Resistance Selection Against Abamectin in Tetranychus cinnabarinus (Boisduval) and Changes in Its Detoxification Enzyme Activity
2019-09-10PingLILianjunLIUHengruiLIHaixiaYANGQiulianNONGZhenghuaLIANGXianhuaMA
Ping LI Lianjun LIU Hengrui LI Haixia YANG Qiulian NONG Zhenghua LIANG Xianhua MA
Abstract The susceptible strain of Tetranychus cinnabarinus (Boisduval) was collected from the cassava germplasm resource garden of Guangxi South Subtropical Agricultural Science Research Institute and raised for 15 generations, during which resistance selection against avermectin was performed through the indoor spraying of avermectin, thereby obtaining an avermectin resistance strain (Ab R) with a resistance ratio of 3.25. After the indoor resistance selection, the determination of detoxification enzyme activity in the Ab R and SS strains showed that the specific activity of carboxylesterases (CarE), glutathione s transferases (GSTs) and multi functional oxidase (MFO) in Ab R 15 was 1.27, 1.69 and 1.92 times of that in SS, respectively. In addition, the specific activity of MFO in Ab R 5, Ab R 10 and Ab R 15 was significantly different from that in SS; there was no significant difference in the specific activity of CarE compared with SS when screened to the F 10 generation; and when screened to the F5 generation, there was no significant difference in the specific activity of GSTs compared with SS. The results showed that the significant increase in MFO activity was an important cause of resistance to avermectin in T. cinnabarinus, and CarE and GSTs were also involved in the formation of avermectin resistant strains.
Key words Cassava; Tetranychus cinnabarinus (Boisduval); avermectin; Resistance selection; detoxification enzyme
Tetranychus cinnabarinus (Boisduval) belongs to Tetranychidae, commonly known as red spider, which is the pest mite with the most serious hazard to cassava, which can cause local cassava to reduce production by 10%-30%, or even 50%-70% in severe case[1]. The spider mites are small in size, strong in reproductive ability, and have a short generation cycle. The annual breeding can reach 15 generations. Due to the long term use of chemical pesticides in large scale production, it is inevitable to cause resistance to T. cinnabarinus[2-3].
Avermectin is a broad spectrum, high efficiency, low residue, antibiotic based pesticide safe for humans and animals[4-5], which interferes with the physiological activity of pests to kill pests, which is different from the action mechanism from commonly used pesticides, and would not produce cross resistance with common pesticides. It is suitable for controlling pests that have developed resistance to other acaricides. The use of avermectin for resistance selection in mites has been reported. For example, Chen et al.[6] have shown that the resistance of T. urticae (Koch) to avermectin developed slowly relatively. Liu et al.[7] found that the field population of T. urticae had a stable resistance to avermectin. And Song et al.[8] used avermectin to screen the resistance in T. truncatus, and the results showed that T. truncatus had stable resistance to avermectin, and overall, the resistance of T. truncatus to avermectin grew slowly.
The formation of drug resistance in mites is closely related to the changes in the activity of various detoxification enzymes in the body[9]. Studies have shown that multifunctional oxidase (MFO), glutathione S transferases (GSTs) and carboxylesterase (CarE) are important detoxification enzymes in animals[10]. MFO has a broad spectrum of substrates, can oxidize and metabolize almost all pesticides and is involved in the formation of drug resistance in many pests; GSTs can make endogenous glutathione bind to toxicologically active electrophilic groups in agro chemicals (including pesticides and acaricides) and eliminate it from the body; and CarE is an important detoxification enzyme system in insects, which plays an important role in the detoxification metabolism of foreign compounds and the formation of resistance to insecticides. He et al.[9,11-12] treated different mites with avermectin, and the results showed that the formation of the resistant strain had a certain relationship with the increase of detoxification enzyme activity in the body, but no studies have been conducted on the mechanism of drug resistance in T. cinnabarinus. In this study, the resistance selection against avermectin in T. cinnabarinus was performed through subculture selection by the indoor spraying method, and the changes in the activity of the three detoxification enzymes in the susceptible and resistance strains were analyzed to explore the formation of drug resistance against avermectin in T. cinnabarinus and the relationship between the changes of detoxification enzyme activity and the formation of in vivo drug resistance, with an attempt to provide a theoretical basis for the comprehensive management of resistance in T. cinnabarinus.
Materials and Methods
Experimental site and time
The experiment was carried out in Agricultural Production Testing Center of Guangxi South Subtropical Agricultural Science Research Institute. The indoor selection of resistant strains was performed from April to December, 2016, and the detoxification enzyme activity was measured in January, 2017.
Source of tested pests
Susceptible T. cinnabarinus strain (SS): The T. cinnabarinus was collected from the field of cassava plantation in Guangxi South Subtropical Agricultural Science Research Institute and was raised indoor for at least 2 to 3 generations before the experiment at the feeding temperature of (27±2) ℃ and relative humidity of (75±2)% under the photoperiod of 16 h/d, during which no agent was contacted.
Resistant T. cinnabarinus strain (Ab R): Partial population was separated from the susceptible A. cinnabarinus strain. After expanding propagation, resistance screening was performed with avermectin EC, during which the mites were raised continuously by water separated feeding method (the cassava leaves were placed on soaked sponge in the trays) to the 15 th generation, which can be regarded as the resistant strain.
Tested reagents and main instruments
Reagents: Avermectin EC (purchased from Guangdong Zhongxun Agri Science Corporation); α naphthol (purchased from Sinopharm Chemical Reagent Co., Ltd.), analytical grade; Fast Blue B salt (purchased from Chengdu Aikeda Chemical Reagent Co., Ltd.), analytically pure; 4 nitroanisole (purchased from Chengdu Aikeda Chemical Reagent Co., Ltd.), analytically pure; sodium dodecyl sulfate (SDS, purchased from Beijing Xinhua Lvyuan Science and Technology Co., Ltd.) , chemically pure; α naphthyl acetate (purchased from Shanghai Ruiyong Biotechnology Co., Ltd.), chemically pure; physostigmine (purchased from Fluka), purity≥98%; 2,4 dichloronitrobenzene (DCNB, purchased from Sigma), purity>98%, chemically pure; reduced glutathione (purchased from Japan), purity≥98%; bovine serum albumin (purchased from Beijing Xinhua Lvyuan Science and Technology Co., Ltd.); Coomassie Blue G 250 (purchased from Fluka); reduced coenzyme II (purchased from Beijing Xinhua Lvyuan Science and Technology Co., Ltd.), purity ≥98%; glutathione (reduced) (purchased from Japan), purity≥98 %; ethylene diamine tetraacetic acid (EDTA) (purchased from Shanghai Yuanye Bio Technology Co., Ltd.); NADPH (purchased from Sigma), purity≥98%. Main instruments: artificial climate incubator (Model LRH 250 GsbI, purchased from Shaoguan Taihong Medical Equipment Co., Ltd.); T6 UV spectrophotometer (purchased from Beijing Persee General Instrument Co. Ltd.).
Experimental methods
Selection of resistant strains
Referring to the method of Li et al. [13], 1.8% avermectin EC was sprayed at a selection pressure of a population mortality rate of about 25%, and the survived spider mites were transferred to the the back of fresh cassava leaves on trays 24 h after spraying and raised by the water separated culture method. The survived individuals were removed 3 d after spawning on fresh cassava leaves. After each spraying, bioassay was performed to calculate the median lethal concentration (LC 50 ), and the spraying concentration of each generation was appropriately increased. When the F 1 generation was in the peak period, the drug was sprayed again, and the above operation was repeated until the F 15 generation.
Indoor toxicity measurement
Referring to the method of Li et al. [13], five different gradients of drug concentration were set for each generation. Each gradient concentration ensured that the mortality rate was 40%-75%, and was repeated for 3 times, with clear water as a control (CK). The female adult mites with uniform size in the raising room were connected to the back of fresh cassava leaves as the F 0 adult. The back surfaces of the cassava leaves were sprayed evenly with a hand held sprayer. The mortality was checked 24 h later, and the survived adult mites were placed in an artificial climate incubator at the temperature of (27±2) ℃ and relative humidity of (75±2)% under the photoperiod of 16 h/d. The total number of adult mites before each application and the number of died mites after the application were recorded after the application, and then, the resistant index was calculated.
Resistant index=Resistant strain LC 50 /Susceptible strain LC 50
Determination of detoxification enzyme activity
Preparation of enzyme solution
The susceptible T. cinnabarinus strain and about 100 female ones undergone the lethal dose were selected and added with 2 ml of corresponding phosphate buffer (0.1 M, PH 7.0), followed by grinding well in ice bath. Each obtained paste was centrifuged in a high speed centrifuge (10 000 r/min, 4 ℃) for 15 min, and the supernatant was put in an ice bath, which was the enzyme solution.
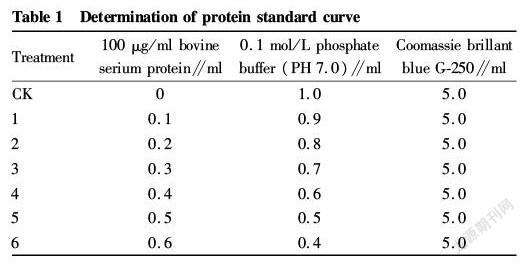
Drawing of protein standard curve
Seven tubes were added in the order of Table 1. The samples were placed in a constant temperature water bath at 37 ℃ for 10 min and colorimetrically determined at 595 nm (Table 1). The bovine serum albumin content (μg/ml) was taken as the abscissa, and the measured OD values were averaged and then plotted on the ordinate to draw a standard curve.
Determination of CarE activity
Referring to the method of Van Astern[14], a NA (3×10 -4 mol/L, containing 10 -4 mol/L a NA) was used as the substrate and added with 3 ml of the enzyme solution, followed by mixing well. The mixture was added with 0.5 ml of a color developer immediately and heated at a constant temperature water bath at 30 ℃ for 10 min to allow the reaction. After the color was stabilized, the absorbance was measured at 600 nm on an ultraviolet spectrophotometer and repeated for 3 times. The specific activity of CarE[mmol/(mg·30 min)]was calculated from the measurement results of the enzyme source protein content.
Determination of GSTs activity
Referring to the method of Clark et al.[15], 66 mmol/L phosphate buffer (pH 7.0), 50 mmol/L glutathione and 0.03 mmol/L 4 dinitrobenzene were mixed with 2.4 , 0.3, 0.1 and 0.2 ml of the enzyme solution, respectively, with the mixture without the enzyme solution as a control. Each mixture was uniformly mixed and heated in a 27 ℃ water bath for 10 min, and the absorbance was measured at 340 nm on a UV spectrophotometer and repeated for 3 times. According to the measurement results of the enzyme source protein content, the absorbance was converted into specific activity[ΔD/(mg·30 min)].
Determination of MFO activity
p nitroanisole (0.1 mol/L, pH 7.0), phosphate buffer and NADPH were mixed with 0.1 , 1.9 , 0.5 and 0.5 ml of the enzyme solution, respectively, with the mixture without the enzyme solution as a control. Each mixture was mixed well and shaken in a 37 ℃ water bath for 30 min, and added with 1.0 ml of HCl solution (1 mol/L) to terminate the reaction. Then, each reaction liquid was extracted with tetrachloromethane and NaOH solution (0.5 mol/L), and after standing at room temperature for 10 min, 2.0 ml of the aqueous phase was taken, added into a cuvette and measured for absorbance at 400 nm. The specific activity of the MFO[nmol/(mg·30 min)]was calculated based on comparison of the absorbance of each treatment with the standard curve.
Data statistics and analysis
The data of resistance selection against abamectin in T. cinnabarinus and the detoxification enzyme activity data were statistically analyzed by SPSS Statistics 22.0 software, and the significant difference analysis was performed by Duncans new multiple range method.
Results and Analysis
Resistance selection against abamectin in T. cinnabarinus
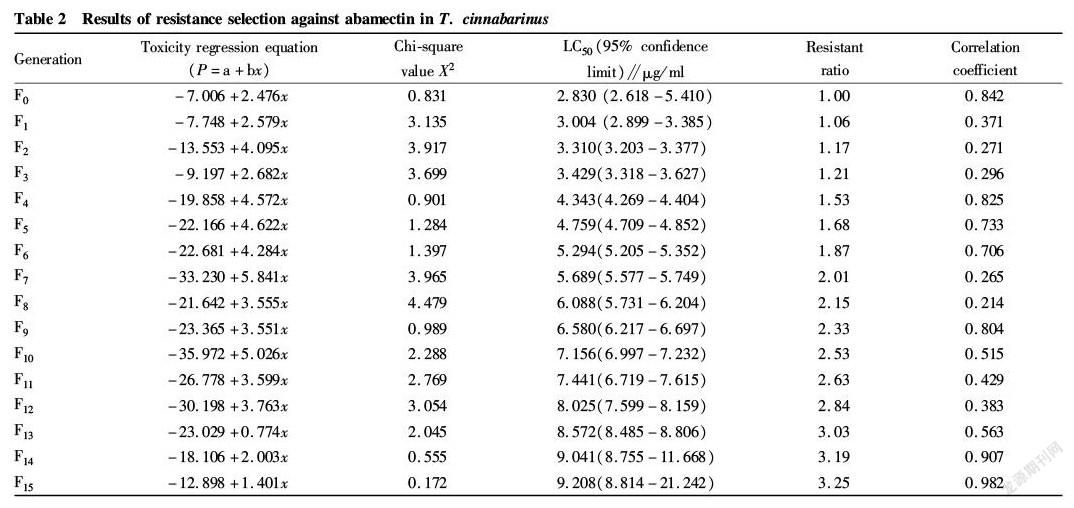
The results of resistance to avermectin in T. cinnabarinus (Table 2) indicated that the F 15 generation acquired the resistance ratio of 3.25 and the LC 50 increased from 2.830 to 9.208 μg/ml by successive selection and rearing. The F 0-F 6, F 7-F 12 and F 13 -F 15 showed a resistance ratio of 1.00-1.87, 2.01-2.84 and 3.03-3.25, respectively, suggesting that the resistance ratio of T. cinnabarinus to avermectin had a slowly increasing trend, and the resistance developed slowly without a sudden increase stage of resistance.
Changes of the specific activity of CarE in different T. cinnabarinus strains
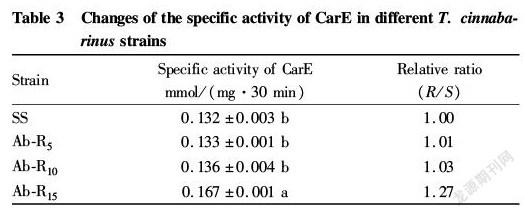
As can be seen from Table 3, only the Ab R15 strain had the specific activity of CarE reaching a significant difference level compared with the SS strain, which was 1.27 times of that of the SS strain. Except for the Ab R15 strain, the differences in the specific activity of CarE between other two resistant strains and the SS strain did not reach a significant level. This indicates that the formation of avermectin resistant strains may have little to do with the specific activity of CarE.
The data shown in the table are the mean ± standard deviation; and different lowercase letters following data within the same column indicate a significant difference (P<0.05), the same below.
Changes of the specific activity of GSTs in different T. cinnabarinus strains
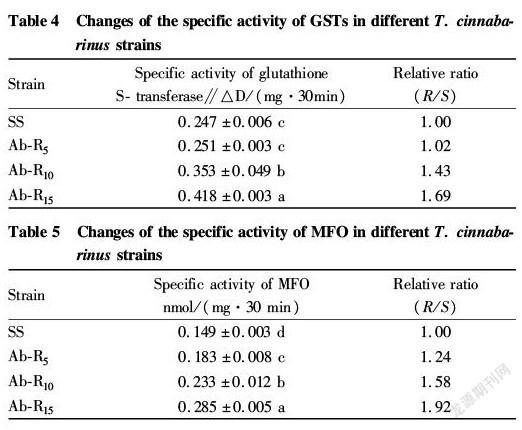
It can be seen from Table 4 that the activity of GSTs had been increasing in the 15 consecutive generations, but the Ab R 5 strain had the smallest increase in the specific activity, and exhibited no significant difference from the SS strain. To the F 10 generation, the Ab R 10 strain showed the specific activity increased rapidly, which was 1.43 times of the SS strain, and its differences from the Ab R 5 and SS strains reached a significant level, indicating that the improvement of the resistance to avermectin in T. cinnabarinus may be partly related to the improvement of GSTs activity.
Changes of the specific activity of MFO in different T. cinnabarinus strains
It can be seen from Table 5 that with the increase of selection generations, the specific activity of MFO was continuously enhanced at a relatively large increase rate. The difference in MFO specific activity between the Ab R strain and SS strain reached a significant level, and the value of the Ab R 15 strain was 1.92 times of that of the SS strain. The specific activity of MFO increased faster from the F 5 to the F 15 generation, each of which had a significant difference from the SS strain, indicating that the resistance of T. cinnabarinus to avermectin was directly related to the enhancement of MFO activity, which was also an important reason for the production of the resistance of T. cinnabarinus to avermectin.
Conclusions and Discussion
Through the indoor selection of resistance in T. cinnabarinus, the population of the F 15 generation with a resistance ratio of 3.25 times was acquired, which was found to have the resistance developed slowly, increased gently, without a sudden increase stage in the resistance selection process. This generation was not easy to produce drug resistance, which is consistent with Chen[6] and Song[8].
Indoor resistance selection is an important means of pest resistance research. The speed and extent of drug resistance development are related to the pest species, the resistance level of the original population, the type of drug and the selection pressure[16]. The results of the research on the resistance of T. cinnabarinus to avermectin showed that in order to prevent the fast generation of resistance in T. cinnabarinus to avermectin in actual production, it is necessary to properly rotate with common agents or use compounding agents accompanied by controlling the dosage and the application times to extend the service life of avermectin, so that the development of pest resistance can be prevented or delayed.
In this study, the activity of three detoxification enzymes (CarE, GSTs and MFO) of the Ab R strain and SS strain was determined after 15 generations of resistance selection using avermectin. The CarE activity did not change before the F 10 generation basically, and there was no significant different in the specific activity between the Ab R strain and SS strain; the specific activity of GSTs changed significantly when selecting the F 10 generation, and the specific activity of the Ab R 10 strain was 1.43 times higher than that of the SS strain; and the specific activity of MFO changed obviously and increased faster, indicating that MFO plays a major role in the drug resistance to avermectin, which verifies the conclusion of Shen et al.[11-12] that the enhancement of MFO is the main reason for the generation of resistance in T. urticae to avermectin. Gao et al.[17] reported that the generation of the resistance to clofentezine in T. urticae was caused by the synergistic effect of the three detoxification enzymes. Liu et al.[18] showed that the formation of the resistance against isocarbophos and fenpropathrin had a certain relationship with the three detoxification enzymes. Similarly, the author believes that the formation of avermectin resistance in T. cinnabarinus is associated with the changes in the specific activity of CarE, GSTs and MFO, but the increase in the specific activity of MFO is an important reason for the formation of resistance to T. cinnabarinus.
In this study, the indoor selection of the resistance in T. cinnabarinus was performed to the F 15 generation. If the resistance screening continues, the resistance in T. cinnabarinus will increase rapidly. With the improvement of resistance, the activity of the three detoxification enzymes in T. cinnabarinus may be changed more significantly. Therefore, this study needs to be further explored in the future, so as to provide a scientific theoretical basis for further research on resistance management.
References
[1] CHEN Q, LU FP, HUANG GX, et al. General survey and safety assessment of cassava pests[J]. Chinese Journal of Tropical Crops, 2010, (5): 819-820. (in Chinese)
[2] HE L, ZHAO ZM, DENG XP, et al. Resistance selection of Tetranychus cinnabarinus to three acaricides and lts management strategy[J]. Scientia Agricultura Sinica, 2003, 36(4): 403-408. (in Chinese)
[3] CAO XF, HE L, ZHAO ZM, et al. Study on esterase isozyme of different resistance strain in Tetranychus cinnabarinus (Boisduval)[J]. Acta Arachnologica Sinica, 2004, 13(4): 95-102. (in Chinese)
[4] MA ZQ. Study on the relationship between the poisoning symptoms and action mechanism of different pesticides[D]. Yangling: Northwest Agriculture & Forestry University, 2002. (in Chinese)
[5] SHOOP WL, MROZIK H, FISHER MH. Structure and activity of avermectins and milbemycins in animal health[J]. Veterinry Parasitology, 1995, 59(2): 139-156.
[6] CHEN WB, SUN L, YANG T, et al. Study on pesticide resistance mechanisms and resistant fitness of Tetranychus turkestani in abamectin and pyridaben[J]. Xinjiang Agricultural Sciences, 2011, 48(2): 229-235. (in Chinese)
[7] LIU YC, WANG L, ZHANG YJ, et al. Abamectin resistance and expression of resistance related genes in field population of Tetranychus urticae (Acari: Tetranychidae)[J]. Acta Entomologica Sinica, 2016, 59(11): 1199-1205. (in Chinese)
[8] SONG LW, LI MW, SHEN HM. Study on resistance selection against pyridaben, avermectin and abamectin·pyridaben in Tetranychus truncatus and its resistance stability[J]. Chinese Bulletin of Entomology, 2016, 53(1): 89-94. (in Chinese)
[9] HE L, TAN SL, CAO XF, et al. Study on resistance selection and activity of detoxification enzyme in Tetranychus cinnabarinus (boiduval)[J]. Chinese Journal of Pesticide Science, 2003, 5(4): 23-29. (in Chinese)
[10] CLAUDIANOS C, RANSON H, JOHNSON RM, et al. A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee[J]. Insect Molecular Biology, 2006, 15(5): 615-636.
[11] SHEN YF, SHEN HM, YUE XL, et al. Resistance selection of Tetranychus urticae to abamectin strain and changes in the activity of detoxification enzymes[J]. Plant Protection, 2014, 40(5): 44-48. (in Chinese)
[12] RU Y, CHEN YN, SHANG SQ, et al. Effect of sublethal dose of avermectin on the activities of detoxifying enzymes in Tetranychus urticae[J]. Journal of Gansu Agricultural University, 2017, 52(1): 87-91. (in Chinese)
[13] LI P, LIU LJ, LI HR, et al. Indoor resistance selection against 1.8% avermectin in Tetranychus cinnabarinus (Boisduval)[J]. China Tropical Agriculture, 2016, (5): 46-48. (in Chinese)
[14] VAN AK. A study of housefly esterase by means of a sensitive colorinmetric method[J]. J Inset Physiol, 1962, 8(2): 401-416.
[15] CLARK AG, DICK GL, SMITH JN. Kinetic studies on a glutathione S transferase from the larvae of Costelytra zealandica[J]. B iochem, 1984, 217: 51-58.
[16] ZHANG XY, HE J. Selection of drug resistance against avermectin B1 and cross resistance in Plutella xylostella[J]. Journal of Plant Protection, 2011, 28(2): 163-168. (in Chinese)
[17] GAO XJ, ZHANG ZG, DUAN XL, et al. Resistance selection against clofentezine in Tetranychus urticae (Koch) and change of its detoxification enzymes activity[J]. Scientia Agricultura Sinica, 2012, 45(7): 1433-1438. (in Chinese)
[18] LIU JX, HAN JC, LIU HP, et al. Study on resistance mechanisms of the Hawthorn spider mite, Tetranychus viennensis Zacher, to isocarbophos and fenpropathrin[J]. Journal of Sichuan University: Natural Science Edition, 2006, 43(6): 1364-1368. (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Study on Chemical Composition of the Ethyl Acetate Extract of Pratia
- Effects of Different Selenium Fertilizer Types on Selenium Content and Quality of “Lingfeng” Grapes
- Protoplast Culture and Its Application in Fruit Breeding
- Indexes of Tree Structure of Cylindrical Pear Orchards at the Sapling Stage
- Carbon Storage and Distribution of the Mature Pinus massoniana Plantation in Northwest Guangxi
- Pollution and Quality Control of Mycotoxins in Foods and Feeds
