Quality Analysis of Lycium ruthenicum Murr. from Different Producing Areas
2019-09-10LeiPANGBinwuZHANGJianlongLUYanhuaRENLixinZHANGXiangGUIYueMALuHAOYuanyuanFANHaimeiWUZhangxiangXIA
Lei PANG Binwu ZHANG Jianlong LU Yanhua REN Lixin ZHANG Xiang GUI Yue MA Lu HAO Yuanyuan FAN Haimei WU Zhangxiang XIA
Abstract [Objectives] The quality of Lycium ruthenicum Murr. from different producing areas was studied.
[Methods] The content of proanthocyanidins (UV spectrophotometry), watersoluble vitamins (VB2, VB6, VB12, Vc, niacinamide, and folic acid) ), fatsoluble vitamins (VD, and VK1) (high performance liquid chromatography), and trace elements Cu, Zn, and Fe (flame atomic absorption method) in L. ruthenicum from Ejin Banner of Inner Mongolia, Nuomuhong of Qinghai and Korla of Xinjiang were measured.
[Results] The content of proanthocyanidins, VB2, nicotinamide and VB12in L. ruthenicum from Ejin Banner was higher, and the content of Vc, VB6, Cu, Zn, VD and VK1 in L. ruthenicum from Nuomuhong was higher, while the content of folic acid and Fe in L. ruthenicum from Korla was higher. The proanthocyanidin content of L. ruthenicum from Ejin Banner was the highest, so it is an ideal plant for extracting proanthocyanidins.
[Conclusions] This study provides a theoretical basis for the research, development and utilization of L. ruthenicum from Nuomuhong, Ejin Banner and Korla.
Key words Lycium ruthenicum Murr.; Proanthocyanidins; Vitamins; Trace elements; Northwest China
Lycium ruthenicum Murr. belongs to the genus Lycium of Solanaceae[1], and the fruit is berry, purpleblack, and globular. It is a unique wild plant that needs to be developed in the desert areas of northwest China[2]. L. ruthenicum is an excellent wild soil and water conservation plant that has shelter forest value, greening value and medicinal value, so it has both ecological and economic benefits[3]. It also plays an important role in greening wasteland and preventing soil erosion. As an excellent plant resource for controlling the ecological environment of the western desert areas, it is one of the alternative plants for desertification control. In some regions of northern China, people also use the fruits of L. ruthenicum to dye cloth, and the fruits can be used to make health drinks. The research and development of L. ruthenicum has certain market base and economic value, and the prospects will be very broad[4].
Proanthocyanidins (PC) are the general term for polyphenolic compounds widely found in plants. They are widely found in vegetative organs such as flowers and fruits and the cytoplasm of vegetative organs such as roots, stems and leaves, and are one of the main pigments that constitute flowers and fruits of plants[5]. Since 1986, China has developed proanthocyanidins, and has undergone more than 30 years of research and development[6]. For plants, proanthocyanidins have the physiological functions of resisting pests and diseases, resisting ultraviolet rays, and regulating seed dormancy and germination, and can affect the digestibility, palatability and health value of crops[7]. Proanthocyanidins have a strong antioxidation effect, can effectively eliminate a large number of free radicals[8], and protect lipids from peroxidation damage. Besides, they can also protect and stabilize vitamin C (Vc), and help the absorption and utilization of vitamin C. As the main antioxidant in plants, vitamin C is found in most plant tissues. In plant antioxidant stress, Vc can directly react with reactive oxygen species such as OH-, O-3 and H2O2. As a coenzyme of many important enzymes in plants, Vc participates in the synthesis of secondary metabolites and plant hormones and hydroxylation of some amino acid residues. Recent studies have shown that vitamin C in plant leaves can play a role in regulating plant defense gene expression[9]. Folic acid (VB9) is a coenzyme of onecarbon unit transferase in biochemical reactions in plants. The main biochemical reactions involved are the synthesis of purines and pyrimidines, the synthesis of DNA and RNA, the synthesis of methyl compounds, and amino acid metabolism[10]. Vitamin B2 is also called riboflavin. The main biochemical reactions involved are the conversion of purines, the oxidation of amino acids and lipids, the synthesis of proteins and certain hormones, and the transport, storage and mobilization of iron, and the metabolism of folic acid, VB6, niacin and pyridoxal is related to VB2. Niacinamide is an amide of niacin (VB3). Niacinamide can be converted from niacin in the body. Nicotinamide, a component of coenzyme I and coenzyme II, plays a role in hydrogen transport in the biooxidation respiratory chain, promotes tissue metabolism and biooxidation, and plays an important role in maintaining the integrity of normal tissues[11]. Vitamin B6, also known as pyridoxine, is not resistant to high temperatures and is easily damaged by light or alkali. The biochemical reactions that VB6 is involved in include protein synthesis and catabolism, amino acid, VB12and folate metabolism, nucleic acid and DNA synthesis, and so on[12]. Vitamin B12, also known as cobalamin, is the only vitamin that contains metal elements. It is easily soluble in water and ethanol, is the most stable in weak acid (pH is 4.5-5.0), and can be decomposed in strong acid or alkaline solution[13]. The main biochemical reactions involved include the synthesis of methionine and thymine, the transfer and storage of folic acid, the metabolism of carbohydrates, fats and proteins, and the biosynthesis of amino acids and nucleic acids. Vitamin K is one of fatsoluble vitamins. Two natural vitamins K1 and K2 have been found, of which VK1 is an oil extracted from plants and VK2 is a crystal obtained from animals. VK1 is chemically stable and resistant to acids and heat, but is sensitive to light and easily decomposed by alkali and ultraviolet light. Vitamin D is a sterol derivative, and can resist rickets. Vitamin D is a fatsoluble vitamin found in some natural foods. As one of the micronutrients necessary for plant growth, zinc can enhance respiration and photosynthesis and stimulate cell viability to improve the saline and alkaline tolerance, disease resistance, stress resistance, cold tolerance and drought tolerance of fruits. Zinc can indirectly affect the synthesis of auxin and is closely related to the photosynthesis of plants; the photochemical reaction of chlorophyll may be catalyzed by zinc[14]. Iron is an essential element in the formation of chlorophyll. However, chlorophyll itself does not contain iron, but chlorophyll can not form when iron is deficient, and it will cause "green deficiency"[15], and affect the growth and development of L. ruthenicum. Iron is also a component of many oxidases such as cytochrome oxidase, succinate dehydrogenase, and catalase[16], and affects respiration and ATP formation. Copper is a component of various oxidases such as polyphenol oxidase and ascorbate oxidase in plants, and affects the redox process and respiration of plants. Copper plays an important role in many enzymes of chloroplasts and can affect photosynthesis of plants and desaturation and hydroxylation of fatty acids.
Materials and Methods
Materials
L. ruthenicum used in this experiment was collected from Ejin Banner of Inner Mongolia, Nuomuhong of Qinghai and Korla of Xinjiang.
Ejina Banner is located in the north of China and at the westernmost end of Inner Mongolia Autonomous Region. The altitude of the area is about 1 000 m on average, and it is between 1 200 and 1 400 m in most areas. It has an inland dry climate with large evaporation, abundant sunshine, drought and little rain, large temperature difference, and frequent wind and sand. The annual average evaporation is 3 841.51 mm, and the annual average precipitation is only 37 mm. The annual average temperature is 8.3 ℃, and the annual sunshine hours are more than 3 400 h. The frostfree period is generally between 179 and 227 d.
Nuomuhong is located in the west of Qinghai Province and in the hinterland of the Qinghai-Tibet Plateau. It is one of the most important "oasis agriculture" areas in the Qaidam Basin. The natural resources in Nuomuhong are unique, with strong sunlight, long sunshine hours, sufficient water resources and fertile soil. It has a plateau arid continental climate. The annual evaporation is 2 800 mm, while rainfall is only 40 mm. The daily average temperature is 4.3 ℃, and the frostfree period is between 90 and 120 d. The annual average sunshine is 3 250 h, and the temperature difference between day and night is large.
Korla, the provincial capital of Bayingol Mongolian Autonomous Prefecture of Xinjiang, is situated on the edge of the Tarim Basin. It is rich in light, heat, water and soil resources. The climate is mild, and the soil is fertile. It has a warm temperate continental arid climate. The annual average precipitation is only 58.6 mm, while the annual maximum evaporation is 2 788.2 mm. The total sunshine duration is 2 990 h, and the annual average frostfree period is 210 d. The annual average temperature is 11.4 ℃, and the lowest temperature is only -28 ℃.
Experimental design
In this experiment, the content of proanthocyanidins, watersoluble vitamins (VB2, VB6, VB12, Vc, nicotinamide, and folic acid), fatsoluble vitamins (VD and VK1), trace elements (Cu, Zn, and Fe) in the L. ruthenicum collected from different producing areas was determined.
Collection of meteorological factors: meteorological data collected by small weather stations were adopted.
Measurement items and methods
Sample processing
Before the experiment, the stems of of L. ruthenicum fruits collected from different producing areas were removed, and the fruits were sealed and preserved.
Determination of proanthocyanidin content——UV spectrophotometry
At room temperature, 10.0 mg of proanthocyanidin standard sample was accurately weighed, to which a certain amountof methanol solution (analytically pure) was added, and the mixture was put in a 10 ml volumetric flask. The volumetric flask was shaken well to get 1 mg/ml standard solution. Afterwards, 0, 0.1, 0.25, 0.5, 1.0, and 1.5 ml of the solution was placed in a 10 ml volumetric flask respectively, and standard application solutions were obtained. 1.0 ml of each of the standard application solutions was placed in a cuvette with a plug, to which 0.2 ml of 2% ammonium ferric sulfate solution (water was added to 2 g of ammonium ferric sulfate until the volume was up to 100 ml) and 6 ml of 95% nbutanol solution (a mixture of nbutanol and hydrochloric acid in a volume ratio of 95∶5) were added. After being shaken well, the cuvettes were heated in a boiling water bath for 30 min, and then cooled for 15 min. The absorbance (A) of the above solutions was measured at 550 nm[17]. Linear regression analysis between absorbance (A) and concentration (C) was conducted to obtain a linear regression equation: y(A)=0.002 2x(C)-0.029 3.
At first, 2.000 gof each of the samples was accurately weighed and placed in a 50 ml volumetric flask, to which 35 ml of methanol was added. After the solutions were processed in ultrasound for 20 min, they cooled to room temperature, to which methanol was added until constant volume. The volumetric flasks were shaken well and stood until the solutions were clarified. The supernatant was collected for use, and the absorbance (A) was measured at 550 nm. The concentration (C) in L. ruthenicum was calculated according to the linear regression equation.
Proanthocyanidin content (mg/g)=C×V×1 000M
where C is the concentration of proanthocyanidins in the samples (μg/ml); V is the total volume of the sample solutions (50 ml); M is sample weight (g).
Determination of watersoluble vitamin content——high performance liquid chromatography (HPLC)
At room temperature, the standard samples of watersoluble vitamins (VB2, Vc, folic acid, VB6, VB12, and nicotinamide) were added to a certain amount of ultrapure water to make up 1.0×10-5g/ml mother liquors. The sample measurement conditions were determined by detection of the standard samples.
Firstly, 1.000 g of L. ruthenicum was weighed and ground in a mortar with liquid nitrogen. It was placed in a 100 ml small beaker, to which 30 ml of 0.2 mol/L hydrochloric acid solution was added, and the mixture was stirred well. After ultrasonic extraction was performed for 30 min, and the solution cooled to room temperature. After suction filtration by pressure reduction, the volume was increased to 50 ml. The samples were diluted 10-5times and assayed using an Agillent 1100 liquid chromatograph[18]. Standard samples with different concentration gradients were prepared according to the interval of peak area of the detected samples. Linear regression analysis between peak area (Area) and concentration (C) was performed, and a linear regression equationwas obtained as follows: VB2: y(Area)=77.848x(C)+128.28; Vc: y(Area)=424.9x(C)-338.03; folic acid: y(Area)=18.329x(C)+7.813 9; VB6: y(Area)=69.535x(C)+1 378.5; VB12: y(Area)=112.51x(C)-65.465; nicotinamide: y(Area)=744.68x(C)+445.27.
The reagents used were glacial acetic acid (analytically pure), sodium acetate (analytically pure), VB2 (standard sample), VB6 (standard sample), VB12(standard sample), Vc (standard sample), nicotinamide (standard sample), folic acid (standard sample) and ultrapure water. The chromatographic column was ZORBAX Eclipse plus C18 column, and the mobile phase was sodium acetateacetate buffer (pH was 5.8). Within 0-5 min, the ratio was 15∶85, and the flow rate was 1.0ml/min. Within 5-15 min, the ratio was 30∶70, and the flow rate was 1.0 ml/min. The ultraviolet detection wavelength of nicotinamide, VB6 and VB12was 218 nm, that of folic acid, VB2 and Vc was 265 nm. The injection volume was 20 μl.
Agricultural Biotechnology2019
Watersoluble vitamin content (mg/g)=C×V×1 000M×10
where C is the concentration of watersoluble vitamins in the samples (μg/ml); V is the total volume of the sample solutions (50 ml); M is sample weight (g).
Determination of fatsoluble vitamin content——high performance liquid chromatography (HPLC)
At room temperature, the standard samples of fatsoluble vitamins (VD and VK1) were added to a certain amount of methanol (chromatographically pure) to make up 1.0×10-5g/ml mother liquors. The sample measurement conditions were determined by detection of the standard samples.
At first, 5.000 g of each of the samples was accurately weighed and ground in a mortar with liquid nitrogen, and the powder was placed in a conical flask. Secondly, 5 ml of 10% ascorbic acid and 10 ml of 50% KOH were added to the conical flask, and they were mixed well. Further, 30 ml of ethanol (dealdehyding) was added to the conical flask, and the mixture was heated for 40 minin a boiling water bath to complete saponification. The saponified supernatant was transferred into a separatory funnel, and the saponified bottle was washed several times with 50 ml of water, and the washing liquid was incorporated into the separatory funnel. Next, the saponified bottle was washed three times with 50 mlof diethyl ether, and the washing liquid was incorporated into the separatory funnel. The separatory funnel was shaken and the extract was allowed to stand for stratification. The ether layer was retained and extracted once more with 30 ml of diethyl ether. Finally, the ether layer was washed with 50 ml of water. This step was repeated until the water was not alkaline (phenolphthalein indicator was not red), and the water was carefully drained to leave the ether layer. The ether layer was filtered into a spherical evaporating flask (250-300 ml) through anhydrous, and the separatory funnel was rinsed three times with about 10 ml of ether, and the ethersolutions were merged in the spherical flask. In a water bath at 55 ℃, the ether was evaporated to dryness under reduced pressure, and it was immediately transferred to a 5 ml volumetric flask, to which methanol was added until the volume was up to 10 ml, and they were mixed well[19]. The solution was passed through a 0.45 μm filter for use. The samples were diluted 10-5times and assayed using an Agillent 1 100 liquid chromatograph[18]. Standard samples with different concentration gradients were prepared according to the interval of peak area of the detected samples. Linear regression analysis between peak area (Area) and concentration (C) was performed, and a linear regression equationwas obtained as follows: VD: y(Area)=38.971x(C)+3.915 2; VK1: y(Area)=283.85x(C)-0.282 8.
The reagents used were VD (standard sample), VK1 (standard sample), ascorbic acid, KOH, ethanol (dealdehyding), diethyl ether, phenolphthalein indicator, and methanol (chromatographically pure). The chromatographic column was ZORBAX EclipseDBC18 column. The mobile phase was methanol (chromatographically pure), and ultrasonic degassing was conducted before use. The ultraviolet detection wavelength was 270 nm, and the flow rate was 1.0 ml/min.
Fatsoluble vitamin content (μg/g)=C×VM×100
where C is the concentration of fatsoluble vitamins in the samples (μg/ml); V is the total volume of the sample solutions (50 ml); M is sample weight (g).
Determination of trace element content——flame atomic absorptionmethod At room temperature, the 1 mg/ml standard solutions of Cu, Zn and Fe were diluted to 10 μg/ml standard mother liquors. The mother liquors were diluted to various concentration gradients (Cu: 0.05, 0.1, 0.15, 0.2, and 0.25 μg/ml; Zn: 0.05, 0.1, 0.15, 0.2, and 0.25 μg/ml; Fe: 0, 2.5, 5.0, 7.5, and 10 μg/ml). Linear regression analysis between absorbance (A) and concentration (C) was conducted to obtain a linearregressionequation: Cu: y(A)=2.158 3x(C)-0.000 2; Zn: y(A)=1.174x(C)-0.005 9; Fe: y(A)=0.078 4x(C)+0.008 4.
L. ruthenicum was washed and dried at 105 ℃. After being smashed, it was placed in a small wildmouth bottle and stored in a dryer. 2.000 g of the dried L. ruthenicum was accurately weighed and put in a crucible. After being carbonized, it became ash at high temperature. After it cooled, 5 ml of HNO3 was added to it. It was carefully boiled and then cooled. The solution was filtered into a 100 ml volumetric flask. The crucible was washed several times with distilled water, and the washing solutions were mixed into the filtrate. Finally, it was diluted to the mark with distilled water and mixed for use, while a blank solution is made at the same time[20].
Results and analysis
Proanthocyanidin content
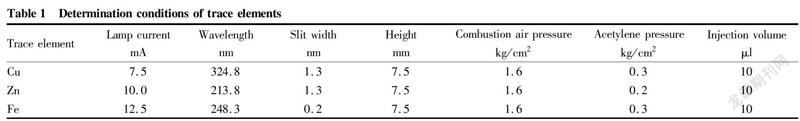
In this experiment, the proanthocyanidin content of L. ruthenicum from different producing areas and the environmental factors affecting the synthesis of proanthocyanidins were mainly studied. Correlation analysis showed that the correlation coefficient between the proanthocyanidin content and sunshine hours in different producing areas was 0.896**(P<0.01). The proanthocyanidin content of L. ruthenicum from Nuomuhong, Ejina Banner and Korla was 21.31, 40.68 and 14.36 mg/g respectively. Seen from Fig. 1, the proanthocyanidin content of L. ruthenicum from Ejina Banner was significantly higher than that of L. ruthenicum from the other two producing areas. The proanthocyanidin content of L. ruthenicum from Nuomuhong was significantly higher than that of L. ruthenicum from Korla.
Light plays an important role in the regulation of plant growth and development. It is also one of the most important environmental factors affecting the synthesis of proanthocyanidins. In most plants, the synthesis of proanthocyanidins requires light induction, and light quality, light intensity, and illumination time all affect the synthesis and accumulation of anthocyanins[5]. The annual sunshine hours of Ejina Banner are more than 3 400 h, and the annual average sunshine hours of Nuomuhong is 3 250 h, while the total annual sunshine hours of Korla is 2 990 h. The sunshine hours of Ejina Banner is more than that of Nuomuhong and Korla, so the proanthocyanidin content of L. ruthenicum from Ejina Banner was significantly higher than that of L. ruthenicum from Nuomuhong and Korla.
Watersoluble vitamin content
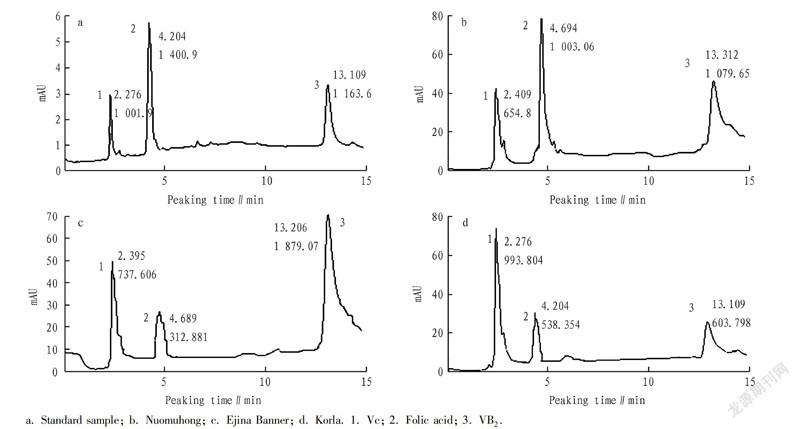
The watersoluble vitamins measured at 265 nm were Vc, folic acid, and VB2, and the separation effect of these three vitamins was satisfactory within 15 min.
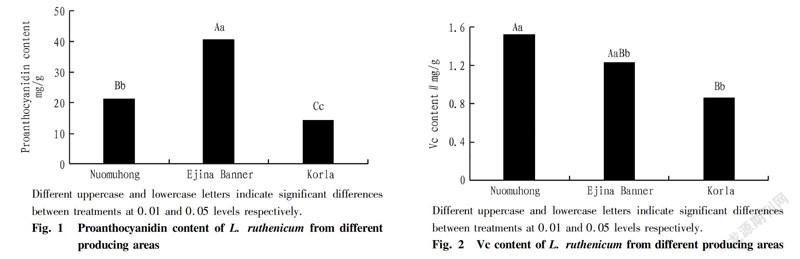
The Vc content of L. ruthenicum from Nuomuhong, Ejina Banner and Korla was 1.53, 1.23 and 0.86 mg/g respectively. As shown in Fig. 2, the Vc content of L. ruthenicum from Nuomuhong was significantly higher than that of L. ruthenicum from Korla. The Vc content of Ejina Banner was between that of Nomhong and Korla, but there was no significant difference.
The folic acid content of L. ruthenicum from Ejina Banner, Korla and Nuomuhong was 8.89 , 24.75, and 14.22 mg/g respectively. Seen from Fig. 3, the folic acid content of L. ruthenicum from Ejina Banner was significantly lower than that of L. ruthenicum from the other two producing areas. The folic acid content of L. ruthenicum from Korla was significantly higher than that of L. ruthenicum from Nuomuhong.
The VB2 content of L. ruthenicum from Ejina Banner, Korla and Nuomuhong was 10.85 ,5.51 and 3.32 mg/g respectively. As shown in Fig. 4, the VB2 content of L. ruthenicum from Ejina Banner was significantly higher than that of L. ruthenicum from the other two producing areas. There was no significant difference between Korla and Nuomuhong in terms of VB2 content of L. ruthenicum.
The differences in the Vc, folic acid and VB2 content of L. ruthenicum from Nuomuhong, Ejina Banner, and Korla may be related to local climate and natural environmental conditions. The specific reasons need further study.
The watersoluble vitamins measured at 218 nm were niacinamide, VB6, and VB12, and the separation effect of these three vitamins was satisfactory within 10 min.
Seen from Fig. 5, the separation effect of Vc, folic acid and VB2 in L. ruthenicum from Korla was the best, and the Vc content of L. ruthenicum from Korla was the highest. The folic acid content of L. ruthenicum from Korla was the highest. The VB2 content of L. ruthenicum from Ejina Banner was the highest.
As shown in Fig. 6, the L. ruthenicum from Nuomuhong contains less niacinamide and VB12and more VB6. The nicotinamide content of L. ruthenicum from Nuomuhong, Ejina Banner and Korla was 0.76, 9.44 and 8.23 mg/g respectively. Correlation analysis showed that the correlation coefficient between nicotinamide content and the producing areas was 0.922**(P<0.01).
As shown in Fig. 7, the nicotinamide content of L. ruthenicum from Nuomuhong was significantly lower than that of L. ruthenicum from Ejina Banner and Korla, and that of L. ruthenicum from Ejina Banner was significantly higher than that of L. ruthenicum from Nuomuhong.
The VB6 content of L. ruthenicum from Nuomuhong, Ejina Banner and Korla was 16.69, 8.40 and 3.04 mg/g respectively. Correlation analysis showed that the correlation coefficient between VB6 content and the producing areas was -0.846**(P<0.01). As shown in Fig. 8, the VB6 content of L. ruthenicum from Nuomuhong was significantly higher than that of L. ruthenicum from Ejina Banner and Korla, and that of L. ruthenicum from Korla was significantly lower than that of L. ruthenicum from Nuomuhong and Ejina Banner.
The VB12content of L. ruthenicum from Nuomuhong, Ejina Banner and Korla was 4.23 , 35.35 and 6.09 mg/g respectively. Correlation analysis revealed that the correlation coefficient betweenVB12content and the producing areas was 0.888**(P<0.01). Seen from Fig. 9, there was a significant difference between Korla and Ejina Banner in terms of VB12content of L. ruthenicum, the VB12content of L. ruthenicum from Nuomuhong was significantly lower than that of L. ruthenicum from Ejina Banner and Korla.
The differences in the niacinamide, VB6, and VB12content of L. ruthenicum from Nuomuhong, Ejina Banner, and Korla may be related to local climate and natural environmental conditions. The specific reasons also need further study.
Fatsoluble vitamin content
The fatsoluble vitamins measured at 270 nm were VK1 and VD, and the separation effect of these two vitamins was satisfactory within 10 min.
As shown in Fig. 10, the VD and VK1 content of L. ruthenicum from Ejina Banner were lower than that of the standard sample, but the separation effect was good. The VK1 content of L. ruthenicum from Nuomuhong, Ejina Banner and Korla was 6.71, 5.56and 0.85 μg/g respectively. Correlation analysis revealed that the correlation coefficient between VK1 content and the producing areas was -0.953**(P<0.01).
Seen from Fig. 11, there was no significant difference between Ejina Banner and Nuomuhong in terms of VK1 content of L. ruthenicum, but the VK1 content of L. ruthenicum from Ejina Banner and Nuomuhong was significantly higher than that of L. ruthenicum from Korla.
The VD content of L. ruthenicum from Nuomuhong, Ejina Banner and Korla was 72.56, 62.62 and 46.52 μg/g respectively. As shown in Fig. 12, there was no significant difference between Ejina Banner and Nuomuhong in terms of VD content of L. ruthenicum, but the VD content of L. ruthenicum from Korla was significantly lower than that of L. ruthenicum from Ejina Banner and Nuomuhong.
The differences in the VD and VK1 content of L. ruthenicum from Nuomuhong, Ejina Banner, and Korla may be related to local climate and natural environmental conditions. The specific reasons also need further study.
Trace element content
The Cu, Zn and Fe content of L. ruthenicum from Nuomuhong, Ejina Banner, and Korla were 15-40, 50-80, and 35-60 μg/g, showing that the Zn content of L. ruthenicum was high, and the Fe content of L. ruthenicum was slightly lower than Zn content.
According to Table 2, the Cu content of L. ruthenicum from Nuomuhong and Korla was significantly higher than that of L. ruthenicum from Ejina Banner. The Zn content of L. ruthenicum from Nuomuhong was higher than that of L. ruthenicum from Ejina Banner and Korla, while that of L. ruthenicum from Ejina Banner was the lowest. The Fe content of L. ruthenicum from Korla was the highest, followed by Nuomuhong, while that of L. ruthenicum from Ejina Banner was the lowest. The Cu content of L. ruthenicum from Nuomuhong, Ejina Banner, and Korla was the lowest. The trace element content of L. ruthenicum from Nuomuhong was higher than that of L. ruthenicum from Ejina Banner and Korla, and that of L. ruthenicum from Ejina Banner was the lowest, which may be related to local climate and natural environmental conditions. The specific reasons also need further study.
Conclusions and discussion
Proanthocyanidins
Proanthocyanidins have the functions of antioxidation, scavenging free radicals and resisting hepatitis B virus, and their antioxidant capacity is 50 times that of vitamin E[21]. L. ruthenicum contains a large amount of proanthocyanidins. The study showed that the proanthocyanidin content of L. ruthenicum from Ejina Banner, Nuomuhong, and Korla was 4.068%, 2.131%, and 1.436%respectively. Seen from proanthocyanidin content, the quality of L. ruthenicum from Ejina Banner was good.
Watersoluble vitamins
Watersoluble vitamins are often part of coenzymes or prosthetic groups and are a class of organic nutrient molecules that are soluble in water. In this experiment, the content of VB2, VB6, VB12, nicotinamide, folic acid and Vc in L. ruthenicum from Ejina Banner, Nuomuhong was measured. There were certain differences between different producing areas in terms of watersoluble vitamin content. The results showed that the Vc and VB6 content of L. ruthenicum from Nuomuhong were higher than that of L. ruthenicum from Ejina Banner and Korla, and the VB2, niacinamide and VB12of L. ruthenicum from Ejina Banner was higher than Nuomuhong and Korla, while the folic acid content of L. ruthenicum from Korla was higher than that of L. ruthenicum from Ejina Banner and Nuomuhong. The total content of watersoluble vitamins in Nuomuhong, Ejina Banner, and Korla was 4.074%, 7.416%, and 4.843%respectively. Seen from the content of watersoluble vitamins, the quality of L. ruthenicum from Ejina Banner was good.
Fatsoluble vitamins
A fatsoluble vitamin is a polypentadiene compound composed of a long hydrocarbon chain, including vitamins A, D, E and K. Since vitamin A and vitamin E are easily decomposed by light, only VK1 and VD were determined in this experiment. The results showed that the total content of fatsoluble vitamins VK1 and VD in L. ruthenicum from Nuomuhong, Ejina Banner, and Korla was 0.007 9%, 0.006 8%, and 0.004 7% respectively. Therefore, seen from the content of fatsoluble vitamins, the quality of L. ruthenicum from Nuomuhong was good.
Trace elements
The content of trace elements in plants is extremely low, but they play an important role in the growth and development of plants. They are the component of enzymes or coenzymes in plants and has strong specificity. In this experiment, the content of copper, zinc and iron in L. ruthenicum from various producing areas was detected. The results showed that the content of the three trace elements in L. ruthenicum from Nuomuhong was higher than that of L. ruthenicum from the other two producing areas, and it was the lowest in Ejina Banner. Seen from the content of trace elements, the quality of L. ruthenicum from Nuomuhong was good.
References
[1] BAI HJ, WANG HB, CHU ZQ, et al. Extraction of polysaccharide from Lycium rethenicum Murr by different methods[J]. Science and Technology of Food Industry, 2007, 28(3): 140-141.
[2] YANG CS, MA MC, LI W. Container seedling test of wild Lycium ruthenicum in different provenances[J]. Shaanxi Journal of Agricultural Sciences, 2007, 15(3): 63-66, 72.
[3] JIAO XL,CHI XF, DONG Q, et al. Analysis of the nutritional components of Lycium ruthenicum[J]. Amino Acids & Biotic Resources, 2011, 33(3): 64-66.
[4] CHEN HK, PU LK, CAO JM, et al. Current research state and exploitation of Lycium ruthenicum Murr[J]. Heilongjiang Agricultural Science, 2008, 155(5): 160-162.
[5] HUANG HM,YUAN LB, PENG ZH, et al. Advances in biosynthesis and environmental regulation of anthocyanin[J]. Hunan Agricultural Sciences, 2011, 25(13): 118-120.
[6] GUO Z, XU L. Proanthocyanidins: botanicals with broad development prospects[J]. World Phytomedicines, 1996, 11(5): 6-14.
[7] ZHAO WJ, ZHANG D, MA LJ, et al. Biosynthetic pathway, functional genes and metabolic engineering of proanthocyanidins[J]. Plant Physiology Communications, 2009, 45(5): 95-105.
[8] JIA LG. Proanthocyanidins inhibit seed germination by maintaining a high level of abscisic acid in Arabidopsis thaliana[J]. Journal of Integrative Plant Biology, 2012, 54(9): 663-673.
[9] LIU YL, HU HT, LAN DW. Advance in research on vitamin C biosynthesis and gene engineering[J]. Journal of Fruit Science, 2006, 23(3): 431-436.
[10] KAN J, LI L, XU JY. Research progress in folic acid biosynthesis and its metabolic engineering[J]. Chinese Journal of Biochemical Pharmaceutics, 2009, 30(4): 284-286.
[11] LI ZY, LI LS. Progress on research of nicotinic acid and nicotinamide[J]. Chemical Industry Times, 2003, 17(2): 9-12.
[12] LIU XL, WU G, ZHONG HB. Determination of vitamins B1, B2, and B6 and nicotinamide in vitamin B complex solution by HPLC[J]. Chinese Pharmaceutical Affairs, 2003, 2(4): 41-42.
[13] MA H, WANG LL, ZHANG CX, et al. Biosynthesis, fermentation and application of vitamin B12[J]. Chinese Journal of Biotechnology, 2008, 24(6): 927-932.
[14] ZHANG XS, SUN AJ. Summary of zinc nutrition in fruit trees[J]. Hebei Fruits, 1995, 26(3): 9-10.
[15] ZHAO MC. Review on the physiological function of nutrient elements and its role in drought resistance of plants[J]. Modern Agricultural Science and Technology, 2015, 6(12): 213-215.
[16] CHEN LZ. Effect of combined appliction of boron and molybdenum microelement fertilizer on cowpea yield and quality[J]. Asian Agricultural Research, 2016, 8(10): 90-92.
[17] CAI X,YANG CX. Determination of procyanidins in health foods by direct ultraviolet spectrophotometry[J]. Chinses Journal of Health Laboratory Technology, 2010, 20(4): 58-59.
[18] ZHANG XZ, FU SM, GAO SL, et al. Isolation, extraction and determination of the watersoluble vitamin from sun papaya[J]. Journal of Anhui Agricultural Sciences, 2009, 37(7): 414-415.
[19] CHENG M. Determination of fatsoluble vitamins A, D, E and K in fortified foods by high performance liquid chromatography[J]. Instrumentation Analysis Monitoring, 1992, 6(2): 37-38, 45.
[20] ZHANG XY, YANG XD, ZHAO Y, et al. Determination of 10 elements in medlar by flame atomic absorption spectrometry[J]. Journal of Mathematical Medicine, 2006, 84(1): 86.
[21] LI FY, CUI RJ, LI CH. Microwaveassisted extraction of procyanidin by from grape seed[J]. Food and Fermentation Industries, 2005, 31(1): 39-42.
Editor: Yingying YANG Proofreader: Xinxiu ZHU
杂志排行
农业生物技术(英文版)的其它文章
- Copyright Authorization Statement
- Optimization of Enzymatic Extraction of Sodium Chondroitin Sulfate From Bovine Nasal Bone
- Optimization of Extraction Process of Anthocyanins from Selenium-enriched Purple Potato by Response Surface Methodology
- Research on Extraction and Purification Technology of Flavonoids from Quinoa (Cheuopodium quinoa) Le
- Effect of Different Processing Techniques on the Content of Total Alkaloids in Toddalia asiatica Lam.
- High Performance Liquid Chromatography Analysis of Sugars and Acid Components in ‘Xintai Tianhong’ Hawthorn Fruit
