in the incidence of skin cancer in the OLT population. This suggests that the characteristics of the analyzed cohort and a more deliberate use of sun blockers,avoidance of direct UV radiation and the type of IS adopted may play a role[38,39].
Human papilloma virus infections, aging, pallor of skin, previous cutaneous malignancies, blue or hazelnut eyes, CD4 lymphocytopenia and history of actinic keratosis are associated to skin tumors after an OLT[18,35,40,41]. In addition, PSC(considered as an indication for transplant[42]), male phenotype, Caucasian ethnicity and monoclonal induction therapy[40]represent relevant assets. A longstanding clinical experience proved CsA to be the strongest predictor; in fact CsA-treated patients developed a skin malignancy in a shorter time than patients treated with tacrolimus,making CsA an independent and specific risk factor for skin cancer[43].

Table 1 Incidence of de novo malignancies in adult orthotopic liver transplant patients
A strong link between IS and DNM development is also found in the Kaposi sarcoma (KS), a multifocal angioproliferative muco-cutaneous tumor[27]that affects immunodeficient patients infected with human herpesvirus-8. However, in contrast with other DNMs[34,44,45], the KS incidence among the OLT population is constantly dropping. KS affects OLT patients around 500-fold more than the general population[27,44,46,47]. Thus, a tailored IS administration and a meticulous use of chemotherapy are crucial to avoid the outset of KS. Of note, a low blood viral concentration often limits the human herpesvirus-8 detection in most affected patients[34,48]. Typical KS diagnosis might also be missed by an inexperienced pathologist[49]. Even though there are ongoing trials on novel treatments for KS,evidence suggests that switching the IS regimen from CsA/tacrolimus to mTORi represents the best option to reduce the growth of KS[44,49,50].
Head and neck cancer are less common, but they are still the most serious DNMs in the OLT population. Although no screening exam is approved to diagnose these malignancies[2,51], specific follow-up guidelines by the European Association for the Study of Liver highly recommended that smokers and former alcoholic OLT patients are screened[52]. A recent study by Piselli et al[53]on 2770 OLT recipients confirmed that these subjects are more prone to develop head and neck cancer especially in those with a previous history of smoking and alcoholic liver disease. The 5-year survival rate has been reported around 35% with a standardized incidence ratio (SIR) that increased to 11.2% in OLT subjects with alcoholic liver disease.
Tobacco seems to be involved in the development of pharyngeal and tongue cancer,whereas alcohol plays a predominant role in the onset of oropharyngeal and upper aerodigestive squamous tumors in OLT individuals[54,55]. Hence, regular screenings should be performed on ears, nose and throat especially if there is a prior history of smoking.
Lung cancer accounts about 26% of the total deaths related to post OLT DNMs[56]. In fact OLT recipients showed between two- and three-fold higher incidence than the general population[34]. Better outcomes in OLT subjects with no history of smoking were observed. Nevertheless, the survival rate in both OLT individuals and in the healthy population after being diagnosed with lung cancer was similar. Therefore, the major gamechanger is mainly represented by cigarette smoking[57].
OLT recipients have a high prevalence of colorectal malignancies usually diagnosed between the 1stand 4thyear after OLT; the risk rises to 5.6% considering the PSC recipients[58]. Although the information about patients suffering from both PSC and inflammatory bowel disease are still scarce, the higher risk in developing colorectal malignancies has been well recognized[59,60]and a special surveillance in these patients is currently strongly recommended[61]. Moreover, after 5 years the risk goes up to 15%, and a closer follow up must be mandatory in order to early detect any tumour development[58,62,63]. Despite being identified at earlier stages, the prognosis of colorectal metastasis in OLT recipients is still worse than the general population mostly due to the IS regimens that reduce the immune cell activity[64,65].
OLT recipients did not show an overall increased risk of prostate cancer when compared to the general population[2,34,66,67]. Non-prostate genitourinary neoplasms are usually more lethal and develop earlier in OLT recipients. Renal malignancies after OLT have a SIR of 3.3, and annual ultrasound screenings after OLT are strongly encouraged[27,34].
Young OLT females under CsA-based IS are more likely to develop breast fibroadenomas compared to males[68,69]. In fact, CsA seems to: enhance the fibroblast activity; influence the hypothalamic-pituitary axis and interfere with the prolactin receptors on lymphocytes[34,70]. Furthermore, the capability of CsA to regulate the expression of pyruvate kinase M2 in different breast cancer cell lines is giving new insights about its role in cancer therapy[71]. A switch to tacrolimus is high advisable because the mass dimension seems to decrease in dimension after conversion[68,69].Non-breast gynecological tumors are often more represented in the OLT patients than in the healthy population[27,72,73]. This might be explained by a pre-OLT stricter screening program towards breast cancer diagnosis that should also be more enforced in gynecological malignancies[27].
De novo malignancy in the pediatric OLT population
DNMs account for 5%-16% of non-hepatic related deaths after pediatric OLT[74]and together with cardiovascular complications are becoming the major cause of late death after transplantation. In children, the risk of developing DNMs is 19-fold higher than adults, and tumors are more aggressive and less responsive to treatments[6].Therefore, the early detection and prompt therapeutic management of DNMs in pediatric recipients is essential to achieve satisfactory results. As in adults, the major risk factors for DNMs after pediatric OLT include IS regimens as well as viral infections such as EBV, cytomegalovirus, human papilloma virus and human herpesvirus-8[75]. Due to the paucity of data in the pediatric population, data on DNMs after OLT in children are reported mainly in registry transplant studies including other solid organ (kidney, lung, heart)[76,77]. Therefore, records on the incidence and types of DNMs after pediatric OLT are limited to single case series and mainly related to PTLDs.
PTLDs:PTLDs are the most frequent DNMs after pediatric OLT with an incidence of 5%-20%. In 90%-95% of cases, PTLDs are related to EBV and cytomegalovirus infections[78]. The risk of developing PTLDs from EBV primary infections increases to 7-fold compared to the reactivation of a pre-existing infection[79,80]. Worldwide,different series confirmed that EBV-related PTLDs were the most common DNM,ranging between 35% and 80% of all neoplasms either in liver and in kidney pediatric transplant recipients[76].
The subtypes of PTLDs might vary from benign polymorphic conditions to aggressive monomorphic states such as lymphomas. From a large registry analysis of DNMs after pediatric OLTs, non-Hodgkin’s lymphoma is the most frequent (71%)type of PTLD, out of which nodal diffuse large-B cell lymphoma and Burkitt’s lymphoma are the most detected, while Hodgkin’s lymphoma and leukemia account for 8% and 4%, respectively[3]. Non-Hodgkin’s lymphoma occurs mainly in younger patients: Estimated SIR is 123 (95% confidence interval 3.12-686) for children aged <17 years old, 55.7 (95% confidence interval 6.74-201) for recipients aged between 17 and 39 years old and 9.42 (95% confidence interval 3.06-22.0) for patients ≥ 40 years old[81]. Several series suggest that donor-derived PTLD might be more likely to relapse in transplanted organs when compared with recipient-derived PTLD. In addition,donor-derived PTLD seems to appear earlier in the post-transplant period and present a more positive 5-year prognosis than the ones arising from recipients[81].
Non-PTLD:Non-PTLD neoplasms are rare in pediatric OLT recipients, so that, the incidence of non-PTLD malignancy is unclear due to paucity of data (Figure 2). Nonmelanoma skin cancer is the most common non-PTLD DNMs represented mainly by squamous cell carcinomas[78]. Cases of melanomas have also been reported with a higher incidence than in adults. Other non-PTLDs include gynecologic neoplasms, KS,papillary thyroid tumors, sarcomas, brain tumors, renal cell carcinoma, liver tumors,testis neoplasms and bladder cancer.
The incidence of PTLD versus non-PTLD malignancies differs among age groups.Data from the Israel Penn International Transplant Tumor Registry[78]reported that children with post-transplant non-PTLD DNMs are older than recipients developing PTLD malignancies (13.2 vs 7.9 years of age, P < 0.0001). Moreover, from the time of transplantation, non-PTLD tumors are diagnosed within 99.2 months (P < 0.0001)while PTLD malignancies are detected within 60.2 months (P < 0.0001), and the latest to onset are usually vulvar and perineal cancer (113 mo)[78].
Modulation of risk: Immunosuppression features
The Consensus on Managing Modifiable Risk in Transplantation group extensively described the main risk factors for graft loss in kidney and OLT recipients and provided useful recommendations to extend the long-term graft survival and to decrease the chances of DNMs onset[82]. IS drugs activate different pathways in the immune system and need to be carefully selected[83]. The primary disease needs to be considered in order to prescribe the most appropriate IS treatment. Of interest,mTORi might play a slight protective role reducing the incidence of DNMs especially within the 1styear of the transplant[84-86]. Similar data were described for mycophenolate mofetil[87]. The use of mTORi, mycophenolate mofetil, and tacrolimus represents the first choice when cancer develops in transplant recipients. There are no reports of such use of mTORi in the pediatric population. On the other hand, CNIs seem to have a cancer-promoting influence that might be related to their blood level concentration. Antilymphocyte medications also influence the onset of DNMs in longlasting IS, while corticosteroids do not directly affect the risk of developing DNMs unless they are associated with chronic IS[88,89]. The association of multiple agents in lifelong IS regimens might be responsible for a substantially higher risk of DNMs. For these reasons, the discontinuation of IS (especially carcinogenic IS) should always be considered in transplant patients[88]. The primary aim is to achieve a COT status defined as a condition of non-reactivity of the immune system with a good graft function and no rejection in the absence of IS[90,91].
On the other hand, the non-compliance to IS considerably reduced the mid- and long-term survival of transplanted organs. It is estimated that about 10% of deaths or graft loss in adult OLT individuals were due to a poor compliance to the IS regimen[92,93]. Therefore, patients unintentionally or surreptitiously do not comply with IS regimens[94-96]due to the most disparate reasons are more likely to lose the graft. The cost and necessity of IS along with the prescribed dosage and the size of daily pills represents irresponsible behaviors that might compromise the patient compliance.Physicians should always be alerted for the possibility of these situations. For these reasons, it is important to establish a positive connection between the recipient and the healthcare provider[82,97].
Therefore, the spectrum of DNMs can also be reduced with a deeper understanding of the reasons for negligent conduct. Earlier studies demonstrated that patients already benefited from reminders of the importance of IS medication combined with counseling and psychological interventions[82,95,96]. Likewise, OLT individuals who do not regularly take daily medications face higher risks of graft rejection and elevated chances of developing DNMs. Consequently, the IS withdrawal must be physiciandriven and always under close clinical surveillance.

Figure 2 De novo malignancy distribution in three main cancer registries.
Role of immunosuppression minimization and withdrawal in liver transplant patients
The “Holy Grail” of transplantation is the achievement of an IFS. As mentioned above, long-lasting IS exposes patients to multiple adverse effects such as infections,tumors and target organ damage. The paramount importance of COT in LT can be achieved in selected recipients starting from a cautious IS minimization and constantly monitoring the liver function tests (LFTs)[9,98]. Unfortunately, as shown in most series only 30% of well-selected LT recipients can be safely weaned from IS[9,98-101](Table 2).
The molecular mechanisms responsible for graft acceptance still need to be fully understood, but the liver seems less likely to cause rejection in their hosts than other organs. Multiple theories were hypothesized: (1) The production of higher levels of major histocompatibility complex might affect the recipient immune response[102]; (2)An OLT donor leukocytes migrating in the recipient blood stream could influence the graft tolerance because their irradiation causes organ rejection[103]; and (3) Donor hematopoietic stem cells might determine a chimeric effect in the recipient[104].Moreover, the huge amount of blood that is constantly flowing in the liver exposes it to plenty of bacteria and antigens that could enhance a COT status[90].
New insights on human leukocyte antigen donor-specific antibody/antibodies(DSA) are emerging in OLT recipients. A recent study described how the IS management and IS withdrawal protocols might affect the onset of de novo DSA(dnDSA) after OLT especially during the transition to IS monotherapy in the 1styear after the OLT[105]. Interestingly, a higher dnDSA prevalence was found in patientsundergoing IS minimization (51.7%) and IS-free patients (66.7%). These findings suggest that monitoring dnDSA is high advisable and the IS minimization or withdrawal should be taken in consideration after at least 1 year from OLT in order to prevent negative consequences on the graft.

Table 2 Clinical operational tolerance literature and clinical trials in adult orthotopic liver transplant recipients[9,90]
The Tor Vergata experience:In the last decade, our Liver Unit from Tor Vergata Institute described multiple trials attempting IS minimization and IS withdrawal after OLTs[9,10,90,106-109]. The first purpose was to minimize the uptake of IS drugs in the first years post-OLT. Afterwards, patients with stable LFTs, no rejection or autoimmune disease who underwent IS minimization were discontinued from IS. Initially, LFTs are monitored every week and then monthly within the 1styear during the IS withdrawal process[90]. IS was resumed in patients who had double the normal LFT levels during follow-up or when a liver biopsy showed features of acute rejection[90].
From April 1998 to December 2014, in the HPB and Transplant Unit, 299 OLT were performed. Of these, 65 (21.7%) patients with a mean follow-up of 81 months were considered for weaning protocol while 234 (78.2%, mean follow-up of 125.6 months)were under CNIs or mTORi and mycophenolate mofetil IS regimens. In unpublished series, data on DNMs were compared in order to address the differences in DNM incidence during a median follow-up of 4 years (Table 3).
Among the 65 recruited patients enrolled in local IS withdrawal protocol[106,108,109], 22(33.8%) were successfully weaned from IS (tolerant; Tol), while 43 (66.2%) were nontolerant (Non-Tol) and needed IS resumption after an observed upsurge of the LFTs or biopsy-proven acute rejection. In the Tol group, none experienced DNMs versus two (4.6%) in the Non-Tol group and thirty-two (13%) in the standard immunosuppressed recipients (Table 4). LT recipients under daily IS showed an increased relative risk of 4.45 of developing DNMs versus Tol and Non-Tol recipients and a SIR of 1.5 when compared to the general population.
Role of immunosuppression minimization and withdrawal in pediatric OLT recipients:Because chronic IS significantly affects the long-term outcomes of pediatric OLT recipients, children were the primary OLT population who experienced IS minimization and withdrawal protocols (Table 4).
Ramos et al[110]reported the first clinical trial of IS weaning where 20 pediatric patients underwent drug discontinuation for long-term IS complications (in two cases for de novo squamous cell carcinomas) reaching COT in 16 patients (27.1%). Takatsuki et al[111]reported the result of a prospective trial where a COT status was reached in 24(38%) out of 63 children after ≥ 2 years from the OLT, and this promising COT rate remained similar in the subsequent trials from the same study group[112-114]. All tolerant patients had normal LFTs after 1-year follow-up, and no rejection episodes were reported. However, almost 6% of selected COT patients showed signs of allograft fibrosis at histological finding, driving the introduction of a protocol liver biopsy for patients undergoing IS withdrawal[115].

Table 3De novo malignancy features in orthotopic liver transplant recipients: The Tor Vergata experience between April 1998 and December 2014
Hurwitz et al[116]described the only report focusing on the effects of IS withdrawal on DNMs after pediatric OLTs. Thirty-eight pediatric OLT recipients affected by PTLDs (n = 19) or severe EBV infection (n = 19) after a mean time of 1.8 ± 2.3 years and 1.1 ± 1.1 years from OLT, respectively, attempted IS withdrawal in combination with antiviral drugs with or without chemotherapy. A complete IS withdrawal was achieved in eight (21%) patients for 4.2 ± 1.7 years with an overall 84% survival rate.Episodes of rejection that did occur after stopping IS were successfully treated with standard therapy with no graft loss. Although the results are tempered by the intrinsic limitations of retrospective studies, the authors state that the mortality risk from cancer well outweighs the risk of graft loss due to acute rejection from IS withdrawal. Also, Lee et al[117]reported in his COT series another case of a successful IS weaning in a child with a de novo PTLD with a 3-year follow-up.
Feng et al[118]published the results from a pilot prospective multi-centric trial aiming to withdraw IS in order to reduce drug-related complications: Out of 20 pediatric OLT recipients attempting COT, 12 (60%) children successfully discontinued (over a period at least of 36 wk) IS, while 8 patients experienced rejection resolved by IS resumption.Recently, the authors reported that after a 5-year follow-up all COT recipients have normal LFTs and no histological inflammation or fibrosis, despite some patients were found with DSA and modest increases in sinusoidal C4d staining[119]. These promising results suggested that in selected pediatric OLT recipients, COT was feasible; yet selection criteria (such as clinical and biomarkers criteria) are needed to identify the children who could successfully attempt IS withdrawal. High rates (40%-42%) of successful COT were also reported by other series[120,121]. Likewise, Waki et al[120]demonstrated that Non-tol patients were associated with post-transplant human leukocyte antigen antibodies. This could represent a future screening criterion to select children who could discontinue IS regimen.
DISCUSSION
The outset of DNMs in LT recipients seems to be connected to the IS regimen. In fact,IS drugs downregulate different pathways both of the adaptive and the innate immune response leading to a higher risk of tumor relapse after OLT. Hepatocellular carcinoma represents one of the indications for OLT. Due to the nature of thetransplant indication itself, it would be beneficial to quickly tailor or withdraw IS because these recipients face a higher risk of recurrent hepatocellular carcinoma[90,122].Thus, immediately after OLT, CNIs should be discontinued to minimize this threat as they seem more likely to trigger DNMs[123,124]. Conversely, mTORi seems to reduce the impact of DNMs at least within 5 years post-OLT[125].

Table 4 Clinical operational tolerance trials in pediatric orthotopic liver transplant recipients
The IS non-adherence must be always avoided due to its dangerous effects often underestimated in the overall graft longevity[126]. Nowadays, COT can be achieved in almost 30% of adult OLT individuals after a meticulous selection, but it is hard to accomplish for other solid organ transplant subjects because COT is organ dependent[127]. Strict criteria from the studies cited in Table 3 include IS regimens and IS drug blood levels, stable allograft function, no history of rejection or autoimmune diseases and a similar human leukocyte antigen match between donors and recipients. All these conditions need to be met in order to attempt COT. The accomplishment of a complete IFS in pediatric OLT recipients proved to be suitable in carefully designated patients albeit the heterogeneous considered cohorts. In fact, up to 60% of the total recipients were successfully withdrawn from IS while preserving a normal graft function.
Histological findings are as important as biochemical assessments in the definition of COT, even if not all studies reported liver biopsies features after weaning off IS.OLT recipients with normal LFTs might hide relevant graft inflammation or fibrosis that offset the risk of organ injury. In addition, modern studies stressed the relevance of histological features when outlining future trials. Considerations on graft fibrosis,independent from IS maintenance or withdrawal, need further investigations to fully understand the etiopathogenetic pathways involved. To the best of our knowledge, no clinical experience has been reported so far on IS withdrawal due to DNMs occurrence. Therefore, we can only speculate that the reconstitution of the immunological pathways can counteract the tumor growing.
The main drawback of the present review is that most COT studies explored have been fitted in order to address the possibility to achieve COT status and not in those who experienced DNMs. In fact, the majority of studies on IS withdrawal is referred to patients who demonstrated a stable clinical pathway with normal LFTs and no rejection post-OLT. An international registry including all adult and pediatric IS weaning experiences might represent an interesting approach to both gain knowledge about the entity of DNMs in OLT subjects and the final outcomes after IS withdrawal in such patients.
The minimization of IS dosages would provide multiple beneficial aspects that include: (1) Releasing from all IS burdens; (2) Remarkable savings in IS drugs[107]; and(3) Increased quality of life after the reduction of daily medications, which can positively influence compliance and graft outcomes in long-term treatments[9,128]. COT immunological biomarkers are constantly researched because their clinical predictor role would represent a game changer in the transplantation field. The blood stream represents the most used source of non-invasive liver tolerance biomarkers due to its potential never-ending amount[129,130]. Unfortunately, the lack of consistent assays and validated biomarkers that might predict graft failure currently represent an arduous issue. Patients are in desperate need of alternative treatments to lifelong IS, and until reliable biomarkers are available the gold standard for rejection diagnosis is still represented by liver biopsies[131].
Conclusion and future prospects
In the last few decades, there have been multiple efforts to reach an IFS in OLT recipients. These attempts might lead to ethical concerns as they shift to a potential unsafe option, which could raise future complications. Patients demand the best longterm quality of life after such a tough experience of an organ transplantation.Researchers methodically commit to fulfill this urgency, and physicians struggle to prevent the recurrence of physical and psychological complications that mainly result from the IS itself or from the primary disease recurrence.
A COT status perfectly frames the overarching goal of transplantation, which aims to provide the best quality of life for transplant recipients who would not be burdened by the IS threats while providing economic benefits. From these perspectives an IFS remains the most enticing path to follow and considered worth it in spite of all the challenges to overcome. Likewise, the relatively recent field of regenerative medicine is constantly gaining ground through new outstanding findings. Specifically, the astonishing capabilities of the extracellular matrix capable of closely emulating the ideal milieu of native organs enhancing cell growth, migration and proliferation is promising to offer innovative hints for future research[132,133].
We are still far away from a translational side of these results, but the immense potential of regenerative medicine surely represents a hope for future therapies and IS avoidance. More than 60 years ago the transplantation era began after the first successful transplantation was performed among identical twins, and the first case of COT was described. Since that moment, tolerance continues to be a grueling problem albeit remarkable steps were taken over the past decades. In fact, when experienced hands were called to action, undeniable evidence proved that a stable IFS is achievable in carefully selected OLT recipients. Clues that COT is no longer intangible is becoming clearer, and the concept that considered IS weaning protocols as detrimental procedures should be now considered out-of-date. However, an in-depth knowledge is certainly required as many immunological pathways responsible for COT still remain arcane, and crucial challenges about tolerance need to be addressed with further investigations.
ARTICLE HIGHLIGHTS

Research methods
A systematic literature examination of DNMs and IS weaning in adult and pediatric OLT recipients was described in the present review. Data from worldwide clinical trials was collected from highly qualified institutions performing OLTs. Patient follow-up, IS discontinuation and incidence of DNMs were reported. Likewise, the review assesses the differences in adult and pediatric recipients by describing the adopted IS regimens and the type of diagnosed solid and blood malignancy.
Research results
Emerging evidence suggests that the liver is an immunologically privileged organ able to support IS discontinuation in carefully selected recipients. Malignancies are often detected in liver transplant patients undergoing daily IS regimens. Post-transplant lymphoproliferative diseases and skin tumors are the most detected DNMs in pediatric and adult OLT patients,respectively. To date, IS withdrawal has been achieved in 40% and 60% of well-selected adult and pediatric recipients, respectively. In both populations, a clear benefit of IS weaning protocols on DNMs is difficult to ascertain because data have not been specified in most of the clinical experiences.
Research conclusions
The selected populations of tolerant pediatric and adult liver transplant recipients greatly benefit from IS weaning. There is still no strong clinical evidence on the usefulness of IS withdrawal in OLT recipients on malignancies. An interesting focus is represented by the complete reconstitution of the immunological pathways that could help in decreasing the incidence of DNMs and may also help in treating liver transplanted patients suffering from cancer.
Research perspectives
Most of the current studies on IS withdrawal describe patients with a stable clinical pathway with normal liver function test levels and no history of rejection post-OLT. In the future, an international registry including all IS weaning experiences in OLT patients would offer a promising database to explore the connections between DNMs and the final outcomes after IS withdrawal in such patients. Seriate graft biopsies should always be considered in future studies to take into account the risk of graft fibrosis. Fibrosis is independent from IS maintenance or withdrawal, and further investigations are strongly suggested to fully understand the etiopathogenetic pathways involved. The minimization of IS dosages may decrease all IS complications and induce remarkable savings in IS drugs. Moreover, the recipient’s quality of life after the reduction of daily medications could significantly boost their compliance and graft outcomes in the long-term. IS withdrawal is still arduous to realize. However, it is possible, and it is supported by the described cases of clinical operational tolerance in OLT individuals. Indepth investigations are needed to study the possibilities of achieving a complete IS-free state and clinical operational tolerance in OLT patients affected by DNMs because few studies explore this possibility. Regenerative medicine and clinical operational tolerance biomarkers are new promising frontiers that could provide novel insights about tolerance mechanisms in order to replace liver biopsies as the currently recognized gold standard method for rejection diagnosis.
杂志排行
World Journal of Gastroenterology的其它文章
- How does Helicobacter pylori cause gastric cancer through connexins: An opinion review
- Colorectal cancer: Parametric evaluation of morphological,functional and molecular tomographic imaging
- Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy
- Significance of tumor-infiltrating immunocytes for predicting prognosis of hepatitis B virus-related hepatocellular carcinoma
- Long non-coding RNA highly up-regulated in liver cancer promotes exosome secretion
- Circular RNA PIP5K1A promotes colon cancer development through inhibiting miR-1273a
