Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy
2019-10-11SilviaNardelliStefaniaGioiaJessicaFaccioliOlivieroRiggioLorenzoRidola
Silvia Nardelli, Stefania Gioia, Jessica Faccioli, Oliviero Riggio, Lorenzo Ridola
Abstract Minimal hepatic encephalopathy (MHE) represents the mildest type of hepatic encephalopathy (HE). MHE is considered as a preclinical stage of HE and is part of a wide spectrum of typical neurocognitive alterations characteristic of patients with liver cirrhosis, particularly involving the areas of attention, alertness,response inhibition, and executive functions. MHE can be detected by testing the patients’ psychometric performance, attention, working memory, psychomotor speed, and visuospatial ability, as well as by means of electrophysiological and other functional brain measures. MHE is very frequent, affecting from 20% up to 80% of patients tested, depending of the diagnostic tools used. Although subclinical, MHE is considered to be clinically relevant. In fact, MHE has been related to the patients’ falls, fitness to drive, and working ability. As a consequence, MHE affects the patients and caregivers lives by altering their quality of life and even their socioeconomic status. Recently sarcopenia, a very common condition in patients with advanced liver disease, has been shown to be strictly related to both minimal and overt HE. Aim of this review is to summarize the most recently published evidences about the emerging relationship between sarcopenia and cognitive impairment in cirrhotic patients and provide suggestions for future research.
Key words: Minimal hepatic encephalopathy; Cognitive impairment; Sarcopenia; Muscle alterations; Cirrhosis
THE BURDEN OF MINIMAL HEPATIC ENCEPHALOPATHY IN LIVER CIRRHOSIS
Hepatic encephalopathy (HE) is a complex neurological syndrome caused by liver failure and shunting of the portal blood into the systemic circulation. HE produces a wide and complex spectrum of nonspecific neurological and psychiatric manifestations[1]. In its milder expression, the so called minimal HE (MHE)[2,3], the manifestations are subclinical and detectable only by means of psychometric testing as well as electrophysiological and other functional brain measures[4,5]. Depending on the population studied and the diagnostic tool used, MHE ranges between 20% and 80% and thus, may be considered the most complication of liver cirrhosis[6-11].Although subclinical, MHE involves the areas of attention, alertness, response inhibition, and executive functions as well as the working memory, psychomotor speed, and visuospatial ability[12-15].
Depending on the population studied and the diagnostic tool used, MHE can be detected in 20%-80% of patients with cirrhosis, MHE is considered as a pre-clinical stage of HE and is part of a wide spectrum of typical neurocognitive alterations in liver cirrhosis, particularly involving the areas of attention, alertness, response inhibition, and executive functions. Although these typical characteristics of MHE reduce the safety and quality of life of patients with cirrhosis together with their caregivers, they are difficult to detect from the clinical point of view. The optimal diagnostic criteria for MHE remain controversial, also because an essential condition for MHE diagnosis is the correct standardization of the tests used by age, education and also employment of the patient. To overcome the difficulties in the execution of complex psychometric batteries, which may require specialist staff for their administration, are time and money consuming, other techniques have been proposed, such as the critical flicker frequency, the smooth pursuit eye movement,and the use of cognitive evoked potentials. Computerized psychometric tests,including the scan test and the inhibitory control test (ICT), which may be more specific and repeatable, have also been proposed. An electroencephalogram is also useful for detecting conditions MHE, especially if a spectral analysis is carried out. A comprehensive summary on the clinical manifestations and diagnosis of MHE has been recently published by our group[12,16].
Low quality of life, falls, sleep disorders, erectile dysfunction in males, impairment in fitness to drive and car accident incidence have been found to be more frequent in patients with MHE than in those without. Moreover, MHE by reducing the patients’working capacity, may affects their socioeconomic status[16,17]. Finally, MHE is a clear risk factor for the development of overt HE and for its recurrence, leading to frequent hospitalization of the patients. In summary, MHE, despite subclinical is a burden for the patients and their caregivers, which results in the use of more health care resource than other manifestations of liver disease[6,18-21]. Recently, a strong statistical correlation between muscle alterations, sarcopenia and myosteatosis, and MHE was found[22-25]. A first consequence of this observation is that muscle alterations should be investigated in all patients with cirrhosis and cognitive impairment.
PREVALENCE AND CAUSE OF SARCOPENIA IN LIVER CIRRHOSIS
In cirrhotic patients, sarcopenia has been assessed by different techniques such as anthropometry (Triceps-Skinfold-Thickness or Mid-Arm-Muscle-Circumference), the bioelectrical impedance analysis and the dual-energy X-ray. The functional consequence of sarcopenia is estimable by the Hand Grip or other more composite tests such the six-minute walk test[26-30]. Moreover, some evidences showed that patients with cirrhosis may develop simultaneously loss of skeletal muscle and gain of intermuscular and intramuscular fat, denominated ‘myosteatosis’[31].
The gold standard to quantify muscle mass is to date represented by the computed tomography (CT) and magnetic resonance image (MRI) analysis[32-34]. The CT scan, by using muscle attenuation, is indirectly able to measure muscle fat infiltration and to add informations not only on quantity but also on the quality of muscle tissue[31,35].MRI is able to quantify the muscle mass and the fat free muscle mass[36]. These diagnostic tools are certainly limited by cost, radiation exposure and logistic concerns[37]. Nevertheless, a CT or MR scan is often performed in cirrhotic patient for clinical reasons, such as the suspicion of hepatocellular carcinoma or portal vein thrombosis. In these patients, sarcopenia can be easily assessed by validated softwares such as SliceOmatic V4.2 software (Tomovision, Montreal, Quebec, Canada), which enables specific tissue demarcation by using previously reported Hounsfield unit(HU) thresholds[38], as shown in Figure 1. Skeletal muscle is identified and quantified by HU thresholds of -29 to +150 as previously described[34]and with these specific HU thresholds, measurements of the SMI are not influenced by the presence of ascites.Cross-sectional areas (cm2) were automatically computed by summing tissue pixels and multiplying by pixel surface area. Muscle cross-sectional area was normalized for stature (cm2/m2) to obtain the L3 Skeletal Muscle Index (L3 SMI). Sarcopenia was defined according to previously validated cutoff values in cirrhotic patients: L3 SMI: <39 cm2/m2for females and < 50 cm2/m2for males[26]. The following HU thresholds were used for adipose tissues: -190 to -30 for subcutaneous and intermuscular adipose tissue[39], and -150 to -50 for visceral adipose tissue[40].
Since muscle attenuation indirectly measures fat infiltration in muscles, mean muscle attenuation in HU was reported for the entire muscle area from the same image used to calculate L3 SMI. To define myosteatosis, we used cutoff values that have been previously associated with mortality: < 41 HU in patients with a body mass index (BMI) up to 24.9, and < 33 in those with a BMI ≥ 25[27,31].
Using these cut off, sarcopenia, with a prevalence ranging between 65 and 90%, has to be considered a very common condition in patients with advanced liver disease[41].The severity and prevalence of sarcopenia correlates with Child-Pugh’s Class[42]and,when added to model for end-stage liver disease (MELD) score, sarcopenia has been shown to improve the prediction of the patients’ survival[22,23]. Sarcopenia contributes to fatigue, limits exercise tolerance and may have a heavy burden on performance status and activities of daily living[24]. Multiple factors are involved in sarcopenia development. In liver disease dietary intake may be inadequate to the energy expenditure; nutrient absorption compromised and substrate utilization impaired[25].Moreover, malabsorption, altered metabolism, hormonal factors, hyperammonemia,may also play a role.
SARCOPENIA AND HE
In Addition to the prognostic impact[32,43,44], sarcopenia is related to several complications of cirrhosis[45-49], including overt HE[22-24,38,50]. The pathophysiological background supporting the relationship between muscle depletion and HE origins from the involvement of muscle tissue in ammonia metabolism and trafficking (Figure 2). Ammonia plays a central role in the pathogenesis of cognitive impairment of cirrhosis. Its concentrations is increased because of the inability of the impaired liver in removing ammonia through urea synthesis due to liver failure and porto-systemic shunts. Skeletal muscle may plays a compensatory role in ammonia clearance[49,50]through glutamine-synthase, which metabolize ammonia into glutamine.Consequently, a muscle depletion may favor the ammonia accumulation.Furthermore, any catabolic increases the glutamine release from muscle. Glutamine metabolism in the small intestine and the kidney to glutamic acid and ammonia, may contribute to the appearance of ammonia in portal circulation where, due to liver failure and the shunts may lead the whole-body ammonia availability[49,51]. Moreover,on the other hand, ammonia impairs muscle protein synthesis in part through the up regulation of myostatin production[52], that is upregulated because skeletal muscle removes large quantities of ammonia from the circulation[53]. Finally, the mitochondrial dysfunction increases reactive oxygen species bringing to autophagy and muscle damage. Therefore, hyperammonemia may be considered the trigger of a vicious circle: By one side the occurrence of hyperammonemia is a consequence of sarcopenia due to the inability of the depleted muscle to metabolize ammonia, on the other side, the increase in ammonia concentrations, through the above processes,induces further muscle wasting.

Figure 1 Computed tomography images used for the muscularity assessment of patients with cirrhosis. Comparison of two cirrhotic patients with (A) and without (B) sarcopenia. Muscle mass is highlighted in red, intra and intermuscular fat in light blue, subcutaneous fat in green and visceral fat in blue.
The studies in which the possible association between muscle alterations and MHE was evaluated are summarized in Table 1. In the first one[47], conducted on 300 hospitalized cirrhotic patients, muscle depletion was evaluated with anthropometry,muscle function with handgrip strength and minimal HE with psychometric tests. At multivariate analysis, muscle depletion was significantly and independently associated with MHE. In another study MHE was found to be higher in patients with sarcopenia than in those without and sarcopenia was an independent predictor of MHE at multivariate analysis[54]. In a further study, a multivariate analysis showed that the time needed to perform number connection test A (one of the tests used to detect MHE) was independently correlated to age, Child Pugh class, malnutrition and diabetes[55]. None of the above studies used the CT scan to determine muscle quantity and quality. This limitation was recently filled by a prospective study which used CT scan for the quantitative estimation of sarcopenia and myosteatosis and the Phychometric Hepatic Encephalopathy Score (PHES) for the estimation of the cognitive impairment in order to investigate the link between muscle quantitative alteration and MHE. In this study both myosteatosis and sarcopenia were strongly related to presence of MHE as well as to the development of overt HE during the follow-up[46]. In particular, a direct correlation was found between the muscle quantity estimated by the SMI and the psychometric performance estimated by the PHES(more muscle = better psychometric performance) while SMI was inversely correlated to ammonia levels. These correlations support the possibility that a reduced muscle mass (and muscle quality) is able at least to contribute to higher ammonia levels and to the cognitive impairment observable in cirrhotic patients. A further evidence on the importance of muscle alteration on both ammonia level and HE is provided by a recently published paper on the modification of muscle mass and the evolution of HE after a transjugular intrahepatic portosystemic shunt (TIPS)[56,57]. TIPS can ameliorate the muscle mass, at least in some patients. In this setting we described that the patients with a muscle wasting amelioration after TIPS placement obtained an improvement of MHE and a lower number of episodes of overt HE in the follow up supporting a causal relationship between muscle alterations and cognitive impairment. Ammonia was also reduced in the patients with muscle mass amelioration and a significant correlation between muscle and ammonia modifications after TIPS was observed. The last study, recently published by Tapper et al[58]evaluated the cognitive impairment with the ICT, a computerized test previously validated on cirrhotic patients. In this study, hand grip correlated strongly with skeletal muscle area and mildly with ICT performance.
HYPOTHESIS AND FUTURE RESEARCH
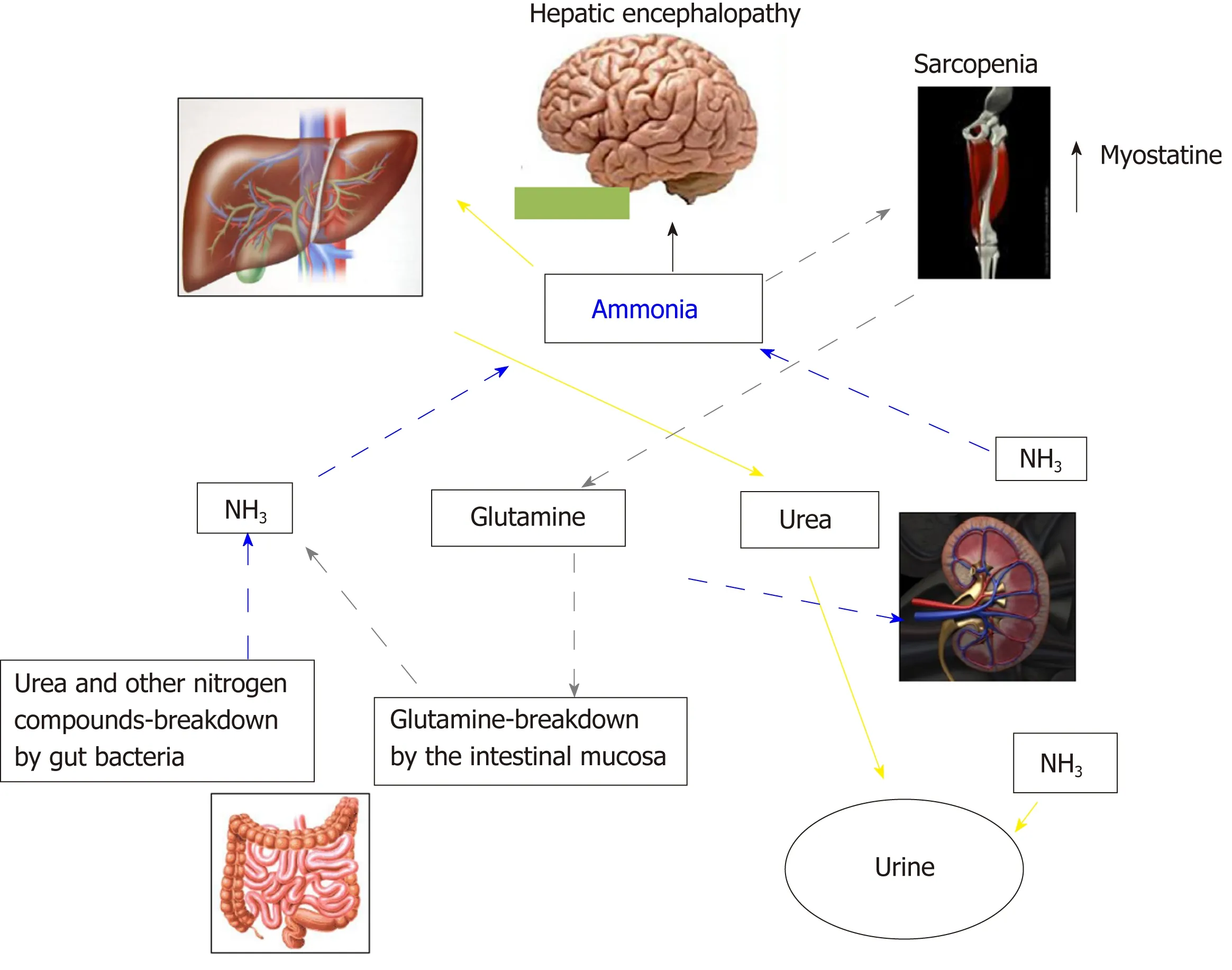
Figure 2 Inter-organ ammonia metabolism showing sites of ammonia generation and disposal. Yellow arrows indicate the ammonia removed by the urea-cycle and disposed in urine. Grey pointed arrows indicate ammonia disposed as glutamine in muscle, which is then broken down in the small intestine to ammonia. This pattern of ammonia disposal does not lead to a net ammonia removal. Ammonia may be also produced by glutamine breakdown in the kidney and by urea and other nitrogen compounds breakdown in the large intestine (blue dashed arrows).
Given the above background, a possible hypothetical point of view is that at least some of the clinical effects attributed until now to the presence of MHE may be partially or totally explained by the contemporary presence of sarcopenia. The falls,which have been shown to be more frequent in the cirrhotic patients with MHE could be due to sarcopenia and to their functional consequences. The same could be hypothesized for the reduced driven capacity and car accidents. Even the reduced working capacity could be considered as an outcome of muscle alterations. This hypothesis should be supported by studies in which the contemporary assessment of the quantity, quality and functionality of the muscle mass are coupled with the assessment of the cognitive impairment. Multivariate analysis should be used to distinguish, at least statistically, the relative role of these two components, which are expected to covariate. This point of view, which must be supported by adequate observations could open a new perspective on the evaluation of the patients’ risk.
Moreover, recent studies showed an improvement of the nutritional assessment after physical exercise in patients with cirrhosis[59-61], but no study investigated the modifications of cognitive impairment after physical activity. Thus, the management of cirrhotic patients could be also seen from a new perspective. In fact, the amelioration of nutritional status may be a possible goal to decrease the prevalence of MHE and its clinical consequences.

Table 1 Studies evaluating the relationship between muscle alterations and minimal hepatic encephalopathy in cirrhosis

MHE: Minimal hepatic encephalopathy; HE: Hepatic encephalopathy; NCT-A: Number connection test part A; NCT-B: Number connection test part B;LTT: Line tracing test; SDT: Symbol digit test; ICT: Inhibitory control test; TIPS: Transjugular intrahepatic portosystemic shunt; PHES: Phychometric Hepatic Encephalopathy Score; CT: Computed tomography; NS: Not significant; BMI: Body mass index.
杂志排行
World Journal of Gastroenterology的其它文章
- How does Helicobacter pylori cause gastric cancer through connexins: An opinion review
- Colorectal cancer: Parametric evaluation of morphological,functional and molecular tomographic imaging
- Significance of tumor-infiltrating immunocytes for predicting prognosis of hepatitis B virus-related hepatocellular carcinoma
- Long non-coding RNA highly up-regulated in liver cancer promotes exosome secretion
- Circular RNA PIP5K1A promotes colon cancer development through inhibiting miR-1273a
- LncRNA-ATB promotes autophagy by activating Yes-associated protein and inducing autophagy-related protein 5 expression in hepatocellular carcinoma
