miR-26a在糖尿病肾病大鼠肾脏的表达及其意义
2019-09-10张岩王祥花李明慧胡晓燕王莹马瑞霞
张岩 王祥花 李明慧 胡晓燕 王莹 马瑞霞

[摘要] 目的 探讨microRNA-26a(miR-26a)在糖尿病肾病(DN)中的表达及其与DN的关系。方法 高脂高糖喂养联合腹腔注射小剂量链脲霉素构建大鼠2型糖尿病模型(T2DM),另设正常对照(NC)组。于动物模型建立后8周收集两组大鼠血、尿及肾脏标本。比较两组血、尿生化指标,苏木精-伊红染色观察肾小球病理变化,实时定量PCR方法检测肾脏miR-26a、Nephrin mRNA表达水平,Western Blot方法测定肾脏Nephrin蛋白表达水平。结果 与NC组比较,T2DM组大鼠空腹血糖(FBG)、胆固醇(TC)、三酰甘油(TG)、内生肌酐清除率(Ccr)、24 h尿蛋白定量均明显升高(t=10.710~28.421,P<0.05)。光镜下观察,T2DM组肾小球体积较NC组增大,基底膜增厚,系膜区扩大,肾小球毛细血管腔受压变窄。与NC组比较,T2DM组Nephrin mRNA和miR-26a表达明显减少(t=9.070、12.270,P<0.05),Nephrin蛋白表达明显减少(t=7.429,P<0.05)。Pearson分析显示,miR-26a与Nephrin表达正相关(r=0.650,P<0.01)。结论 miR-26a 在DN大鼠肾组织表达降低,并与大鼠DN足细胞的损伤密切相关。
[关键词] 糖尿病肾病;足细胞;miR-26a;大鼠
[中图分类号] R587.24 [文献标志码] A [文章编号] 2096-5532(2019)04-0447-05
[ABSTRACT] Objective To investigate the expression of microRNA-26a (miR-26a) in diabetic nephropathy (DN) and its relationship with DN. Methods A rat model of type 2 diabetes mellitus (T2DM) was established by high-fat and high-sugar diet combined with intraperitoneal injection of low-dose streptozotocin, and a normal control (NC) group was also established. Blood, urine, and kidney specimens were collected at 8 weeks after the establishment of the animal model. The blood and urine biochemical parameters were compared between the two groups. Glomerular pathology was observed by HE staining. The expression of miR-26a and Nephrin mRNA was determined by qPCR, while the protein expression of Nephrin in the renal cortex was measured by Western blot. Results Compared with the NC group, the T2DM group had significantly increased fasting blood glucose, cholesterol, triglyceride, endogenous creatinine clearance, and 24 h urinary protein excretion (t=10.710-28.421,P<0.05). As observed under light microscopy, the T2DM group had a larger glomerular volume, a thicker basement membrane, an enlarged mesangial region, and a smaller glomerular capillary lumen compared with the NC group. Meanwhile, the T2DM group had significant reductions in the expression of Nephrin mRNA and miR-26a (t=9.070,12.27;P<0.05), as well as Nephrin protein (t=7.429,P<0.05), as compared with the NC group. Pearson analysis showed that miR-26a was positively correlated with the expression of Nephrin (r=0.650,P<0.01). Conclusion In rats with DN, the expression of miR-26a in renal tissue is reduced, which is closely associated with the damage of podocytes.
[KEY WORDS] diabetic nephropathies; podocytes; miR-26a; rats
糖尿病腎病(DN)是糖尿病(DM)最严重和常见的慢性并发症之一,研究显示其为导致终末期肾病(ESRD)的最常见病因[1-2]。因此,防治DN、延缓肾功能的减退,越来越受到医学界关注。足细胞、肾小球毛细血管内皮细胞与肾小球基底膜共同构成肾小球滤过屏障,足细胞在肾小球滤过功能中发挥着重要作用。足细胞病变是DN发病机制的中心环节及影响DN病人预后的主要因素之一[3-4]。高葡萄糖干预足细胞、钙离子内流增加诱发足细胞凋亡[5]。Nephrin与podocin等分子共同参与裂孔隔膜信号转导[6]。检测Nephrin的表达可以评估DN足细胞病理损伤[7]。近期有研究结果显示,microRNA-26a(miR-26a)在狼疮肾炎及IgA肾病病人肾小球足细胞中表达显著降低[8]。miRNA是高度保守的小非编码RNA家族,可以通过降解或抑制翻译下调靶蛋白的表达[9-10]。然而,目前miR-26a与DN足细胞病变的关系国内外研究甚少。本课题通过构建2型糖尿病(T2DM)模型研究miR-26a在DN中的表达水平,探讨miR-26a与DN的关系,为DN防治提供依据。现将结果报告如下。
1 材料与方法
1.1 DN大鼠模型的制备及分
清洁级SD雄性大鼠30只,体质量为180~220 g(青岛大学附属医院动物实验室提供),SPF级环境饲养,环境温度为(22±2)℃、相对湿度45%~65%,每日光照12 h。随机分为正常对照组(NC组,n=10)和T2DM组(n=20)。NC组常规饲料喂养;T2DM组给予高脂高糖饮食(Research Diet公司,USA,每100 g饲料含:常规饲料66.5 g,蔗糖20.0 g,猪油10.0 g,胆固醇2.5 g,胆酸盐1.0 g)喂养8周后,联合小剂量链脲佐菌素(30 mg/kg,Sigma,USA)一次性腹腔注射1周后,于禁食不禁水12 h后鼠尾静脉取血,测定空腹血糖(FBG)及空腹胰岛素(FINS),并计算胰岛素敏感指数(ISI)。以FBG≥NC組大鼠FBG均值+3个标准差(血糖≥10.0 mmol/L)及ISI≤NC组大鼠的均值为T2DM模型建立成功[11-12]。
1.2 血液和尿液生化指标检测
T2DM造模成功后,继续高脂喂养8周,用代谢笼收集大鼠24 h尿液以测定清蛋白和肌酐,测鼠尾收缩压(SBP)并采集大鼠尾静脉血。参照相关文献方法[11],检测大鼠血标本FBG、胆固醇(TC)、三酰甘油(TG)、低密度脂蛋白(LDL)、高密度脂蛋白(HDL)、血肌酐(Scr),尿液标本尿肌酐(Ucr)、24 h尿蛋白定量。根据Ucr、Scr计算内生肌酐清除率(Ccr)[13]。Ccr=Ucr/Scr×24 h尿量。用ELISA检测试剂盒(Novus公司,美国)检测FINS,按说明书方法操作,用酶标仪检测450 nm波长处吸光度。
1.3 大鼠肾脏组织病理检查
处死大鼠,取部分肾皮质用40 g/L多聚甲醛固定,梯度乙醇脱水,二甲苯透明,浸蜡包埋,4 μm切片,苏木精-伊红(HE)染色后光镜下观察肾组织病理学变化。
1.4 实时定量PCR检测Nephrin mRNA表达
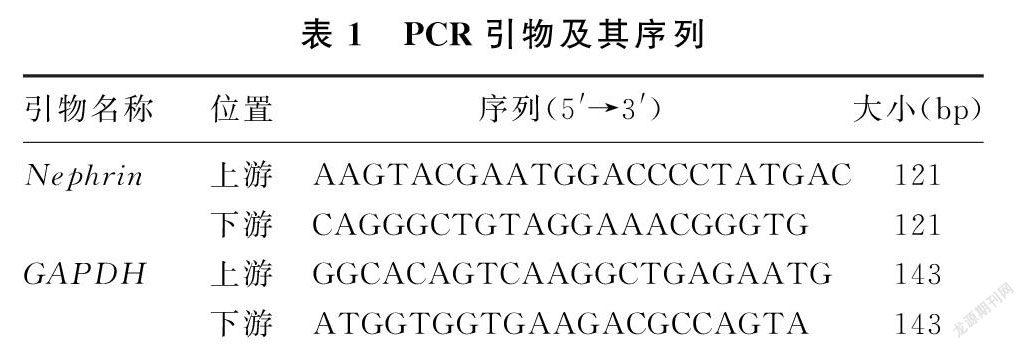
采用TRIZOL试剂(TaKaRa公司,日本)提取肾皮质总RNA,通过紫外线分光光度计检测RNA的浓度和纯度。应用RNA反转录逆转录试剂盒(TaKaRa公司,日本)合成cDNA。以GAPDH作为内参照,应用实时荧光定量PCR仪(美国ABI 7500)进行PCR扩增,引物由TaKaRa公司合成,引物的序列见表1。实时定量PCR反应条件为:95 ℃、10 min,95 ℃、30 s,95 ℃、5 s,60 ℃、30 s,扩增45个循环。用2-△△CT计算相对表达量,△△CT=(CT实验组目的-CT实验组内参)-(CT对照组目的-CT对照组内参)。每个实验组均重复检测3次。
裂解肾皮质,用BCA试剂盒测定蛋白浓度,蛋白煮沸变性。取30 μg总蛋白,行十二烷基硫酸钠聚丙烯酰胺凝胶电泳,转膜,室温下以5 g/L脱脂奶粉封闭1 h,加一抗摇匀后置于4 ℃冰箱内孵育过夜。一抗Nephrin(1∶1 000,美国Novus),一抗GAPDH(1∶2 000,英国Abcam)。TBST洗膜3×12 min后加入二抗(1∶10 000,中国Elabscience)室温摇床孵育1 h。电化学发光液(ECL)显色并扫描结果。所有实验均进行3次。用Image J软件分析实验条带。
1.6 统计学分析
应用SPSS 22.0软件进行统计学分析,符合正态分布的计量资料结果用±s表示,两组数据比较用t检验;相关性分析用Pearson分析。P<0.05为差异有统计学意义。
2 结 果
2.1 两组大鼠一般情况比较
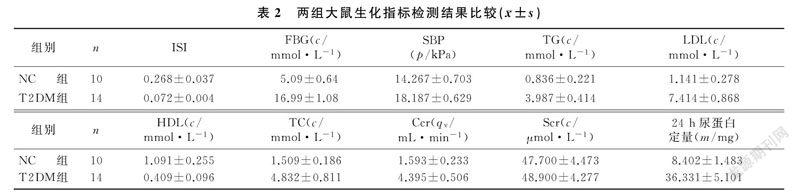
本文14只大鼠T2DM造模成功,造模成功率70%。T2DM组大鼠的FBG、TC、TG、SBP、LDL、Ccr、24 h尿蛋白定量较NC组明显升高,ISI、HDL显著低于NC组,差异有统计学意义(t=5.387~28.421,P<0.05);而两组间Scr差异则无统计学意义(P>0.05)。见表2。
2.2 两组肾脏组织病理比较
NC组大鼠肾小球结构完整、清晰,并无明显异常。
4期张岩,等. miR-26a在糖尿病肾病大鼠肾脏的表达及其意义449
与NC组比较,T2DM组大鼠肾小球体积增大,基底膜增厚,系膜区扩大,肾小球毛细血管腔受压变窄。
2.3 两组Nephrin mRNA及miR-26a表达比较
与NC组相比较,T2DM组Nephrin mRNA、miR-26a表达量减少(t=12.270、9.070,P<0.05),见表3。Pearson分析表明,miR-26a与Nephrin表达正相关(r=0.65,P<0.01)。
2.4 两组Nephrin蛋白表达比较
与NC组比较,T2DM组Nephrin蛋白表达量减少(t=7.429,P<0.05)。见图1、表3。
3 讨 论
DN为1型和2型DM的严重并发症之一[14],最终可发展为ESRD[1,15]。目前关于DN发病机制尚不明确。足细胞是附着在肾小球基底膜外侧的终末分化的上皮细胞,是肾小球滤过屏障的重要组成部分[16]。高糖通过刺激NADPH氧化酶和线粒体途径增加细胞内活性氧的产生,刺激钙调磷酸酶TRPC6激活,促使凋亡基因的活化导致足细胞凋亡,并且是导致小鼠1型DM和T2DM的早期病理机制[17]。DKD大鼠模型肾脏足细胞钙调磷酸酶激活和活性氧增多可刺激TRPC6诱导的足细胞凋亡[18],而抑制TRPC6可降低NFAT mRNA改善高葡萄糖诱导的足细胞损伤,保护肾小球滤过屏障完整性[19]。有研究表明,雷公藤多甙通过上调自噬和下调β-arrestin-1改善足细胞损伤,延缓小鼠DN发生[20]。国外有研究证实,足细胞凋亡是DN发病机制中的中心环节[3-4]。
Nephrin与podocin等分子共同参与足细胞裂孔隔膜信号转导[5]。Nephrin的表达变化可以用来评估大鼠DN病变及足细胞的损伤[7]。本文实验结果显示,T2DM组的造模成功率为70%,T2DM组FBG、TC、TG、SBP、LDL、24 h尿蛋白定量较NC组大鼠均明显升高,伴胰岛素抵抗;与NC组比较,T2DM组肾脏组织出现病理改变,Nephrin基因和蛋白表达降低,提示DN肾脏组织病理损伤。有研究显示,miR-26a在自身免疫性肾小球肾炎小鼠以及IgA肾病病人肾小球足细胞中表达显著降低[8],并且与非DN相关的肾小球足细胞损伤密切相关。miRNA是高度保守小非编码RNA家族,miRNA与靶mRNA的3′非翻译区(3′UTR)配对结合从而负向调节靶基因的表达,可通过降解或抑制翻译下调靶蛋白[9-10]。國内外研究结果证实,miRNA参与多种肾脏疾病的发生发展,miR-23b在1型DM组及T2DM组外周血及肾脏组织中的表达下降[21];miR-146a在急性肾损伤组织的表达水平降低[22]。在人和小鼠肾脏组织miR-26a呈高水平特异性表达[23],足细胞是小鼠肾脏中miR-26a表达的主要位点。目前国内外关于miR-26a与DN病变关系的研究甚少。本文研究结果表明,T2DM组miR-26a表达降低,并且miR-26a与Nephrin表达正相关,提示miR-26a表达降低与大鼠DN病变及足细胞的损伤密切相关。ICHII等[8]的研究显示,miR-26a在小鼠自身免疫性肾小球肾炎中表达降低并与足细胞的损伤紧密相关,本文结果与其相一致。另有研究结果表明,miR-217通过靶向调节TNFSF11改变人足细胞的凋亡,是膜性肾病一种有用的诊断生物标志物[24]。尿miR-30c-5p和miR-192-5p为缺血再灌注诱导的肾损伤的潜在生物标志物[25]。尿miR-377和miR-216a也是DN风险的生物标志物[26]。
因此推测miR-26a可能对DN诊断有一定价值,但需进一步研究证明。
综上所述,miR-26a在DN大鼠肾组织表达降低,并与大鼠DN病变及足细胞的损伤密切相关。目前,国外已有学者研究miR-26a在狼疮型肾小管间质炎症、肾衰竭、免疫性肾病等疾病表达变化及作用机制[8,24]。本实验研究miR-26a与DN足细胞损伤的关系,在国内外研究甚少。本课题后续将进一步深入研究miR-26a在DN足细胞损伤中的作用机制,为DN的治疗提供依据。
[参考文献]
[1] GUO Yinfeng, SONG Zhixia, ZHOU Min, et al. Infiltrating macrophages in diabetic nephropathy promote podocytes apoptosis via TNF-alpha-ROS-p38MAPK pathway[J]. Oncotarget, 2017,8(32):53276-53287.
[2] WANG Xiaodan, GAO Lihui, LIN Hua, et al. Mangiferin prevents diabetic nephropathy progression and protects podocyte function via autophagy in diabetic rat glomeruli[J]. European Journal of Pharmacology, 2018,824(4):170-178.
[3] SHI Jianxia. Glucagon-like peptide-1 protects mouse podocytes from high glucose-induced apoptosis and inhibits the production of reactive oxygen species and the secretion of pro-inflammatory cytokines by sirtuin 1 activation in vitro[J]. Molecular Medicine Reports, 2018,18(2):1789-1797.
[4] WANG Liming, JING Yonggang, BAI Yi. Podocyte-specific knockout of cyclooxygenase 2 exacerbates diabetic kidney di-sease[J]. American Journal of Physiology-renal Physiology, 2017,313(2): F430-F439.
[5] SZABO T, AMBRUS L, ZAKANY N, et al. Regulation of TRPC6 ion channels in podocytes Implications for focal segmental glomerulosclerosis and acquired forms of proteinuric diseases[J]. Acta Physiologica Hungarica, 2015,102(3):241-251.
[6] LI Sutong, LIU Xiaoxia, LEI Jie, et al. Crocin protects podocytes against oxidative stress and inflammation induced by high glucose through inhibition of NF-kappa B[J]. Cellular Physio-logy and Biochemistry, 2017,42(4):1481-1492.
[7] NEW L A, MARTIN C E, SCOTT R P, et al. Nephrin tyrosine phosphorylation is required to stabilize and restore podocyte foot process architecture[J]. Journal of the American Society of Nephrology, 2016,27(8):2422-2435.
[8] ICHII O, OTSUKA-KANAZAWA S, HORINO T, et al. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis[J]. PLoS One, 2014,9(10): e110383-e110388.
[9] NEUMANN A, NAPP L C, KLEEBERGER J A, et al. MicroRNA 628-5p as a novel biomarker for cardiac allograft vasculopathy[J]. Transplantation, 2017,101(1): E26-E33.
[10] NEAULT M, COUTEAU F, BONNEAU , et al. Molecular regulation of cellular senescence by microRNAs:implications in cancer and age-related diseases[J]. International Review of Cell&Molecular Biology, 2017,334(5):27-29.
[11] MA Ruixia, LIU Liqiu, JIANG Wei, et al. FK506 ameliorates podocyte injury in type 2 diabetic nephropathy by down-regulating TRPC6 and NFAT expression[J]. International Journal of Clinical and Experimental Pathology, 2015,8(11):14063-14074.
[12] ZHANG Li, ZHANG Qianmei, LIU Shuangxin, et al. DNA methyltransferase 1 may be a therapy target for attenuating diabetic nephropathy and podocyte injury[J]. Kidney International, 2017,92(1):140-153.
[13] 武國华,马瑞霞,张伟,等. 他克莫司对2型糖尿病大鼠足细胞氧化应激的影响[J]. 中华肾脏病杂志, 2016,32(4):300-301.
[14] ZHANG Yinghui, BING Wang, FENG Guo, et al. Involvement of the TGFβ1-ILK-Akt signaling pathway in the effects of hesperidin in type 2 diabetic nephropathy[J]. Biomedicine & Pharmacotherapy, 2018,105(6):766-772.
[15] TENG B N, DUONG M, TOSSIDOU I, et al. Role of protein kinase C in podocytes and development of glomerular damage in diabetic nephropathy[J]. Frontiers in Endocrinology, 2014,5(3):179-183.
[16] WANG Yao, LI Han, SONG Shuping. Beta-Arrestin 1/2 aggravates podocyte apoptosis of diabetic nephropathy via Wnt/beta-Catenin pathway[J]. Medical Science Monitor, 2018,24(3):1724-1732.
[17] LU H, LI Y, ZHANG T, et al. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upre-gulating heme oxygenase-1 (HO-1) expression[J]. Med Sci Monit, 2017,23(8):4067-4076.
[18] YAO Xingmei, LIU Yujun, WANG Yunman, et al. Astragaloside Ⅳ prevents high glucose-induced podocyte apoptosis via downregulation of TRPC6[J]. Molecular Medicine Reports, 2016,13(6):5149-5156.
[19] ZHAI Limin, GU Junfei, DI Yang, et al. Metformin ameliorates podocyte damage by restoring renal tissue podocalyxin expression in type 2 diabetic rats[J]. Journal of Diabetes, 2015,9(5):231825-231831.
[20] ZHAN Huifang, JUAN Jin, LIANG Shikai, et al. Triptery-gium glycoside protects diabetic kidney disease mouse serum-induced podocyte injury by upregulating autophagy and downre-gulating β-arrestin-1[J]. Histology and Histopathology, 2019,6(3):18097-18102.
[21] ZHAO Binghai, LI Hongzhi, LIU Jieting, et al. MicroRNA-23b targets Ras GTPase-activating protein SH3 Domain-binding protein 2 to alleviate fibrosis and albuminuria in diabetic nephropathy[J]. Journal of the American Society of Nephrology: JASN, 2016,27(9):2597-2608.
[22] DING Ying, GUO Feng, ZHU Tao, et al. Mechanism of long non-coding RNA MALAT1 in lipopolysaccharide-induced acute kidney injury is mediated by the miR-146a/NF-κB signaling pathway[J]. International Journal of Molecular Medicine, 2018,41(1):446-454.
[23] KOGA K, YOKOI H, MORI K, et al. MicroRNA-26a inhi-bits TGF-β-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy[J]. Diabetologia, 2015,58(9):2169-2180.
[24] LI Jing, LIU Bin, XUE Hen, et al. miR-217 is a useful diagnostic biomarker and regulates human podocyte cells apoptosis via targeting TNFSF11 in membranous nephropathy[J]. BioMed Research International, 2017,32(3):1-9.
[25] ZOU Yanfang, WEN Dan, ZHAO Qian, et al. Urinary MicroRNA-30c-5p and MicroRNA-192-5p as potential biomarkers of ischemia-reperfusion-induced kidney injury[J]. Experimental Biology and Medicine, 2017,242(6):657-667.
[26] EL-SAMAHY M H, ADLY A A, ELHENAWY Y I, et al. Urinary miRNA-377 and miRNA-216a as biomarkers of nephropathy and subclinical atherosclerotic risk in pediatric patients with type 1 diabetes[J]. Journal of Diabetes and Its Complications, 2018,32(2):185-192.
(本文編辑 黄建乡)
