Liver stiffness and serum markers for excluding high-risk varices in patients who do not meet Baveno Vl criteria
2019-10-11HongZhouJunLongHanHuCaiYunTianShiDeLin
Hong Zhou, Jun Long, Han Hu, Cai-Yun Tian, Shi-De Lin
Abstract
Key words: Baveno VI; Esophageal varices; Liver cirrhosis; Liver stiffness measurement;Serum markers of liver fibrosis
INTRODUCTION
About 30% of cases of liver cirrhosis worldwide result from chronic hepatitis B virus(HBV) infection[1]. Esophageal varices (EV) and esophagogastric variceal bleeding(EVB) are major complications among patients with liver cirrhosis and are associated with high morbidity and mortality[2]. Six-week mortality rates range between 15% and 25% in patients with EVB[3,4]. High-risk varices (HRV) are medium or large EV or small EV with red wale signs. Prophylactic therapy with beta-blockers or elastic band ligation benefits patients with HRV and is the standard of care in patients with cirrhosis to identify those with HRV[5,6].
Gastroscopy is the gold standard for diagnosing EV and assessing bleeding risk[7].However, gastroscopy is an invasive and expensive procedure with associated risks[6].In the last 10 years, evidence has accumulated regarding the usefulness of noninvasive methods for stratifying EV risk in patients with compensated advanced chronic liver disease[8]. The 2015 Baveno VI consensus workshop recommended that patients with liver stiffness measurement (LSM) < 20 kPa and platelet (PLT) count >150 × 109/L could safely avoid gastroscopy screening[3,4]. These criteria were verified in several clinical studies and can safely spare 20%-30% of liver cirrhosis patients from undergoing gastroscopy[9,10]. The American Association for the Study of Liver Disease recently recommended using the Baveno VI criteria to stratify EV risk in patients with liver cirrhosis[3]. However, up to 40% of gastroscopies are still unnecessary[11]. It is therefore imperative to finding a new strategy to identify more patients without HRV.
Several studies reported that adjusting the LSM and PLT thresholds could spare more patients from undergoing gastroscopy. The expanded Baveno VI criteria (PLT >110 × 109/L and LSM < 25 kPa) further spare 19% of gastroscopies compared to the original Baveno VI criteria, with a risk of missing 1.6% of HRV cases[12]. Jangouk et al[9]recently reported a 12% increase in spared gastroscopies (with no additional HRV cases missed) by expanding the Baveno VI criteria to include a Model for End-Stage Liver Disease (MELD) score of 6. Using cutoff values of LSM ≤ 25 kPa and PLT ≥ 100 ×109/L, Ding et al[13]found that 21% of gastroscopies were spared. However, these criteria have not been confirmed.
Because liver inflammation can significantly increase LSM, it is recommended that the interpretation of LSM is based on the levels of alanine transaminase (ALT) and total bilirubin (TBil)[14,15]. Clinically, most patients with HBV-related liver cirrhosis have concomitant liver inflammation. However, it remains unknown whether adjusting the LSM and PLT cutoffs according to ALT and TBiL levels would increase the number of spared gastroscopies among patients who do not meet the Baveno VI criteria.
Serum markers of liver fibrosis such as PLT count, aspartate aminotransferase(AST)-to-PLT ratio index (APRI), Fibrosis-4 (FIB-4), and the Lok index are useful in predicting severe liver fibrosis or cirrhosis[16-18], EV risk, and variceal bleeding risk in patients with HBV infection[19]. It remains unknown whether these serum markers of liver fibrosis can identify patients without HRV among those who do not meet the Baveno VI criteria. In this study, we aimed to evaluate the utilities of LSM, PLT count,APRI, FIB-4, and the Lok index stratified by ALT and TBil levels for ruling out HRV in patients with HBV-related compensated cirrhosis who did not meet the Baveno VI criteria.
MATERIALS AND METHODS
Study population
From September 2016 to June 2018, we applied the Baveno VI criteria to patients with compensated liver cirrhosis who were admitted to the Affiliated Hospital of Zunyi Medical University and Suining Central Hospital. All the patients with compensated liver cirrhosis who did not meet the Baveno VI criteria underwent gastroscopy screening during this period. We retrospectively reviewed records of 183 hospitalized patients with HBV-related compensated liver cirrhosis who underwent a FibroScan procedure and gastroscopy within 6 months and had complete clinical, laboratory,and imaging data. A total of 132 patients were included in the study. The remaining 51 were excluded from the study because they had the following concomitant conditions: 19, alcoholic liver disease; 15, hepatocellular carcinoma; 6, invalid LSM; 4,human immunodeficiency virus infection; 3, cardiovascular disease; 3, splenectomy;and 1, primary biliary cholangitis.
Diagnostic criteria for HBV-related compensated liver cirrhosis
The diagnosis of cirrhosis was based on previous liver biopsy findings or a composite of clinical signs and findings provided by laboratory tests, gastroscopy, radiologic imaging, and FibroScan procedures. Decompensated liver cirrhosis was defined as the presence of one of the following: New onset of hepatic encephalopathy, EVB, or ascites[2].
Two professionally trained operators performed each transient elastography (TE)measurement using a FibroScan device (Echosens, Paris, France). The M probe was used in all measurements. Only cases with 10 valid measurements obtained with a success rate ≥ 60% and an interquartile range-to-median ratio ≤ 30% were considered valid. The median valid LSM value is expressed in kPa.
Grading criteria for EV
Gastroscopy procedures were performed by two experienced endoscopists who were unaware of the LSM results. EV stage was classified as none (no veins above the esophageal mucosal surface; F0), small (minimally elevated veins above the esophageal mucosal surface; F1), medium (large tortuous veins occupying < 1/3 of the lumen; F2), or large (large coil-shaped veins occupying ≥ 1/3 of the lumen; F3).HRV was defined as F2/F3 EV or F1 EV with red wale signs[20].
Candidate predictor variables
Laboratory parameters included white blood cell (WBC) count, PLT count, ALT, AST,gamma-glutamyl transpeptidase (GGT), TBil, albumin (ALB), globulin (GLB),prothrombin (PT), prothrombin time activity (PTA), international normalized ratio(INR), HBV DNA, serum sodium (Na+), blood urea nitrogen (BUN), creatinine (Cr),and alpha-fetoprotein (AFP). For patients with multiple laboratory parameter measurements, we used the results obtained nearest in time to the TE procedure.
The serum markers of liver fibrosis were calculated according to the following formulas: APRI = [AST/upper limit of normal (ULN)] × 100/PLT; FIB-4 = (age ×AST)/(PLT × square root of ALT); Lok index = exp (log odds)/[1 + exp(log odds)], log odds = -5.56 - 0.0089 × PLT + 1.26 × (AST/ALT) + 5.27 × INR. The following formula was used for calculating the MELD scores: MELD score = 3.8 × ln[TBil (mg/dL)] +11.2 × ln(INR) + 9.6 × ln[Cr (mg/dL)] + 6.4 × (constant for liver disease etiology: 0 if cholestatic or alcoholic, otherwise 1).
Ethics statement
The protocol conformed to the provisions of the Declaration of Helsinki and was approved by the Human Ethical Committee of the Affiliated Hospital of Zunyi Medical University and Suining Central Hospital. All patients were informed in writing regarding the potential use of their data for clinical research purposes, and all accepted.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, USA) and MedCalc®15.8 (MedCalc Software BVBA, Ostend, Belgium). Patient characteristics were compared between patients with and without HRV, using χ2tests for categorical variables, t-tests for variables with normal distributions, and Mann-Whitney U tests for variables with non-normal distributions. Logistic regression analysis was used for univariate and multivariate analyses. MedCalc 15.8 was used to calculate receiver operating characteristic (ROC) curves, and the accuracy of each diagnostic criterion was evaluated according to the area under each ROC curve (AUROC). Given that the aim of this study was to identify patients without HRV, we defined the cutoff values of LSM, PLT, MELD score, and serum markers of liver fibrosis based on the values corresponding to a negative predictive value (NPV) of 100%. The main results calculated were sensitivity, specificity, positive predictive value (PPV), and positive likelihood ratio (PLR) as well as the number of unnecessary gastroscopy procedures.Since we set the cutoff values based on an NPV of 100%, we did not calculate the negative likelihood ratio (NLR). P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics and HRV prevalence
Among the 132 patients enrolled in the study, 59 (44.7%) had EV. Among them, 32(24.2%) had small EV without red wale signs and 27 (20.5%) had HRV (medium or large EV). Regarding Child-Pugh class, 95 and 37 patients had classes A and B,respectively. Of the 132 patients, 99 (26 with HRV) did not meet the Baveno VI criteria due to having both LSM ≥ 20 kPa and PLT ≤ 150 × 109/L; 11 (1 with HRV) due to having only LSM ≥ 20 kPa; and 22 (0 with HRV) due to having only PLT ≤ 150 ×109/L. As shown in Table 1, PT, INR, LSM, and MELD score were significantly higher in patients with HRV than in those without, whereas ALT, AST, GGT, PLT, PTA, and HBV DNA levels were significantly lower.
Independent risk factors for HRV and performance of LSM and serum markers of liver fibrosis for ruling out HRV
As shown in Table 2, the risk factors for HRV in patients who did not meet the Baveno VI criteria included PT, PLT, ALT, GGT, LSM, and MELD score. Independent risk factors for HRV were LSM and ALT. We then explored whether adjusting the LSM and PLT cutoff values and using serum markers of liver fibrosis could exclude more patients without HRV among those who did not meet the Baveno VI criteria. As shown in Table 3, the Lok index had an AUROC of 0.753 [95% confidence interval(CI): 0.671-0.824]. The AUROCs of LSM, APRI, FIB-4, and MELD score were all < 0.7.The Lok index cutoff value of 0.4531 could further spare 32/132 (24.2%) of gastroscopies without missing HRVs. Although PLT had an AUROC of 0.712 (95%CI:0.627-0.787), PLT > 151 × 109/L could only spare 10/132 (7.6%) of gastroscopies without missing HRVs. The AUROC of LSM was 0.637 (95%CI: 0.548-0.718), and LSM< 20.9 kPa could spare 31/132 (23.5%) of gastroscopies without missing HRVs.
If the expanded Baveno VI criteria (LSM < 25 kPa with a PLT count > 110 × 109/L)were applied to patients who did not meet the Baveno VI criteria, they only spared 8(6.1%) gastroscopies without missing HRVs. In our study, only 7 patients had an MELD score of 6, and a stepwise strategy using PLT > 150 × 109/L and MELD = 6 only led to 7/132 (5.3%) additional patients avoiding gastroscopies without missing HRVs.Using cutoff values of LSM ≤ 25 kPa and PLT ≥ 100 × 109/L, as suggested by Ding et al[13], only led to 16/132 (12.1%) more patients avoiding gastroscopies without missing HRVs.
Effects of ALT and TBil on LSM in patients who did not meet the Baveno VI criteria
LSM in patients with ALT < 2 ULN (26.77 ± 12.08 kPa) was significantly lower thanthat in patients with ALT ≥ 2 ULN (32.59 ± 13.25 kPa) (P = 0.011). The mean LSM value in patients with TBil < 2 ULN (26.40 ± 8.66 kPa) was also significantly lower than that in patients with TBil ≥ 2 ULN (38.80 ± 16.77 kPa) (P = 0.000). The LSM in patients with both ALT and TBil < 2 ULN (23.66 ± 8.44 kPa) was significantly lower than that in patients with ALT or TBil ≥ 2 ULN (33.23 ± 13.71 kPa) (P = 0.000).

Table 1 Biochemical characteristics of patients with hepatitis B virus-related liver cirrhosis with and without high-risk varices
LSM and serum markers of liver fibrosis for ruling out HRV in patients with ALT and TBil < 2 ULN
To explore the possibility of increasing the number of patients spared gastroscopy by adjusting the cutoff values of LSM and PLT according to ALT and TBil levels, we divided them into patients with ALT and TBil < 2 ULN (n = 41) and patients with ALT or TBil ≥ 2 ULN (n = 91). Among the 41 patients with both ALT and TBIL < 2 ULN, 14 (34.1%) had HRV. As shown in Table 4, LSM had an AUROC of 0.821(95%CI: 0.670-0.923, P < 0.0001). At a cutoff value of 20.6 kPa, LSM further spared 16/41 (39.0%) of gastroscopies without missing HRVs. PLT, Lok index, MELD score,APRI, FiB-4, and PLT had no predictive value for HRV.
LSM and serum markers of liver fibrosis for ruling out HRV in patients with ALT or TBil ≥ 2 ULN
Among the 91 patients with ALT or TBil ≥ 2 ULN, the prevalence of HRV (13/91,14.3%) was significantly lower than that among patients with ALT and TBil < 2 ULN(14/41, 34.1%, P < 0.05). As shown in Table 5, only the AUROC of the Lok index for identifying HRV was > 0.800. The AUROC of LSM was significantly reduced to 0.672,which was lower than those for the Lok index (0.814), PLT (0.741), and MELD score(0.735).
Using a Lok index cutoff ≤ 0.5596 could spare 36/91 (39.6%) of gastroscopieswithout missing HRVs. Using PLT > 100 × 109/L could spare 40/91 (43.9%) of gastroscopies without missing HRVs. An MELD score ≤ 7 could only spare 10/91(11.0%) of gastroscopies without missing HRVs. These results suggested that the Lok index and PLT performed satisfactorily in identifying patients without HRV among patients with ALT or TBil ≥ 2 ULN.
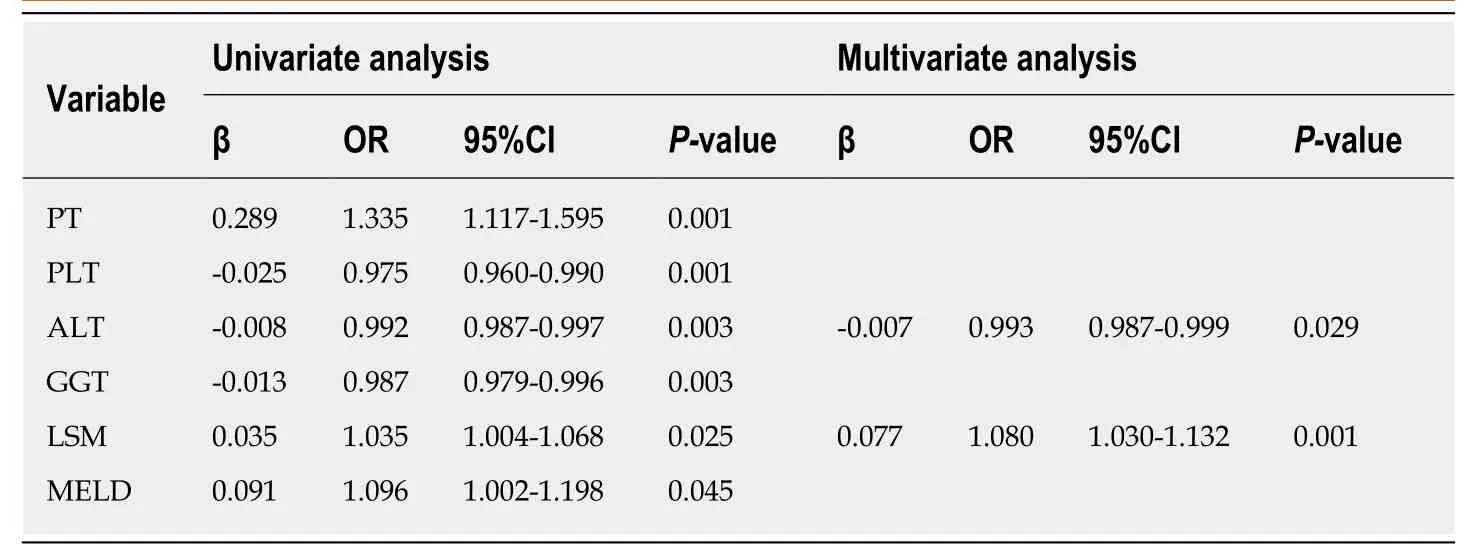
Table 2 Univariate and multivariate analyses of risk factors associated with high-risk varices in patients who did not meet the 2015 Baveno Vl criteria
DISCUSSION
Although many studies have validated the accuracy of the Baveno VI criteria for ruling out HRV[9,21-23], studies have also found that the criteria are conservative and need to be refined to accurately identify more patients without HRV[10,24,25]. In this study, we focused on patients with HBV-related compensated liver cirrhosis who did not meet the 2015 Baveno VI criteria because the prevalence of HRV is relatively high in this population and the possible HRV risk factors may be different from those for patients who fulfill the 2015 Baveno VI criteria[3,26]. We found that only 20.5% of the patients had HRV. Moreover, among the 91 patients with ALT or TBil ≥ 2 ULN, only 14.3% had HRV. Thus, our results demonstrated that many unnecessary gastroscopies are conducted in patients with HBV-related compensated liver cirrhosis despite excluding patients at low risk for EV using the 2015 Baveno VI criteria, especially among patients with ALT or TBil ≥ 2 ULN.
Although LSM was still one of the independent factors associated with HRV, it only had an AUROC of 0.637 for predicting HRV among patients who did not meet the Baveno VI criteria. This result indicated that LSM was not a good predictor of HRV in the overall cohort of patients who did not meet the Baveno VI criteria.
We also found that ALT was independently negatively associated with HRV,because most patients in our study had concomitant liver inflammation. Previous studies showed that LSM can be elevated by liver inflammation and cholestasis[27,28].Our results suggested that concomitant liver inflammation might be an important factor that interfered with the predictive accuracy of LSM in patients with HBVrelated compensated liver cirrhosis who did not meet the Baveno VI criteria.
It was previously unknown whether adjusting the LSM cutoff value according to ALT and TBil levels could improve its utility for predicting HRV among patients with compensated liver cirrhosis and obvious liver inflammation. We found that LSM could accurately identify patients with HRV in those with ALT and TBil < 2 ULN. By slightly increasing the LSM cutoff to 20.6 kPa, LSM further spared 16/41 (39.0%) of gastroscopies without missing HRVs. PLT, Lok index, MELD score, APRI, FiB-4, and PLT had no value for predicting HRV. However, in the patients with ALT or TBil ≥ 2 ULN, LSM had no HRV predictive value.
Previous studies have found that serum markers of liver fibrosis have moderate value for predicting liver cirrhosis in patients with HBV infection[29,30]. However, there is disagreement on the performance of serum markers of liver fibrosis in EV or HRV prediction[8,19,31]. In this study, we found that APRI and FIB-4 could not predict HRV.We also found that PLT and the Lok index performed better in predicting HRV in patients with ALT or TBil ≥ 2 ULN compared with ALT and TBil < 2 ULN. As previous studies found that the Lok index could better predict liver fibrosis in patients with a slightly higher ALT level compared with a normal ALT level[16,32], it isreasonable that the Lok index performed better in predicting HRV in patients with ALT or TBil ≥ 2 ULN. However, it is difficult to explain the different performances of PLT in patients with ALT or TBil ≥ 2 ULN vs < 2 ULN. The different prevalence rates of HRV in these two groups may be a possible explanation, as the prevalence in the patients with ALT and TBil < 2 ULN (34.1%) was significantly higher compared to that in patients with ALT or TBil ≥ 2 ULN (14.3%). Indeed, previous studies reported that the utility of serum markers of liver fibrosis in predicting EV or HRV is greatly affected by the prevalence[29,33,34]. Because patients with ALT or TBil ≥ 2 ULN had obvious liver inflammation, which could elevate LSM, they were difficult to fulfill the Baveno VI criteria, and as a result, the prevalence rate of HRV in patients with ALT or TBil ≥ 2 ULN was lower than that in patients with ALT and TBil < 2 ULN.
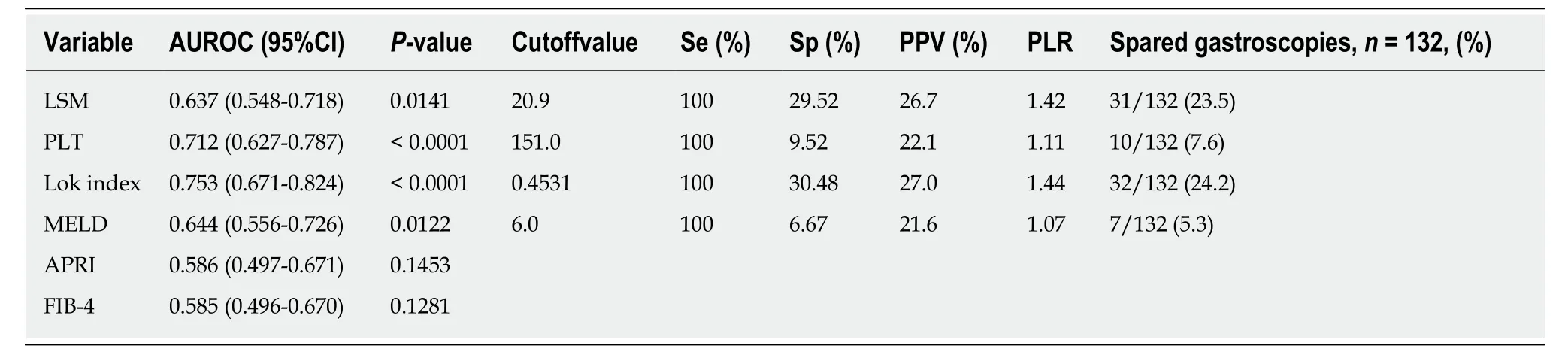
Table 3 Performance of liver stiffness measurement and serum markers of liver fibrosis in predicting high-risk varices in patients who did not meet the 2015 Baveno Vl criteria
Our study had several limitations. First, it was a two-center, retrospective study based on LSM assessed and gastroscopies performed by different operators, although all were experienced. Second, because we only included the patients with HBVrelated compensated liver cirrhosis who did not meet the Baveno VI criteria, the number of patients in this study was small, and the cutoff values were not tested in a validation cohort. Further studies are required to verify our findings.
In conclusion, our study found that in patients with HBV-related compensated cirrhosis who did not meet the Baveno VI criteria, using the cutoff value of LSM, PLT,or the Lok index stratified by ALT and TBil levels accurately identified more patients without HRV. An algorithm-based screening process for HRV in patients with HBVrelated compensated liver cirrhosis is shown in Figure 1.
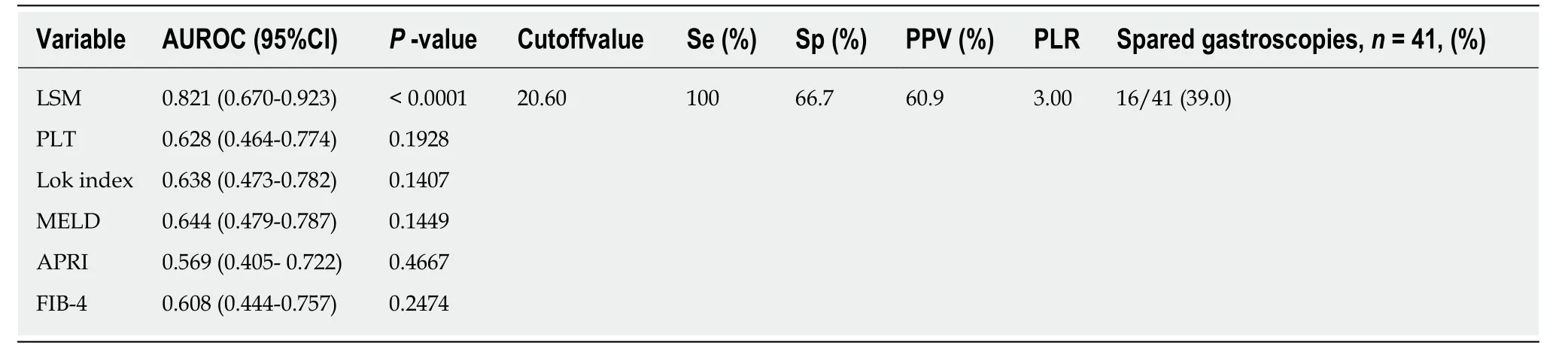
Table 4 Performance of liver stiffness measurement and serum markers of liver fibrosis in predicting high-risk varices in patients with alanine aminotransferase and total bilirubin < 2 upper limit of normal

Table 5 Performance of liver stiffness measurement and serum markers of liver fibrosis in predicting high-risk varices in patients with alanine aminotransferase or total bilirubin ≥ 2 upper limit of normal

Figure 1 Algorithm-based screening process for high-risk varices in patients with HBV-related compensated liver cirrhosis. ALT: Alanine aminotransferase;HBV: Hepatitis B virus; LSM: Liver stiffness measurement; PLT: Platelet; TBil: Total bilirubin; ULN: Upper limit of normal.
ARTICLE HIGHLIGHTS
Research conclusions
In HBV-related compensated cirrhosis patients who do not meet Baveno VI criteria, the LSM,PLT, or Lok index cutoff stratified by ALT and TBil accurately identified more patients without HRV.
Research perspectives
Our study found that in patients with HBV-related compensated cirrhosis who did not meet the Baveno VI criteria, using the cutoff value of LSM, PLT or the Lok index stratified by ALT and TBil levels accurately identified more patients without HRV.
杂志排行
World Journal of Gastroenterology的其它文章
- How does Helicobacter pylori cause gastric cancer through connexins: An opinion review
- Colorectal cancer: Parametric evaluation of morphological,functional and molecular tomographic imaging
- Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy
- Significance of tumor-infiltrating immunocytes for predicting prognosis of hepatitis B virus-related hepatocellular carcinoma
- Long non-coding RNA highly up-regulated in liver cancer promotes exosome secretion
- Circular RNA PIP5K1A promotes colon cancer development through inhibiting miR-1273a
