Study on Invitro Production Technology of Embryos from Young Dorper Sheep
2019-09-10HuidiLUOYangyiMAOHuihuiGUOLiZHANGZhiwuWANGZhijunWANGJunLIHongyuGUOLinaXUEWenqingDANG
Huidi LUO Yangyi MAO Huihui GUO Li ZHANG Zhiwu WANG Zhijun WANG Jun LI Hongyu GUO Lina XUE Wenqing DANG

Abstract This study was conducted to investigate the invitro production technology of embryos from young Dorper sheep, so as to provide technical support for the utilization of ovarian follicles in young Dorper sheep. Tests were conducted from the induction of Dorper sheep of 4 to 8 weeks old using follicle stimulating hormone (FSH) and pregnant horse serum (PMSG), collection of oocytes, invitro oocyte maturationfertilizationzygote cultivation and 2-4cellstage fertilized ovum transfer. The results showed that 585 oocytes were collected from eight Dorper sheep at the age of 4 and 8 weeks, with an average of 73.13 oocytes/sheep. 346 of the 2-4cellstage fertilized eggs were obtained, whose cleavage rate was 59.15%. 77 invitro fertilized eggs at 2-4cell stage were transplanted into 17 recipient sheep, seven of which were pregnant and gave birth to 13 "testtube sheep" with a conception rate of 41.18%. It is indicated that the hormone induction technique, invitro oocyte maturationfertilizationzygote cultivation technique and 2-4cellstage fertilized ovum transfer technique used in this study can serve as effective techniques for the invitro production of embryos from Dorper sheep of 4-8 weeks old.
Key words Young Dorper sheep; Invitro production technology of embryos; Invitro fertilization; 2-4cell fertilized ovum; Testtube sheep
Received: July 27, 2019Accepted: October 10, 2019
Supported by Earmarked Fund for Construction of National Wool Sheep Industry Technology Research System (CARS3924); Program for Science and Technology Development of Shanxi Province (201203110241); Fund for Science and Technology Innovation Team in Shanxi Province (201705D13102820); Shanxi Agricultural Industry Development Technology Leading Fund (2017CYYL08).
Huidi LUO (1959-), female, P. R. China, researcher, devoted to research about genetic breeding of sheep.
*Corresponding author. Email: lhd2638@126.com.
With the development of social economy and the improvement of peoples living standards, the demand for highquality mutton in the market is increasing, and the demand for highquality mutton sheep is also increasing. Therefore, the rapid propagation technology of superior mutton sheep has become one of the hotspots of current research on mutton sheep. In the late 20th and early 21st centuries, scientists conducted indepth research on the embryo transfer technology of superior mutton sheep, which doubles the number of superior mutton sheep. However, the traditional multiple ovulation and embryo transfer (MOET) technique is only used for adult sheep, and 8 to 10 embryos can be obtained per sheep in superovulation. The generation interval is long and the reproduction speed is still slow.
The juvenile in vitro embryo technology researched and developed by the South Australia Reproduction Institute (SARD) is a new rapid propagation technology system integrating superovulation and egg collection of young animals, invitro maturation and fertilization of oocytes and invitro culture and transfer of fertilized eggs. The principle is that the number of antral follicles in the ovary of lambs peaks at the age of 1 to 2 months, the follicular development is rarely blocked, and the ovaries are very sensitive to reproductive hormones. However, because the function of the pituitary gland of lambs is not yet perfect, they cannot secrete a sufficient amount of gonadotropin to stimulate ovarian activity, and the follicles eventually cannot mature and be ovulated. If at this stage, foreign gonadotropin induction technology is used, the average number of oocytes per lamb can be 80-160, which is about 10 times of that of adult sheep, and the generation interval is only 1/3 of that of adult sheep, which means that the breeding speed of the superior sheep is greatly accelerated. At present, scientists at aboard have deeply studied the JIVET technology of excellent sheep breeds, and have achieved gratifying results[1-5]; and scientists at home, An Xiaorong, Wang Liqin, Guo Hong, Liu Zongzheng, Ma Shike, Chen Xiaoyong, etc., also have carried out studies on the JIVET technology in Suffolk sheep, Kazakh sheep, Liaoning cashmere goats and smalltailed Han sheep raised in Xinjiang Autonomous Region, Liaoning Province, Shandong Province, Qinghai Province and Hebei Province[6-16]. However, so far, there has been no report on the technical research of JIVET in Shanxi Province. Therefore, Institute of Animal Husbandry and Veterinary of Shanxi Academy, Shanxi Academy of Agricultural Sciences, studied the invitro production technology of embryos in young Dorper Sheep in cooperation with China Agricultural University, so as to optimize the JIVET technology system of superior sheep.
Materials and Methods
Test time and location
The invitro maturation and fertilization test of oocytes was carried out in the Animal Genetic Engineering Laboratory of Institute of Animal Husbandry and Veterinary, Shanxi Academy of Agricultural Sciences from May 5 to 23, 2014. The invitro fertilized egg transfer test was carried out in the sheep farm of Jinzhong Linshan Cooperative.
Materials
Test sheep
Male Dorper sheep, 48weekold Dorper sheep and recipients were provided by the Jinzhong Linshan Cooperative.
Frozen semen and estrus sheep serum (ESS)
Frozen semen and ESS of Dorper sheep were made in Animal Genetic Engineering Laboratory of Institute of Animal Husbandry and Veterinary, Shanxi Academy of Agricultural Sciences.
Main hormones, reagents and consumable items
Luteinizing hormone (LH), 17 βestradiol (17βE2), follicle stimulating hormone (FSH) and pregnant horse serum (PMSG) were purchased from Beijing Luxin Agriculture and Animal Husbandry Technology Co., Ltd. M199, biological agents such as heparin sodium, bovine serum albumin (BSA), essential amino acids (EAAs) and nonessential amino acids (NEAAs) and chemical agents such as CaCl2·2H2O, NaHCO3 and KH2PO4 were all purchased from Shanxi Cell Biotech Co., Ltd. Fourwell plates were purchased from Nunc, and the bottom tube, centrifuge tube and petri dish were purchased from Corning.
Main instruments
Stereomicroscope (OLYMPUS SZX10), inverted microscopy system (OLYMPUS IX71), ultraclean workbench (SWCJ2FD) and carbon dioxide incubator (SANYO MCO5AC) were all provided by the Animal Genetic Engineering Laboratory of Institute of Animal Husbandry and Veterinary, Shanxi Academy of Agricultural Sciences.
Culture media
Eggcollecting liquid: M199 (containing 20 mmol/L Hepes, 2% ESS, 10 mg/ml heparin sodium); mature liquid: M199 (containing 20% ESS, 10 μg/ml FSH, 10 μg/ml LH, 1 μg/ml 17βE2); capacitation liquid: synthetic oviduct fluid (SOF) (containing 20 μg/ml heparin sodium); fertilizing liquid: SOF(containing 2% ESS); development liquid of fertilized eggs: SOF (containing 8 mg/ml BSA, 2% EAA, 1% NEAA).
Methods
Frozen semen preparation
The pelleted semen was prepared by the conventional dry ice freezing method from the Dorper sheep semen with vitality of 0.8 or more.
ESS preparation
Blood was collected from the jugular vein of sheep in estrus for 1 d, and stood in a refrigerator for about 20 h at 4 ℃. The serum was obtained and inactivated in a water bath at 56 ℃ for 30 min, filtered through a 0.22 μm filter, and stored at -20 ℃.
Lamb superovulation
The lamb ovulation method of Kelly et al.[1] was used, except that the PMSG injection volume was 320 U per sheep.
Capacitation
Invitro capacitation was performed according to the method of Bai[6].
Collection of oocytes
After the donor lambs were anesthetized, fixed and disinfected at the surgical site, a scalpel was used to cut a 3-5 cm small opening in the anterior part of the breast slightly approximating the median. The ovary was gently pulled out of the abdominal cavity, and the 18# needle of a 10 ml syringe containing 2 ml of eggcollecting liquid was used to pierce the follicles over 2 mm in diameter on the ovary, and the cumulusoocyte complexes (COCs) were sucked out. After collecting the eggs, the opening was sutured and disinfected.
Oocyte maturationfertilizationfertilized ovum cultivation
The collected COCs of grade A and B were placed in the mature liquid in the wells of fourwell culture plates, covered with mineral oil, and matured in a 5% carbon dioxide incubator (38.5 ℃, saturated humidity) for about 24 h, with the excretion of the first polarbody as the criterion for oocyte maturation. The mature oocytes were gently blown and beaten in a 0.2% hyaluronidase solution to remove part of the cumulus cells, and then placed in the fertilizing liquid in the wells of fourwell culture plates, and the capacitated sperms were added at a concentration of 1×106-2×106 sperms/ml. The mixture was then covered with mineral oil to allow fertilization and incubation in a 5% carbon dioxide incubator for 24 h.
After removing the residual sperms and cumulus cells attached to the fertilized egg, they were placed in the fertilized egg development liquid in the wells of fourwell culture plates and then covered with mineral oil. The fourwell plates were placed in aluminum foil bags (inflated with 5% CO2, 7% oxygen and 88% nitrogen), which were then sealed. The cells were cultured in a 5% carbon dioxide incubator for about 24 h. The cleavage was observed under a microscope, and the cleavage rate was calculated.
Estrus synchronization of recipient sheep
CIDR was implanted in recipient sheep for 12 d, and PMSA 330 U was injected intramuscularly into each recipient sheep when the CIDR was withdrawn.
Fertilized egg transfer
The surgical method was the same as the previous"Collection of oocytes". The ovary and the fallopian tube on the side of the ovary with the ovulation point were gently pulled outside the abdominal cavity, and 4 to 5 fertilized eggs at the 2-4cell stage were implanted with an embryo transfer device into the ampulla of the uterine tube.
Data processing methods
The test data were subjected Excel statistical analysis.
Results and Analysis
Inducing effect of young Dorper sheep with gonadotropin
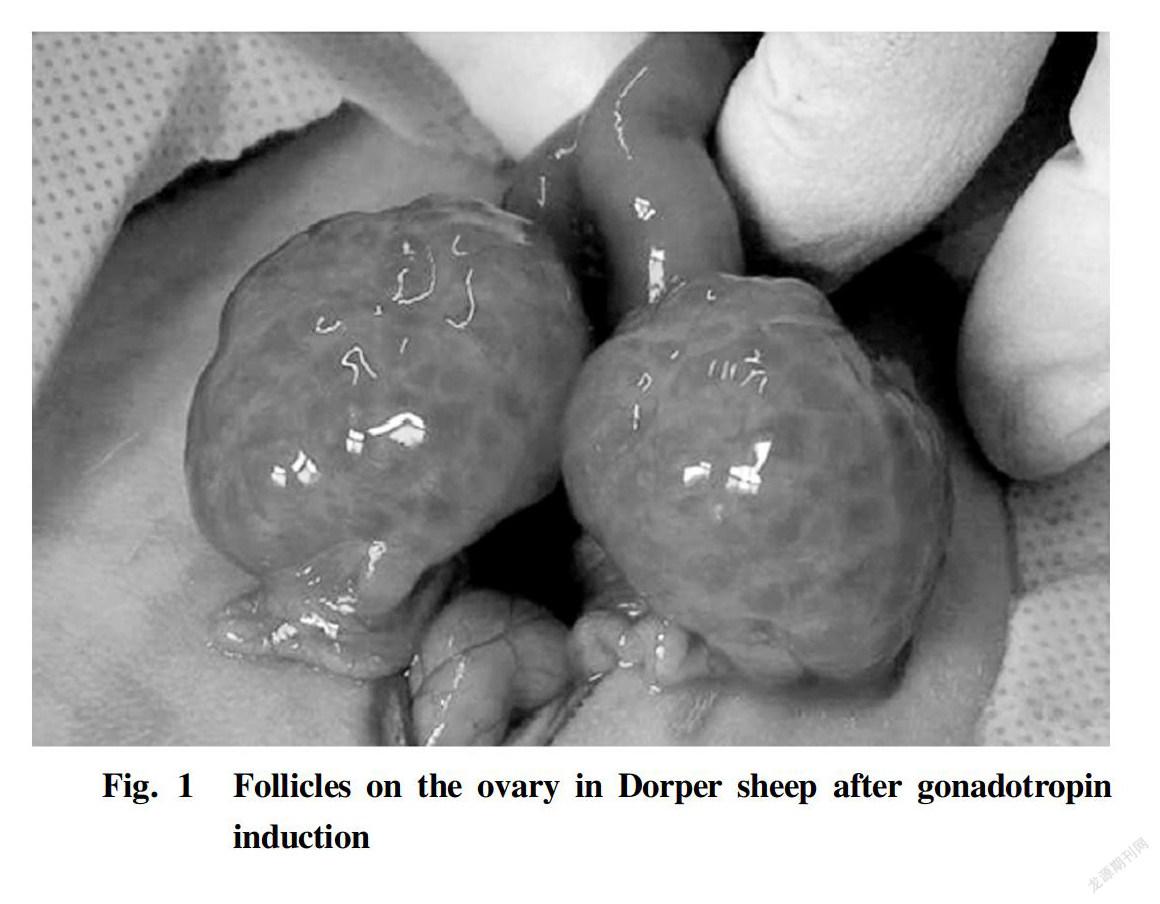
The oocyte samples collected from the Dorper sheep of 4 to 8 weeks old are shown in Table 1. The development conditions of the ovaries and follicles are shown in Fig. 1.
It can be seen from Table 1 among the eight Dorper sheep of 4-8 weeks old induced by gonadotropin, the ovaries of seven sheep were developed, and the superovulation rate was 87.50%. A total of 605 COCs were obtained, with an average of 75.63 COCs/sheep; and there were 585 A and Bgrade COCs, 73.13 COCs/sheep averagely. It can be seen from Fig. 1 that for the seven sheep of 4-8 weeks old responding to the gonadotropin induction, the ovary of each Dorper sheep increased in volume, and was in red color and densely covered by developed follicles.
Invitro development of oocytes
585 usabe oocytes (COCs) were matured in the mature liquid in vitro, of which 439 oocytes matured and the maturation rate was 75.04%. The mature oocytes were coincubated with capacitated sperms, followed by culture of fertilized eggs, after which there were 346 fertilized eggs split into 2-4 cells, showing a cleavage rate of 59.15% and an average of 43.25 invitro fertilized eggs/sheep at 2-4cell stage. The development of fertilized eggs is shown in Fig. 2.
Effect of 2-4cellstage fertilized egg transfer
Seventytwo invitro fertilized eggs of the 2-4cell stage were transplanted into the ampulla of the fallopian tube of 17 estrus recipient sheep, that is, 4-5 fertilized eggs/sheep. The results showed that seven sheep were pregnant and 13 lambs were born. Among the seven recipients, six female sheep gave birth to double lambs, and 1 sheep gave single lamb. The conception rate was 41.18%. An average of six invitro fertilized eggs at the stage of the 2-4cell stage produced 1 JIVET (test tube) lamb. The "test tube sheep" is shown in Fig. 3.
Agricultural Biotechnology2019
Conclusions and Discussion
Effects of the hormone induction method on the invitro production technology of embryos from young Dorper sheep
Inducing follicular development on the ovary of young Dorper sheep by gonadotropin and obtaining a large number of oocytes is the basis for establishing an invitro production technology system for embryos from young Dorper sheep. In the field of inducing young sheep with hormones, Gou et al.[5] induced lambs of 1 month old with FSH and PMSG, obtaining 79.1 oocytes/sheep. Kelly et al.[1,3] studied follicular development of lambs of 1 to 2 months old by injecting FSH 160 mg and PMSG 500 U, and the results showed that each lamb produced more than 80.0 oocytes. Wu et al.[17] conducted a follicular development study by the equal amount method and diminishing method through the injection of 120 U of FSH into Xinjiang fine wool lambs of 30-60 d old, and the results showed that the equal amount method produced 101.0 oocytes/sheep, which was significantly higher than the diminishing method (39.0 oocytes/sheep).Yu et al.[18] studied the follicular development of Tao Han hybrid sheep of 1 and 3 months old by injectingFSH 160 mg into each sheep by the equal amount method for 2 d, 4 times in total, the last of which was accompanied with the injection of PMSG 500 U, and the results showed that the FSH level in the blood of the 1monthold lamb was significantly higher than that in the 3monthold lambs, and the former produced 79.0 oocytes/sheep, which was significantly higher than that of 3monthold lambs (18.5 oocytes/sheep). In this study, the FSH produced in Canada and PMSG produced in Ningbo Hormone Factory were used to induce the follicular development on the ovary of Dorper sheep of 4 to 8 weeks old. The FSH was injected at intervals of 12 h for 2 d, four times (40 mg each time) in total, the last time of which was accompanied by the injection of PMSG at 320 U/sheep, and an average of 75.63 COCs/sheep were obtained. The results of this study are basically consistent with the results from lambs of 1 to 2 months old studied by Gou et al.[5] and Kelly et al.[1,3], and the research results from the follicular development of Tao Han hybrid sheep of 1 month old and Xinjiang finewool lambs of 30-60 d old by the equalamount hormone induction method conducted by Wu et al.[17] and Yu et al.[18]. This study demonstrates the feasibility of using gonadotropin to induce ovarian follicular development in Dorper sheep of 4 to 8 weeks old.
Effects of invitro oocyte maturationfertilizationfertilized ovum cultivation on the invitro production technology system of embryos from young Dorper sheep
Invitro oocyte maturation, fertilization and incubation and fertilized ovum cultivation of young sheep constitute a culture system for the invitro production of embryos from young sheep, and any problem in any link will affect the invitro embryo production. In the invitro oocyte culture system, Bai et al.[4] added 600 μmol/L cysteine and 100 μmol/L βmercaptoethanol to the Mongolian lamb oocyte mature liquid, and the fertilization rate and blastocyst rate were, respectively, 86.9% and 23.9%, which were significantly higher than 67.9% and 14.9% in the control group (P<0.05). Studies also have shown that adding cysteine and βmercaptoethanol to the mature liquid can reduce oocyte parthenogenetic activation and the proportion of polyspermy, and speed up the development of fertilized eggs to blastocysts. Guo et al.[19] added 100 μmol/L βmercaptoethanol to the invitro mature liquid of oocytes from sheep of 4 to 8 weeks old, and the results showed that the cleavage rate (68.0%) was higher than that of the control group (64.2%), and the blastocyst rate (19.3%) was also significantly higher than that of the control group (9.8%) (P<0.05), indicating that βmercaptoethanol significantly increased the developmental capacity of lamb oocytes. Wang et al.[7] conducted a study on the invitro development of Suffolk lamb oocytes, and the results showed that the cleavage rate was 73.93%. Chang et al.[20] conducted a study on the invitro fertilization techniques of Boer goats and found that the CIDR+FSH superovulation combination achieved a good cleavage effect on oocytes, and the cleavage rate was 69.0%. In this study, conventional invitro oocyte fertilization and culture system and culture method were used to study the development of oocytes from Dorper sheep of 4-8 weeks old. The results showed that the maturation rate was 75.04%, and the cleavage rate was 59.15%, which was slightly lower than the research results of Bai et al.[4], Guo et al.[19], Wang et al.[7] and Chang et al.[20] on young Mongolian sheep, 4-8weekold sheep, young Suffolk sheep and Boer goats. The reason may be that the purity of carbon dioxide gas affected the development of oocytes, and the oocyte maturation and cleavage were somewhat delayed, resulting in a decrease in oocyte maturation rate and cleavage rate of fertilized eggs. It is indicated that the invitro production and culture method of embryos from Dorper sheep in this study is feasible, but further improvement is still needed.
Effects of 2-4cellstage fertilized egg transfer method on the invitro production technology system of embryos from young Dorper sheep
Sheep ova are fertilized in the ampulla of the fallopian tube, and the development of fertilized eggs to the 16cell stage is completed in the fallopian tube. Therefore, the invitro transfer of 2-4cellstage fertilized eggs should be performed in the fallopian tube. Wang et al.[7], Guo et al.[8], Liu et al.[9] and Ma et al.[10] conducted the research on the application of JIVET technology in Suffolk sheep, Kazakh sheep, Dorset sheep and smalltailed Han sheep, and the conception rate ranged from 16.6% to 53.6%. In this study, the invitro fertilized eggs of the 2-4cell stage were transplanted into the ampulla of the fallopian tube of estrus recipient sheep, and the conception rate was 41.18%, which is consistent with the findings of the above researchers. It indicates that the 2-4cellstage fertilized egg transfer method used in this study is feasible.
Conclusions
In this study, the invitro production technology of embryos from the Dorper sheep of 4-8 weeks old was studied. The results showed that 73.13 A and Bgrade oocytes and 43.25 fertilized eggs in the 2-4cell stage were obtained per sheep, and the cleavage rate was 59.15%. 77 invitro fertilized eggs at the 2-4cell stage were transplanted into 17 recipient sheep, seven of which were pregnant and gave birth to 13 "testtube sheep" with a conception rate of 41.18%. It is indicated that the hormone induction technique, invitro oocyte maturationfertilizationzygote cultivation technique and 2-4cellstage fertilized ovum transfer technique used in this study can serve as effective techniques for the invitro production of embryos from Dorper sheep of 4-8 weeks old.
Acknowledgement
Prof. An Xiaorong, an expert in the embryo engineering of National Wool Sheep Industry Technology Research System, and team members Hou Jian, Guan Hong, Yan Fengxiang, Zheng Li and Wang Chunxin, supported the experiment.
References
[1] KELLY JM, KLEEMANN DO, WALKER SK. Enhanced efficiency in the production of offspring from 4 to 8weekold lambs[J]. Theriogenology, 2005, 63(7): 1876-1890.
[2] RANGELSANTOS R, MCDONALD MF, WICKHAM GA. Evaluation of the feasibility of a juvenile MOET scheme in sheep[J]. Proceedings of the New Zealand Society of Animal Production, 1991, 51(1): 139-142.
[3] KELLY JM, KLEEMANN DO, WALKER SK. The effect of nutrition during pregnancy on the in vitro production of embryos from resulting lambs[J].Theriogenology,2005, 63(7): 2020-2031.
[4] BAI JH, HOU J, GUAN H, et al. Effect of 2 mercaptoethanol and cysteine supplementation during in vitro maturation on the developmental competence of oocytes from hormone stimulated lambs[J]. Theriogenology, 2008, 70(5): 758-764.
[5] GOU KM, GUAN H, BAI JH, et al. Field evaluation of juvenile in vitro embryo transfer(JIVET)in sheep[J].Animal Reproduction Science, 2009, 112(3/4): 316-324.
[6] BAI JY. Premature sheep and cattle JIVET technology research[D]. Beijing: China Agricultural University, 2008. (in Chinese)
[7] WANG LQ, HE ZL, LIN JP, et al. Study on the induced development effect of Suffolk lamb follicular[J]. Journal of China Agricultural University, 2015, 20(4): 141-146. (in Chinese)
[8] GUO H, WANG PC, SHI WY, et al. study on in vitro production (ivp) of embryo utilizating superovulated oocytes from different breed lambs[J]. Animal Husbandry and Veterinary Medicine, 2012, 44(10): 20-24. (in Chinese)
[9] LIU ZZ, LI HG, CHENG M, et al. Study on rapid propagation of Suffolk sheep by using JIVET technology[J]. Animal Husbandry and Feed Science, 2016, 37(3): 1-4. (in Chinese)
[10] MA SK, QIAO HS, MA LQ, et al. Application research on JIVET in lamb in Qinghai plateau area[J]. Chinese Qinghai Journal of Animal and Veterinary Sciences, 2015, 45(2): 13-14. (in Chinese)
[11] CHEN XY, TIAN SJ, SANG RZ, et al. Inducement of lamb follicular development and embryo production in vitro[J]. Chinese journal of agricultural biotechnology, 2008, 16(3): 456-460. (in Chinese)
[12] ZHANG XH, ZHANG SW, SONG XC, et al. Study on follicular development of female lamb and technology of oocyte maturation[J]. Chinese animal husbandry and veterinary medicine, 2011, 38(11): 136-139. (in Chinese)
[13] WU HF, LI FD, CHANG WH, et al. The effect of different treatments on superovulation and in vitro fertilization(IVF)efficiency[J].Journal of Gansu Agricultural University, 2011, 46(6): 1-5. (in Chinese)
[14] LI YS, CAO HG, LIU Y, et al. Advance in goat and sheep juvenile in vitro embryo transfer[J]. Progress in Veterinary Medicine, 2011, 32(11): 99-104. (in Chinese)
[15] GUO H, WANG PC, DAI R, et al. Analysis of influencing factors on the oocyte in vitro embryo production in lambs[J]. Hubei Agricultural Sciences, 2013, 52(19): 4726-4729. (in Chinese)
[16] MIN JT, DU WH, ZHAO XM, et al. Oocyte recovery, in vitro maturation and embryo culture systems influence the efficiency of ovine in vitro fertilization[J]. Chinese animal husbandry and veterinary medicine, 2014, 41(5): 202-207. (in Chinese)
[17] WU WW, XU XM, ZHANG TH, et al. Study on the factors affecting Xinjiang fine wool lamb JIVET[J]. Chinese animal husbandry and veterinary medicine, 2011, 38(4): 144-147. (in Chinese)
[18] YU Y, ZHONG X, WANG FL, et al. Comparative study on follicular development of sheep of different ages[J]. Chinese Journal of Animal Science, 2012, 48(9): 17-21. (in Chinese)
[19] GUO H, WANG PC, SHI WY, et al. Effects of EGF and βmercaptoethanol on invitro fertilization and development of oocytes collected from hormonestimulated lambs[J]. Journal of Anhui Agricultural Sciences, 2012, 40(11): 6537-6540. (in Chinese)
[20] CHANG D, GAO Y, LUO GH, et al. Investigation of in vitro fertilization of Boer goat lambs oocytes obtained from different superovulation[J]. Journal of Beijing University of Agriculture, 2009, 24(4): 28-31. (in Chinese)
杂志排行
农业生物技术(英文版)的其它文章
- Observation on Cardiac Opening of the Inferior Vena Cava in Goat Fetuses
- Evaluation on Application and Spraying Effect of AirAssisted Sprayer in Apple Orchard with Dwarfing Rootstocks
- Problems in the Development of Traditional Chinese Medicinal Materials Planting Industry in Shiyan City and Countermeasures
- Study on Practical Mature Age of Individual Pinus thunbergii×P. densiflora
- Effects of Different Densityreducing Methods on Canopy Microenvironment, Tree Growth and Fruit Quality in Closed Apple Orchard
- Development of Whole Potato Flour Fish Noodles
