Advances of Allelopathic Autotoxicity in Rehmannia glutinosa L.
2019-09-10XiaoranWANGWeixiLIZhenLIYuhongWANGZhongyiZHANGXinjianCHEN
Xiaoran WANG Weixi LI Zhen LI Yuhong WANG Zhongyi ZHANG Xinjian CHEN

Abstract Allelopathic autotoxicity occurs when a plant releases toxic chemical substances into the environment which inhibits development and growth of the same plant species. Rehmannia glutinosa L. (R. glutinosa) is one of the most common traditional Chinese medicines, whose productivity and quality, however, are seriously impacted by consecutive monoculture obstacle. Allelopathic autotoxicity is one reason for consecutive monoculture obstacle. In this paper, we reviewed the categories of allelochemicals, the methods of allelochemicals identification, and the mechanisms of allelopathic autotoxicity, which provides clues for further study of the molecular mechanisms of allelopathic autotoxicity and consecutive monoculture obstacle.
Key words Rehmannia glutinosa; Allelopathic autotoxicity; Consecutive monoculture obstacle; Autotoxins; Self DNA
Consecutive monoculture obstacle refers to the continuous planting of the same crop or close relatives crops in the same piece of land. Under normal cultivation and management measures, there are phenomena such as decreased yield and quality, increased pests and diseases, and poor stress resistance[1-3]. The consecutive monoculture obstacle is widespread in the plant kingdom. For many food crops (wheat and rice), cash crops (cotton and peanuts), melons and vegetables (cucumber and tomato), ornamental flowers (tulip and lily) and Chinese herbal medicines (Panax notoginseng (Burk.) F. H. Che, Panax ginseng C. A. Mey and Rehmannia glutinosa L.), there are different degrees of consecutive monoculture obstacle[4-5], and about 70% of root tuber medicinal materials suffer from serious consecutive monoculture obstacle[6].
R. glutinosa, a perennial herb in Scrophulariaceae, is one of the oldest medicinal plants in China. According to the "Compendium of Materia Medica", since the Ming Dynasty, Huaiqing of Henan (now Wen County, Qinyang, Xiuwu, Boai, Wuzhi, etc.) has been the Geo authentic product area of R. glutinosa. Today, the area planted with R. glutinosa is still 15 000 hm2, with an annual output value of several billion yuan[5]. However, the problem of consecutive monoculture obstacle has a long term influence on the planting and cultivation of R. glutinosa. As early as the book "Bencao Chengya Banjie", it was recorded that after the planting of R. glutinosa, the soil would be bitter, the next year, Achyranthes bidentata Blume. could be planted, followed by Chinese yam, and after ten years, the soil turned sweet and was suitable for the replanting of R. glutinosa, otherwise, the R. glutinosa replanted was bitter and thin and cannot be used as medicine[7]. Consecutive monoculture R. glutinosa shows poor growth, more fibrous roots formed in the underground part, and the tuberous roots could not expand normally, resulting in a significant decrease in the yield and quality or even total crop failure. In order to improve the impact of consecutive monoculture obstacle on the yield and quality of R. glutinosa, large amounts of fertilizers and pesticides applied by farmers have not only seriously damaged the soil ecological environment, but also failed to fundamentally solve the problem of consecutive monoculture obstacle in the planting of R. glutinosa. Therefore, it is imperative to explore the mechanism of consecutive monotulture obstacle in R. glutinosa planting.
Allelopathy was first proposed by Molisch in 1937, and then Rice[8] defined allelopathy in 1984 as the phenomenon that a plant (or microorganism) releases chemicals to the environment which directly or indirectly, favorably or adversely affect another plant (or microorganism). Allelopathy can occur between species. However, if the donor and recipient are the same species, it will become an internalized allelopathy, directly or indirectly inhibiting or injuring itself or the same species planted again, which will cause autotoxicity, which is called allelopathic autotoxicity[9-12]. In recent years, a lot of research has been carried out the effects of consecutive monoculture obstacle of R. glutinosa from soil nutrient change, soil physical and chemical properties and pH value change, soil microbial population change and allelopathic autotoxicity[13-16]. The results confirmed that the autotoxins secreted by the tubers of R. glutinosa are the key factors causing the consecutive monoculture obstacle, and allelopathic autotoxicity has become one of the hotspots of the mechanism of consecutive monoculture obstacle in recent years.
Kinds of Allelopathic Autotoxins Secreted and Released by R. glutinosa
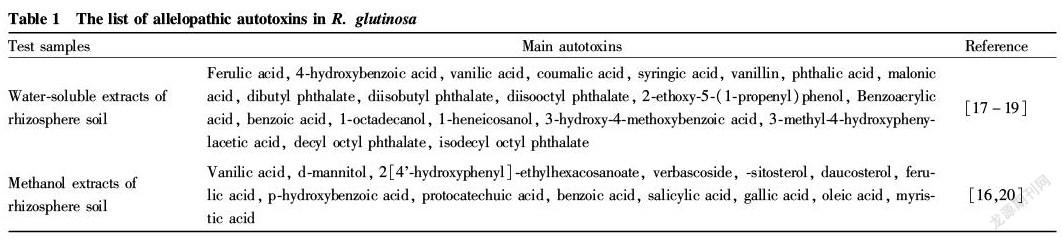
During the growth and development stage of R. glutinosa, the tuberous roots secrete and release allelopathic autotoxins into the soil. The extract of the consecutive monoculture soil (water soluble extract and organic solvent extract) was analyzed by HPLC and GC MS, which confirmed that the tuberous root secreta of R. glutinosa contains organic compounds such as organic acids, alcohols, phenols, aldehydes and phenolic acids[16-20] (Table 1). From the studies on the tuberous root exudates of R. glutinosa, it is generally found that ferulic acid, vanillic acid, coumaric acid, syringic acid, p hydroxybenzoic acid and vanillin are important allelochemicals in the process of consecutive monoculture obstacle of R. glutinosa[16,20-23]. Du et al.[22] confirmed that the phenolic acids in the soil decreased with the increase of the time interval (years), and meanwhile, the yield of the underground stem, plant height and fresh weight of R. glutinosa increased accordingly. Ferulic acid, vanillic acid, vanillin and p hydroxybenzoic acid not only significantly inhibit the growth of the tuberous roots of R. glutinosa , but also affect the chlorophyll synthesis of R. glutinosa. The activity of superoxide dismutase (SOD) and peroxidase (POD) in phenolic acid treated R. glutinosa seedlings increased at first and then decreased, and the content of malondialdehyde increased. Among the treatments, the ferulic acid treatment led to the decrease of the enzyme activity in the roots to the lowest level, and the roots finally rotted and died[23].
Test samplesMain autotoxinsReference
Water soluble extracts ofrhizosphere soilFerulic acid, 4 hydroxybenzoic acid, vanilic acid, coumalic acid, syringic acid, vanillin, phthalic acid, malonic acid, dibutyl phthalate, diisobutyl phthalate, diisooctyl phthalate, 2 ethoxy 5 (1 propenyl)phenol, Benzoacrylic acid, benzoic acid, 1 octadecanol, 1 heneicosanol, 3 hydroxy 4 methoxybenzoic acid, 3 methyl 4 hydroxyphenylacetic acid, decyl octyl phthalate, isodecyl octyl phthalate[17-19]
Methanol extracts ofrhizosphere soilVanilic acid, d mannitol, 2[4 hydroxyphenyl] ethylhexacosanoate, verbascoside, sitosterol, daucosterol, ferulic acid, p hydroxybenzoic acid, protocatechuic acid, benzoic acid, salicylic acid, gallic acid, oleic acid, myristic acid[16,20]
Separation and Identification Methods of Allelopathic Autotoxins Secreted and Released by R. glutinosa
Allelopathic autotoxins are mainly sourced from plant root exudates, plant tissue residues and metabolites of soil microorganisms. The soil environment is extremely complicated, which brings many obstacles to the study of allelochemicals. At present, the methods of separating autotoxins commonly used in the study of consecutive monoculture obstacle are solvent dissolution method, sand culture method and water culture method. In the solvent dissolution method, allelochemicals in the soil are extracted from aqueous solutions or organic reagents. This method is widely used, but due to the influence of complex soil environment, the extract composition is the most complicated. In the case of the sand culture method, R. glutinosa is cultured in the sand and irrigated with the nutrient solution, and the sand is periodically flushed to collect the root exudates which are then absorbed by macroporous resin. This method eliminates the interference of the complex components of the soil, which is greatly different from the soil extract analysis method. Because the water culture method can easily lead to the death of R. glutinosa roots and cannot culture the plant for a long time, as the most commonly used method for the separation of plant root exudates, it is less used for the analysis of root exudates of R. glutinosa[24]. The allelochemicals obtained by separation were usually further identified by HPCL or GC MS[25-26], and combined with the effects of allelochemicals on the indicators including seed germination rate, biomass (root length, root weight, fresh weight and dry weight) and nutrient absorption of R. glutinosa, the specific allelopathic autotoxins of R. glutinosa can thus be screened[20-23,27].
The Action Mechanism of Allelopathic Autotoxins Secreted and Released by R. glutinosa
The consecutive monoculture obstacle of R. glutinosa is the result of the combination of factors such as allelopathic autotoxins, soil environment and soil microorganisms. However, a large amount of evidence indicates that allelopathic autotoxicity is the main reason for the consecutive monoculture obstacle of R. glutinosa. The accumulation of allelochemicals can lead to changes in enzyme activity and soil pH in the soil, destroying the normal soil ecological environment and affecting the growth and development of R. glutinosa; and the identification and response of the tuberous roots to the allelopathic autotoxins further aggravates the inhibitory effect of consecutive monoculture obstacle on the growth of R. glutinosa[28-29].
Effects of Allelopathic Autotoxins on Soil Nutrient Structure
Du et al.[22] studied the autotoxicity of soils with different time intervals by year, and found that the total content of the five phenolic acids in the soil of the two year interval was 4.42 times of that in the soil of the eight year interval, while the available phosphorus content in the soil of the two year interval was 2.94 times of that in the soil of the eight year interval, and the contents of available potassium, available nitrogen, total nitrogen and organic matter in the R. glutinosa soil of the eight year interval was 1.79 , 1.19 , 1.05 and 1.08 times of those in the soil of the two year interval, respectively, which accords with the result of Chen et al.[30]. Urease is one of the main enzymes in the soil, which can accelerate the chemical reaction of soil organic matter and rapidly hydrolyzes urea into CO 2 and NH 3, producing nitrite and ammonia which are toxic to seedlings. Therefore, a too high urease activity is not beneficial to soil fertility and crop growth. Chen et al.[31] found that the urease activity in the soil planted with R. glutinosa for one year and the soil consecutively monocultured with R. glutinosa was significantly higher than that of the control. Li et al.[32] also found that the urease activity in the soil after the planting of R. glutinosa increased significantly, and thus speculated that the allelochemicals secreted by R. glutinosa to the soil during development may change the soil nutrient structure by changing soil enzyme activity and pH, which indirectly affected the nutrient uptake and utilization by plant roots, thus affecting the normal growth and development of R. glutinosa, but the mechanism remains to be further studied.
Effects of allelopathic autotoxicity on rhizosphere microorganisms
Allelopathic autotoxins can cause microbial composition changes in the roots of R. glutinosa, which in turn affects the interaction between roots and soil microorganisms. Studies have found that the addition of grinding liquid of stems and ground leaf, and root exudates of R. glutinosa in the soil all can change the soil microbial population. The microbial populations of the three treatments had obvious carbon source preference, and the root exudate treatment had the poorest microbial diversity and inhibited the growth of microorganisms with multiple carbon sources as substrates. As a result, the microbial populations had a tendency towards changing from the "bacterial type" to the "fungal type"[14,33]. This indicates that the microbial population changes induced by the root exudates of R. glutinosa are not conducive to the growth and development of R. glutinosa roots. Zhang et al.[34] used GC MS and denaturing gradient gel electrophoresis (DGGE) to analyze the allelochemicals and microbial community structure in the rhizosphere soil and outside of root soil of R. glutinosa. Compared with the outside of root soil, the rhizosphere soil contained many unique compounds, including terpenoids, alcohols, amines, organic acids, etc.; and the rhizosphere soil had the highest diversity index and the highest abundance of bacteria and fungi, and contained 10 endemic bacteria and 5 endemic fungi, and no specific microbial population was found in the outside of root soil. It indicates that the root zone of R. glutinosa contains a variety of potential allelochemicals, and its distribution may have a certain impact on the microbial community structure, both of which play a role in the process of the occurrence of consecutive monoculture obstacle of R. glutinosa. Li et al.[21] found that ferulic acid, as a verified autotoxin of R. glutinosa, can promote the secretion of trichothecenes from Fusarium oxysporum in soil, causing wilting of R. glutinosa. The above studies again showed that the allelopathic autotoxins secreted by the tuberous roots of R. glutinosa not only affect the soil microbial population, but also the growth characteristics of specific microorganisms, which indirectly affects the growth and development of R. glutinosa.
Agricultural Biotechnology2019
Effects of allelopathic autotoxicity on the gene expression of R. glutinosa
The aseptic culture of the stems of R. glutinosa confirmed that the allelopathic autotoxins, in addition to the soil microenvironment (soil nutrient and soil microbes), indirectly affected the development of the tuberous roots of R. glutinosa by destroying the interaction of "plant soil microorganism". The more important is that the inhibition is to poison R. glutinosa specifically in the manner of abiotic stress, which breaks the stability of the genome and the normal gene expression process, inhibits the normal growth and development of R. glutinosa (Fig. 1), and even reduces the resistance of R. glutinosa to biotic and abiotic stress, thereby leading to consecutive monoculture obstacle including yield decrease and high incidence of pests and diseases. In recent years, omics research has found that consecutive monoculture obstacle causes changes in the expression of a large number of genes, miRNAs and proteins related to the growth and development of the tuberous roots of R. glutinosa[35-39], laying a foundation for revealing the mechanism of the consecutive monoculture obstacle of R. glutinosa at the molecular level.
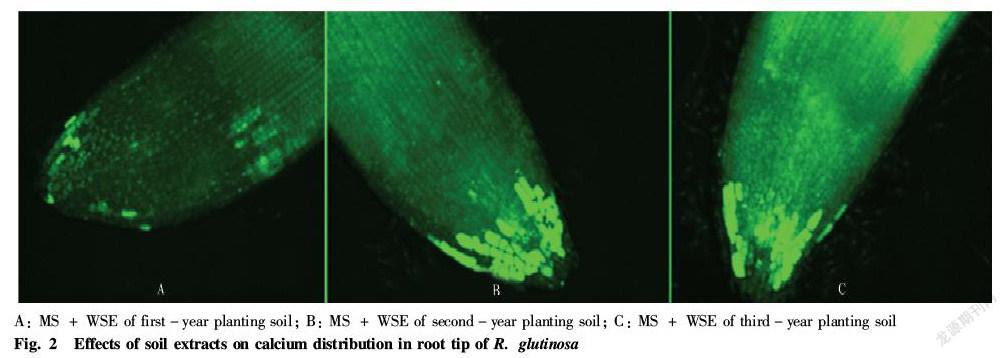
Due to the specificity of allelopathic autotoxicity, the recipient plants must have a signal system that specifically recognizes, transmits and induces responses to autotoxins[40]. Based on this, it is one of the cores of omics research in recent years to find the signal pathway of R. glutinosa to specifically recognize allelopathic autotoxins. Through the combination of the cDNA subtraction library of R. glutinosa and its transcriptome analysis, it was found that the genes specifically responding to consecutive monoculture obstacle were blocked in the process of gene expression such as DNA replication, RNA transcription and protein translation; and meanwhile, the calcium signaling system called the second messenger of organisms and the ethylene signaling system regulating plant growth and development changed[41]. Specifically, the expression levels of calcium signaling system related calcium dependent protein kinase (CDPK) and calmodulin (CaM) were significantly up regulated, accompanied by the up regulation of the expression level of the key rate limiting enzyme 1 aminocyclopropane 1 carboxylic acid (ACC) oxidase in the ethylene synthesis pathway, suggesting that calcium signaling and ethylene signaling systems are involved in the recognition and signaling of autotoxins by the tuberous roots of R. glutinosa. At the protein level, it was found that calcium ion ATPase 9 (ACA9), calmodulin dependent protein kinase 2 (MKK2), calcium dependent protein kinase 6 (CPK6) and calreticulin (CRT2), which were directly involved in calcium signaling, suffered from phosphorylation modification and were localized on the cell membrane, confirming that the tuberous roots of R. glutinosa may change through the covalent modification of calcium signal related proteins on the cell membrane to complete the process of recognition of allelopathic autotoxins; and meanwhile, phosphatidylinositol participating in the regulation of calcium signaling system changed, affected intracellular calcium channels and may induce intracellular calcium accumulation[42]. After treating the tuberous roots of R. glutinosa with the medium supplemented with the extract of consecutive monoculture soil, it was found from the calcium ion fluorescence indicator detection[43] that the extract of consecutive monoculture soil could cause calcium accumulation in the tuberous roots of R. glutinosa, and the calcium ions increased with the consecutive monoculture years, confirming the results of the omics analysis (Fig. 2). Excessive accumulation of calcium ions indicates that the allelopathic autotoxins lead to disturbance of the calcium signaling system and affect the intracellular metabolic regulation process of the tuberous roots of R. glutinosa, and high concentrations of calcium ions may also destroy the growth and development of root cells by the damage to root cells[44-46].
Prospects
Allelopathic autotoxicity is widespread in a variety of plants, and the types of allelopathic autotoxins are different in different species. Studies have confirmed that the allelopathic autotoxicity of R. glutinosa has no toxic effects on other plants such as wheat, maize and rice[47]. However, many kinds of allelopathic autotoxins obtained by HPCL and GC MS are often found in other plants, and therefore, immobilizing the allelopathic autotoxins with higher specificity accurately by optimizing the extraction technology and identification technology of allelochemicals is still an important part of the research on the allelopathic autotoxicity of R. glutinosa.
Recently, studies have confirmed that after the degradation of plant tissues (roots, stems, leaves, etc.) in the cultivated soil, they can release self DNA into the soil, which naturally degrades into DNA fragments of 50-2 000 bp in size. Fragmented self DNA can be recognized by its own roots and has a significant inhibitory effect on its own growth[48]. Stefano et al.[49] analyzed the multiple forest and flower plants and found that the fragmented self DNA can inhibit the root growth and seed germination of itself rather than other species. Francesca Barbero et al.[50] used electrophysiology and laser confocal microscopy to expose lima beans and maize to self DNA and non self DNA, respectively, and assessed changes in calcium plasma membrane potential (Vm) and detected intracellular calcium flux. They found that self DNA induced Vm depolarization and increased calcium flux, while non self DNA could not trigger any of these early signaling events. Although the molecular mechanisms of the above studies are still unclear, the self DNA induced specific inhibition is a typical plant allelopathic autotoxicity. So far, there is no research report showing that self DNA induces consecutive monoculture obstacle in R. glutinosa , but the research of this research group has confirmed that the water soluble extract of consecutive monoculture soil can significantly inhibit the growth of the tuberous roots of R. glutinosa and induce calcium accumulation (Fig. 1, Fig. 2), and it was not excluded that the soil extract contained fragmented self DNA derived from the degradation of the leaves or tubers of R. glutinosa. Therefore, self DNA may serve as a new allelopathic autotoxin, which has become a new focus and direction of the molecular mechanism of the allelopathic autotoxicity of R. glutinosa.
The remarkable feature of allelopathic autotoxicity is autologous poisoning, and it is essential to explore the extracellular "sense system" of R. glutinosa while searching for allelopathic autotoxins. As the first barrier for cells to receive and transmit external information, cell membrane is the key link to study this "sense system". Proteomics studies have confirmed that some calcium signaling proteins undergo phosphorylation modification, and the related proteins are localized on the cell membrane. Does this suggest that focusing on membrane proteins for the screening and analysis on the "sense system" of the autotoxins of R. glutinosa may provide new insights into the mechanism in R. glutinosa for recognizing allelopathic autotoxins. Intracellular signaling is as important as extracellular recognition. Although studies have found that important signaling pathways including the calcium signaling system and ethylene signaling system are all involved in the response to allelopathic autotoxicity, the exact molecular mechanism is still worth exploring.
However, due to lack of complete genomic information of R. glutinosa , it is difficult to obtain a more precise molecular mechanism of allelopathic autotoxicity of R. glutinosa simply by genomic analysis. Under the premise of accurately locking the allelopathic autotoxins of R. glutinosa, establishing a stable genetic transformation system of R. glutinosa and creating a library of allelopathic autotoxins and a sensitive mutant library by means of forward and reverse genetics combined with multi omics screening may become an important means to reveal the exact molecular mechanism of allelopathic autotoxins in R. glutinosa from the intracellular "sensing" to intracellular signaling in depth.
References
[1] WANG Z, LI Y, DING WL. Advances in allelopathic autotoxicity and continuous cropping obstacle of Panax ginseng[J]. Modern Chinese Medicine, 2017, 19(7):1040-1044. (in Chinese)
[2] Chon SU, Jang HG, Kim DK, et al. Allelopathic potential in lettuce (Lactuca sativa L.) plants[J]. Sci Hort, 2005, 106(3): 309-317.
[3] ZHAO YP, LIN S, CHU L, et al. Insight into structure dynamics of soil microbiota mediated by the richness of replanted Pseudostellaria heterophylla[J]. Scientific Reports, 2016(6): 26175.
[4] ZHANG XL, PAN ZG, ZHOU XF, et al. Autotoxicity and continuous cropping obstacles[J]. Chinese Journal of Soil Science, 2007, 38(4): 781-784. (in Chinese)
[5] WU HM, WU LK, WANG JY, et al. The mechanisms of the rhizosphere management on the remission in consecutive monoculture problem and the improvement of soil quality[J]. Ecological Science, 2016, 35(5): 225-232. (in Chinese)
[6] LI XL, HUA ZR. Research progress on allelopathy of medicinal plants[J]. Journal of Anhui Agricultural Sciences, 2009, 37(5): 2022-2023. (in Chinese)
[7] LU ZY. Bencao Chengya Banjie[M]. Beijing: China Medical Science and Technology Press, 2014. (in Chinese)
[8] RICE EL. Allelopathy[M]. New York: Academic Press, 1984.
[9] MILLER DA. Allelopathy in forage crop systems[J]. Agronomy Journal, 1996, 88(6): 854-859.
[10] SINGH HP, BATISH DR, KOHLI RK. Autotoxicity: Concept, organisms, and ecological significance[J]. Crit Rev Plant Sci, 1999, 18(6): 757-772.
[11] GERSHENZON J. Changes in the levels of plant secondary metabolites under water and nutrient stress[J]. Recent Advances in Phytochemistry, 1984, 18: 273-320.
[12] WANG BL, SHI L, LI YX, et al. Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings[J]. Planta, 2010, 231(6): 1301-1309.
[13] QI XH. Study on continuous cropping autotoxicity of Rehmannia glutinosa Libosch[D]. Fuzhou: Fujian Agriculture and Forestry University, 2008.
[14] WU LK, WANG JY, HUANG WM, et al. Plant microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture[J]. Scientific Reports, 2016(6): 19101.
[15] WANG MD, WU ZW, YUAN ZY, et al. Effects of Rehmannia glutinosa Libosch. continuous cropping on microbial communities[J]. Journal of Henan Agricultural University, 2008(5): 532-538. (in Chinese)
[16] Li ZF, Yang YQ, Xie DF, et al. Identification of autotoxic compounds in fibrous roots of Rehmannia (Rehmannia glutinosa Libosch.)[J]. Plos One, 2012, 7(1): e28806.
[17] ZHANG ZY, LI MJ, CHEN XJ, et al. Research advancement and control strategy of consecutive monoculture problem of Rehmannia glutinosa L.[J]. Modern Chinese Medicine, 2013, 15(1): 38-44. (in Chinese)
[18] HAO QH, LIU HY, WANG F, et al. The GC MS analysis of extracts from the rhizosphere soil of Rehmannia glutinosa[J]. Journal of Henan Agricultural Sciences, 2007(2): 78-80. (in Chinese)
[19] ZHU GJ, WANG MD, WU ZW, et al. Analysis of potential allelochemicals in soils around rhizosphere of Rehmannia glutinosa Libosch. by GC MS[J]. Henan Science, 2007(2): 255-257. (in Chinese)
[20] LI ZF, QI XH, LI QS, et al. Extraction, bioassay, and chemical identification of Rehmannia glutinosa L. autotoxins[J]. Acta Ecologica Sinica, 2010, 30(10): 2576-2584. (in Chinese)
[21] LI ZF, HE CL, WANG Y, et al. Enhancement of trichothecene mycotoxins of Fusarium oxysporum by ferulic acid aggravates oxidative damage in Rehmannia glutinosa Libosch[J]. Scientific Reports, 2016(6): 33962.
[22] DU JF, YIN WJ, ZHANG ZY, et al. Autotoxicity and phenolic acids content in soils with different planting interval years of Rehmannia glutinosa[J]. Chinese Journal of Ecology, 2009, 28(3): 445-450. (in Chinese)
[23] WU ZW, WANG MD, LIU XY, et al. Phenolic compounds accumulation in continuously cropped Rehmannia glutinosa soil and their effects on R. glutinosa growth[J]. Chinese Journal of Ecology, 2009, 28(4): 660-664. (in Chinese)
[24] CHEN F. Study on screening of allelochemicals in rhizosphere soil of Rehmannia glutinosa and their allelopathy[D]. Zhengzhou: Henan Agricultural University, 2016.
[25] YUAN ZY. Isolation and identification of autotoxins in Rehmannia glutinosa Libosch[D]. Zhengzhou: Henan Agricultural University, 2010.
[26] YE YS. Application of HPLC fingerprinting technique in Rehmannia glutinosa[C]//Proceedings of the 8 th annual conference of Chinese Medicine Chemistry Branch of China Association of Chinese Medicine, Chinese Medicine Chemistry Branch of China Association of Chinese Medicine: China Association of Chinese Medicine, 2013: 6.
[27] LI J, NIE M, LI L. Effects of adding exogenous phenolic acids to soil on the growth of Rehmannia glutinosa[J]. Journal of Zhejiang Agricultural Sciences, 58(6): 986-989. (in Chinese)
[28] LI ZF, YANG YQ, XIE DF, et al. Effects of continuous cropping on the quality of Rehmannia glutinosa L. and soil micro ecology[J]. Chinese Journal of Eco Agriculture, 2012, 20(2): 217-224. (in Chinese)
[29] LI JJ, LI XZ, LI ZF, et al. Effects of different cultivation soils on growth and enzyme activity of Rehmannia glutinosa and microecology of its rhizosphere soil[J]. Journal of Plant Resources and Environment, 2015, 24(1): 42-47. (in Chinese)
[30] CHEN LC, LIAO LP, WANG SL, et al. Effect of exotic toxin on the nutrition of woodland soil[J]. Chinese Journal of Ecology, 2002(01): 19-22. (in Chinese)
[31] CHEN H, HAO HR, XIONG J, et al. Effects of successive cropping Rehmannia glutinosa on rhizosphere soil microbial flora and enzyme activities[J]. Chinese Journal of Applied Ecology, 2007, 18(12): 2755-2759. (in Chinese)
[32] LI YH, DENG PY, LEI ZH, et al. Effects of growing Rehmannia glutinosa on activities of urease, polyphenol oxidase and alkaline phosphatase[J]. Southwest China Journal of Agricultural Sciences, 2018(5). (in Chinese)
[33] LI JJ, LI XZ, ZHANG B, et al. Effects of allelochemicals from different parts of Rehmannia glutinosa L. on the functional diversity of microbial flora in soil[J]. Guangdong Agricultural Sciences, 2014, 41(10): 48-50. (in Chinese)
[34] ZHANG B, LI XZ, ZHANG LJ, et al. Study on allelochemical distribution and microbial community structure in the root zone of Rehmannia glutinosa[C]//Annual conference of Ecological Committee of Resources of Chinese Herbal Medicine, Ecological Society of China. 2014.
[35] YANG YH, CHEN XJ, CHEN JY, et al. Differential miRNA expression in Rehmannia glutinosa plants subjected to continuous cropping[J]. BMC Plant Biology, 2011, 11(1): 53.
[36] WU LK, WANG HB, ZHANG ZX, et al. Comparative metaproteomic analysis on consecutively Rehmannia glutinosa monocultured rhizosphere soil[J]. PLOS ONE, 2011, 6.
[37] ZHANG ZY, CHEN XJ, LI MJ, et al. Transcriptome wide identification of the genes responding to replanting disease in Rehmannia glutinosa L. roots.[J]. Molecular Biology Reports, 2015, 42(5): 881-892.
[38] YANG YH, CHEN XJ, CHEN JY, et al. Identification of novel and conserved microRNAs in Rehmannia glutinosa L. by solexa sequencing[J]. Plant Molecular Biology Reporter, 2011, 29(4): 986-996.
[39] WANG FQ, TIAN YH, WEI H, et al. Identification and expression analysis of Rehmannia glutinosa mediator complex genes in response to continuous cropping[J]. Acta Physiologiae Plantarum, 2015, 37(12): 264.
[40] ZHENG ZY, NIU MM, CHEN T, et al. Enlightenment gained from research on allelopathic autotoxicity of medicinal plants to cultivation technology innovation[J]. Modern Chinese Medicine, 2011, 13(1): 4-7. (in Chinese)
[41] YANG YH, ZHANG ZY, FAN HM, et al. Construction and analysis of different expression cDNA libraries in Rehmannia glutinosa plants subjected to continuous cropping[J]. Acta Physiologiae Plantarum, 2013, 35(3): 645-655.
[42] WANG CY. Difference analysis of phosphorylated proteome in roots of continuously cropped Rehmannia glutinosa[D]. Zhengzhou: Henan Agricultural University, 2016.
[43] LI MJ, YANG YH, LI XY, et al. Analysis of integrated multiple ‘omics’ datasets reveals the initiation and determination mechanisms of tuberous root formation in Rehmannia glutinosa[J]. Journal of Experimental Botany, 2015, 66(19): 5837-5851.
[44] GUO GY, LI MJ, WANG PF, et al. Abnormal change of calcium signal system on consecutive monoculture problem of Rehmannia glutinosa[J]. China Journal of Chinese Materia Medica, 2013(10): 22-29. (in Chinese)
[45] LECOURIEUX D, RANJEVA R, PUGIN A. Calcium in plant defence signalling pathways[J]. New Phytologist, 2006, 171(2): 249-269.
[46] BOSE J, POTTOSIN II, SHABALA SS, et al. Calcium efflux systems in stress signaling and adaptation in plants[J]. Frontiers in Plant Science, 2011, 2.
[47] LI MJ, FENG FJ, ZHANG B, et al. Advances on molecular mechanisms of Rehmannia glutinosa consecutive monoculture problem formation in multi omics era[J]. China Journal of Chinese Materia Medica, 2017, 42(03): 413-419. (in Chinese)
[48] CARTENì F, BONANOMI G, GIANNINO F, et al. Self DNA inhibitory effects: Underlying mechanisms and ecological implications[J]. Plant Signaling & Behavior, 2016, 11(4):e1158381.
[49] MAZZOLENI S, BONANOMI G, INCERTI G, et al. Inhibitory and toxic effects of extracellular self DNA in litter: a mechanism for negative plant soil feedbacks[J]. New Phytologist, 2015, 205(3): 1195-1210.
[50] BARBERO F, GUGLIELMOTTO M, CAPUZZO A, et al. Extracellular self DNA (esDNA), but not heterologous plant or insect DNA (etDNA), induces plasma membrane depolarization and calcium signaling in lima bean (Phaseolus lunatus) and maize (Zea mays)[J]. International Journal of Molecular Sciences, 2016, 17(10): 1695.
杂志排行
农业生物技术(英文版)的其它文章
- Study on Chemical Composition of the Ethyl Acetate Extract of Pratia
- Effects of Different Selenium Fertilizer Types on Selenium Content and Quality of “Lingfeng” Grapes
- Protoplast Culture and Its Application in Fruit Breeding
- Indexes of Tree Structure of Cylindrical Pear Orchards at the Sapling Stage
- Carbon Storage and Distribution of the Mature Pinus massoniana Plantation in Northwest Guangxi
- Pollution and Quality Control of Mycotoxins in Foods and Feeds
