黔产昆明山海棠化学成分及其抑菌活性研究
2019-09-10李江李��穆淑珍张仕林王莉云郝小江邓璐璐
李江 李�� 穆淑珍 张仕林 王莉云 郝小江 邓璐璐
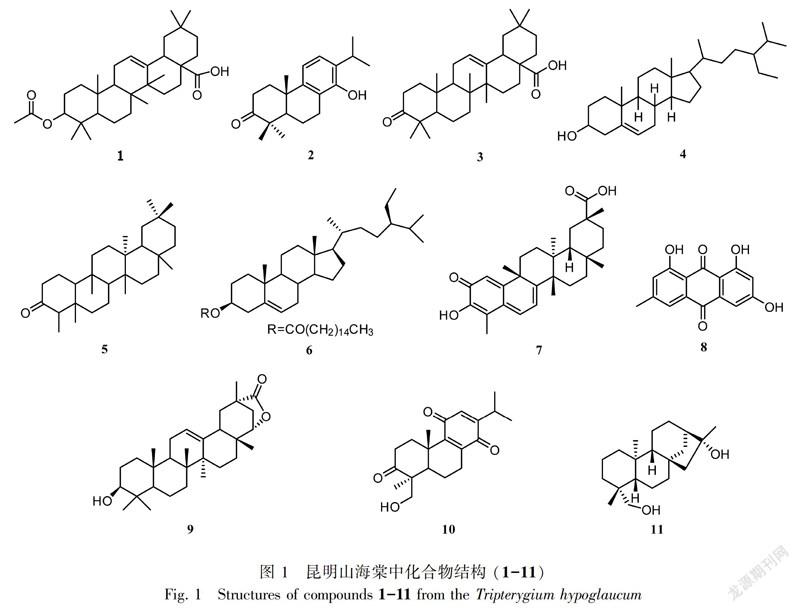
摘 要:该研究采用硅胶柱色谱、Sephadex LH-20 凝胶柱色谱、半制备型高效液相色谱和重结晶等方法分离纯化,从黔产昆明山海棠乙醇提取物中分离得到11个化合物,并采用96孔板微量稀释法对化合物进行抑菌活性测定。结果表明:利用 NMR,MS 等现代波谱技术以及化合物的理化性质并结合参考文献分别鉴定为3-O-乙酰基齐墩果酸(1),雷酚萜(2),3-氧代齐墩果酸(3),β-谷甾醇(4),木栓酮(5),β-谷甾醇棕榈酸酯(6),雷公藤红素(7),大黄素(8),雷公藤内酯甲(9),雷藤二萜醌 B(10),ent-kauran-16 β,19-diol(11)。抑菌活性结果显示,化合物3、7和8具有较好的抑菌作用,MIC值为2~16 μg·mL-1。其中,化合物6、11为首次从该植物中分离得到,化合物11为首次从雷公藤属植物中分离得到,且首次发现了化合物3、7对绿脓杆菌和青枯菌具有明显的抑制作用。
关键词:昆明山海棠, 雷公藤属, 雷公藤红素, 雷藤二萜醌 B, 抑菌作用
中图分类号:Q946.91
文献标识码:A
文章编号:1000-3142(2019)11-1505-07
Abstract:Eleven compounds were isolated and purified from ethanol extract of Tripterygium hypoglaucum from Guizhou Province by silica gel column chromatography, Sephadex LH-20 gel column chromatography, semi-prepared HPLC and recrystallization. Their antibacterial activity were determined by 96-well plate microdilution method. The results were as follows:The structures were identified as 3-O-acetyloleanolicacetic anhydride(1), triptonoterpene(2), 3-Oxo-olean-12-en-28-oic acid(3), β-sitosterol(4), friedelin(5), β-sitosteryl palmitate(6), celastrol(7), emodin(8), wilforlide A(9), triptoquinone B(10), and ent-kauran-16 β, 19-diol(11) by NMR, MS, physicochemical properties and some reported data. Based on the results of antibacterial activity, compounds 3, 7 and 8 showed potential activity, and their MIC values were 2-16 μg·mL-1. Compounds 6 and 11 were isolated from this plant for the first time, and meanwhile compound 11 was also isolated from the genus of Tripterygium for the first time. Furthermore it was found firstly that compounds 3 and 7 had significant inhibitory effects on Pseudomonas aeruginosa and Rastonia solanacearum.
Key words:Tripterygium hypoglaucum, Tripterygium, celastrol, triptoquinone B, antibacterial activity
昆明山海棠(Tripterygium hypoglaucum),又名紫金皮、大方藤、洋道藤、粉背雷公藤、九團花、火把花等,是卫矛科(Celastraceae)雷公藤属(Tripterygium)有毒木质藤本植物。产于贵州、湖南、云南、广西、江西等地,贵州主要分布在梵净山、雷公山、台江、龙里、兴仁、兴义等地。目前临床上相关药品及制剂主要作为免疫抑制剂,用于治疗类风湿性关节炎、慢性肾炎、麻风反应、白血病和红斑狼疮等多种胶元性疾病及自身免疫性疾病,且疗效确切,效果良好(国家中医药管理局《中华本草》编委会,1999)。近年来药理学研究表明昆明山海棠还具有抗生育作用(周激文等,1991;Zhen et al., 1995)。
目前,针对昆明山海棠,国内外均以抗肿瘤方面的研究为主,而其抑菌活性少见报道。为开发利用该植物资源,探索昆明山海棠的化合物结构及其活性成分,本文对黔产昆明山海棠抑菌活性明显的乙酸乙酯萃取部位化学成分进行初步研究。该研究进一步丰富了昆明山海棠的化学研究,为综合利用昆明山海棠资源奠定了基础。
1 材料、仪器与试剂
1.1 材料
昆明山海棠2016年10月采集于贵州省雷山县,经贵阳中医学院孙庆文教授鉴定为昆明山海棠(Tripterygium hypoglaucum)的根,标本存放于省部共建药用植物功效与利用国家重点实验室中。
金黄色葡萄球菌(Staphyloccus aureus)、绿脓杆菌(Pseudomonas aeruginosa)、枯草芽孢杆菌(Bacillus subtilis)、青枯菌(Rastonia solanacearum)由省部共建药用植物功效与利用国家重点实验室李老师提供。
1.2 仪器与试剂
仪器:恒温振荡器HZQ-F160A(上海一恒科学仪器有限公司),立式压力蒸汽灭菌锅LDZX-50KBS(上海申安医疗器械厂),SW-CJ-2FD型双人单面净化工作台(苏州净化设备有限公司),电子天平FA2204B(上海精科天美科学仪器有限公司),INOVA-400 MHz 核磁共振波谱仪,Bruker AM-400和DRX-500 核磁共振仪(TMS为内标,瑞士Bruker公司),Si-HPLC 半制备色谱仪(美国Waters公司)。材料:Sephadex LH-20 凝胶(40~70 μm)(瑞士Amersham Pharmacia Biotech AB 公司),柱层析硅胶(200~300目和300~400目),硅胶H(10~40 μm)和薄层层析用硅胶GF254(0.20~0.25 mm)(青岛海洋化工厂)。
试剂:本实验所用HPLC试剂均为色谱纯试剂,二氯甲烷、石油醚、乙酸乙酯、甲醇、乙醇(分析纯,上海泰坦科技股份有限公司),氯仿(工业级,使用前经重蒸处理),其余试剂为国产分析纯。LB培养基:胰蛋白胨10.0 g ·L-1,酵母提取物5.0 g ·L-1,NaCl 10.0 g ·L-1,pH=7.4,在高压下121 ℃湿热灭菌21 min。阳性对照药:环丙沙星(萨恩化学技术有限公司)。
2 研究方法
2.1 提取和分离
取昆明山海棠干燥根19.4 kg,用无水乙醇提取3次,时间分别为4、3、2 h,合并提取液,用水乙醇提取液=11减压回收乙醇至无醇味,得到昆明山海棠水溶液;用乙酸乙酯萃取上述水溶液3次,浓缩乙酸乙酯层萃取液得到浸膏275 g,经常压硅胶柱层析, 以石油醚-乙酸乙酯、 氯仿-甲醇溶剂系统梯度洗脱,得到Fr. 1-7。Fr. 2(9.4 g)经硅胶柱层析,以石油醚∶乙酸乙酯=200∶1洗脱,并通过重结晶得到化合物5(18 mg)和6(56 mg)。Fr. 5(69.2 g)经硅胶柱层析,以石油醚-乙酸乙酯、氯仿-甲醇溶剂系统梯度洗脱,得到Fr. 5.1-5.5。Fr.5.1经硅胶柱层析,以石油醚∶乙酸乙酯=30∶1洗脱以及 Sephadex LH-20 凝胶柱色谱分离(甲醇),得到化合物1(39 mg)、2(5 mg)、3(563 mg)、4(803 mg)和8(12 mg)。Fr. 5.2经硅胶柱层析,以氯仿∶甲醇=40∶1洗脱,Si-HPLC(甲醇水=8812)以及 Sephadex LH-20 凝膠柱色谱(氯仿甲醇=11,甲醇),分离得到化合物7(140 mg)、9(9 mg)、10(22 mg)和11(16 mg)。
2.2 最小抑菌浓度(MIC)的测定
2.2.1 菌悬液的制备 将活化的各供试菌种分别接种3~5个单菌落于30 mL LB培养基的100 mL三角瓶中,增殖6 h,再另取提前装好3 mL LB培养基用于稀释菌液至1.5×108个·mL-1,再用LB 培养基稀释1 000倍即为1.5×105个·mL-1备用。
2.2.2 最小抑菌浓度(MIC)的测定 在无菌操作台上将灭菌后的LB培养基,加入100 μL于96孔板每个孔中,A,B,C 3排的第1孔加入配制的药液A(浓度为1 024 μg·mL-1)100 μL;D,E,F 3排的第1孔加入配制的药液B(浓度为1 024 μg·mL-1)100 μL,用移液枪对A~F排充分吹打使药液与培养基混匀,然后进行2倍稀释,照此重复至第12孔,第12孔混匀后吸100 μL弃去;将配制的菌悬液加100 μL于96孔板中,重复3次(A,B,C 3排样品);在同一96孔板上的G排为空白对照(空白培养基)和F排为阴性对照(菌液和培养基),于37 ℃恒温培养18~24 h,96孔板中没有混浊的最后1孔的最低药液浓度为该化合物的最小抑菌浓度(μg ·mL-1),分别记录实验结果。
3 结果与分析
3.1 结构鉴定
化合物1 白色粉末,EI-MS(m/z):498[M]+,分子式为C32H50O4。1H-NMR(400 MHz,CDCl3) δ:5.27(1H,t,J=4.0 Hz,H-12),4.49(1H,t,J=8.0 Hz,H-3),2.83(1H,dd,J=13.8,4.6 Hz,H-18),2.05(3H,s,Me-OAc),1.12(3H,s,Me),0.94(3H,s,H-30),0.93(3H,s,H-29),0.90(3H,s,H-25),0.86(3H,s,H-23),0.85(3H,s,H-24),0.74(3H,s,H-26);13C-NMR(100 MHz,CDCl3) δ:38.0(C-1),23.4(C-2),80.9(C-3),37.7(C-4),55.2(C-5),18.1(C-6),32.4(C-7),39.2(C-8),47.5(C-9),36.9(C-10),22.8(C-11),122.5(C-12),143.6(C-13),41.5(C-14),27.6(C-15),23.5(C-16),45.8(C-17),40.8(C-18),46.5(C-19),30.6(C-20),33.7(C-21),32.5(C-22),28.0(C-23),16.6(C-24),15.4(C-25),17.1(C-26),25.9(C-27),184.2(C-28),23.6(C-29),33.0(C-30),171.1(C=O),21.3(C-CH3)。以上数据与Carvalho et al.(1993)的报道一致,故鉴定化合物1为3-O-乙酰基齐墩果酸。
化合物2 白色结晶,EI-MS(m/z):300[M]+,分子式为C20H28O2。1H-NMR(400 MHz,CDCl3) δ:7.04(1H,d,J=8.3 Hz,H-12),6.86(1H,d,J=8.3 Hz,H-11),4.69(1H,s,-OH),3.12(1H,sept,J=7.0 Hz,H-15),2.89(1H,m,H-5),2.70(2H,m,H-7),2.58(2H,m,H-2),1.92(2H,m,H-1),1.87(2H,m,H-6),1.33(3H,s,H-20),1.27(3H,s,H-19),1.25(3H,s,H-18),1.17(3H,d,J=7.0 Hz,H-16),1.14(3H,d,J=7.0 Hz,H-17);13C-NMR(100 MHz,CDCl3) δ:37.1(C-1),34.7(C-2),217.2(C-3),47.3(C-4),50.0(C-5),19.5(C-6),24.6(C-7),120.8(C-8),146.4(C-9),37.6(C-10),117.3(C-11),123.6(C-12),130.3(C-13),150.1(C-14),26.9(C-15),22.5(C-16),22.7(C-17),24.4(C-18),26.6(C-19),21.2(C-20)。以上数据与(Kutney & Han, 1996)的报道一致,故鉴定化合物2为雷酚萜。
化合物3 白色粉末,EI-MS(m/z):454[M]+,分子式为C30H46O3。1H-NMR(400 MHz,CDCl3) δ:5.30(1H,t,J=4.0 Hz,H-12),2.83(1H,dd,J=13.7,4.1 Hz,H-18),2.54(1H,m,H-2),2.36(1H,m,H-2),1.14(3H,s,CH3),1.08(3H,s,H-30),1.04(3H,s,H-29),1.03(3H,s,H-25),0.93(3H,s,H-23),0.91(3H,s,H-24),0.81(3H,s,H-26);13C-NMR(100 MHz,CDCl3) δ:39.1(C-1),34.1(C-2), 217.8(C-3),46.6(C-4),55.3(C-5),19.5(C-6),32.1(C-7),39.2(C-8),47.4(C-9),36.8(C-10),22.9(C-11),122.3(C-12),143.6(C-13),41.7(C-14),27.7(C-15),23.6(C-16),46.9(C-17),41.0(C-18), 45.8(C-19),30.7(C-20),33.1(C-21),32.4(C-22),21.4(C-23),26.4(C-24),15.0(C-25),17.0(C-26), 25.8(C-27),183.9(C-28),33.8(C-29),23.5(C-30)。以上数据与Sun et al.(2008)的报道一致,故鉴定化合物3为3-氧代齐墩果酸。
化合物4 白色针晶,EI-MS(m/z):414[M]+,分子式为C29H50O。1H-NMR(500 MHz,CDCl3) δ:5.30(1H,br d,H-6),3.65(1H,m,H-3),0.96(3H,s,H-19),0.88(3H,d,J=6.6 Hz,H-21),0.82(3H,t,J=7.5 Hz,H-29),0.80(3H,d,J=6.8 Hz,H-27),0.77(3H,d,J=6.8 Hz,H-26),0.64(3H,s,H-18);13C-NMR(125 MHz,CDCl3) δ:37.2(C-1),31.4(C-2),71.6(C-3),42.1(C-4),140.8(C-5),121.6(C-6),31.8(C-7),31.8(C-8),50.0(C-9),36.4(C-10),21.0(C-11),39.7(C-12),42.2(C-13),56.7(C-14), 24.2(C-15),28.2(C-16),55.9(C-17),11.8(C-18),19.3(C-19),36.1(C-20),18.9(C-21),33.8(C-22), 29.0(C-23),45.7(C-24),25.9(C-25),18.7(C-26),19.7(C-27),23.0(C-28),11.8(C-29)。以上數据与Li et al.(2008)的报道一致,故鉴定化合物4为 β-谷甾醇。
化合物5 白色针晶,EI-MS(m/z):426[M]+,分子式为C30H50O。1H-NMR(500 MHz,CDCl3) δ:2.31(1H,m,H-2),2.25(1H,m,H-4),1.18(3H,s,H-28),1.05(3H,s,H-27),0.98(3H,s,H-26),0.99(6H,d,J=5.1 Hz,H-29,30),0.95(3H,s,H-25),0.87(3H,s,H-23),0.72(3H,s,H-24);13C-NMR(125 MHz,CDCl3) δ:22.3(C-1),41.5(C-2),213.3(C-3),58.2(C-4),42.1(C-5),41.3(C-6),18.2(C-7), 53.1(C-8),37.4(C-9),59.5(C-10),35.6(C-11),30.5(C-12),38.3(C-13),39.7(C-14),32.4(C-15), 36.0(C-16),30.0(C-17),42.8(C-18),35.3(C-19),28.2(C-20),32.8(C-21),39.2(C-22),6.8(C-23), 14.7(C-24),17.9(C-25),20.3(C-26),18.7(C-27),32.1(C-28),35.0(C-29),31.8(C-30)。以上数据与Ageta et al.(1995)的报道一致,故鉴定化合物5为木栓酮。
化合物6 白色晶体,EI-MS(m/z):653[M]+,分子式为C45H80O2。1H-NMR(500 MHz,CDCl3) δ:5.37(1H,d,J=4.6 Hz,H-6),4.62(1H,t,J=5.5 Hz,H-3),2.31(2H,m,H-4),2.27(2H,t,J=7.5 Hz,H-2′),1.85(2H,m,H-2),1.26,1.62(2H,m,H-3′),1.02(3H,s,H-19),0.92(2H,d,J=6.5 Hz, H-24),0.89(2H,m,H-21),0.84(2H,m,H-26),0.82(3H,s,H-29),0.68(3H,s,H-18);13C-NMR(125 MHz,CDCl3) δ:37.0(C-1),27.8(C-2),73.7(C-3),38.2(C-4),139.7(C-5),122.6(C-6),31.9(C-7),31.9(C-8),50.0(C-9),36.6(C-10),21.0(C-11),39.7(C-12),42.3(C-13),56.7(C-14),24.3(C-15),28.2(C-16),56.0(C-17),11.8(C-18),19.3(C-19),36.2(C-20),18.8(C-21),33.9(C-22),26.1(C-23),45.8(C-24),29.1(C-25),19.8(C-26),19.0(C-27),23.1(C-28),12.0(C-29),173.3(C-1′),34.7(C-2′),25.1(C-3′),29.3(C-4′),29.4(C-5′),29.5(C-6′),29.6(C-7′),29.7(C-8′),29.7(C-9′),29.7(C-10′),29.7(C-11′),29.7(C-12′),29.7(C-13′),31.9(C-14′),22.7(C-15′),14.1(C-16′)。以上数据与孙红祥等(2002)的报道一致,故鉴定化合物6为 β-谷甾醇棕榈酸酯。
化合物7 红色结晶,ESI-MS(m/z):473[M+Na]+,分子式为C29H38O4。1H-NMR(500 MHz,CDCl3) δ:7.06(1H,d,J=5.2 Hz,H-6),6.49(1H,br s,H-1),6.32(1H,d,J=6.3 Hz,H-7),2.21(3H,s,H-23),1.43(3H,s,H-24),1.27(3H,s,H-28),1.24(3H,s,H-25),1.09(3H,s,H-27),0.58(3H,s,H-26);13C-NMR(125 MHz,CDCl3) δ:120.4(C-1),178.3(C-2),147.0(C-3),120.5(C-4),127.5(C-5),135.4(C-6),118.3(C-7),172.7(C-8),43.1(C-9),165.0(C-10),33.8(C-11),29.3(C-12),39.3(C-13),45.3(C-14),28.7(C-15),36.3(C-16),30.6(C-17),44.2(C-18),31.0(C-19),39.9(C-20),29.5(C-21), 34.5(C-22),10.5(C-23),38.3(C-24),21.4(C-25),18.7(C-26),31.5(C-27),182.5(C-28),32.4(C-29)。以上數据与倪慧艳等(2014)的报道一致,故鉴定化合物7为雷公藤红素。
化合物8 黄色粉末,ESI-MS(m/z):269[M-H]-,分子式为C15H10O5。1H-NMR(500 MHz,CDCl3) δ:7.52(1H,br s,H-4),7.18(1H,d,J=3.2 Hz,H-5),7.03(1H,d,J=1.8 Hz,H-2),6.57(1H,d,J=3.2 Hz, H-7),2.43(3H,s,H-11);13C-NMR(125 MHz,CDCl3) δ:165.8(C-1),124.9(C-2),148.8(C-3),121.5(C-4),133.9(C-4a),109.8(C-5),166.8(C-6),108.9(C-7),162.8(C-8),114.3(C-8a),191.0(C-9), 110.2(C-9a),183.1(C-10),136.0(C-10a),22.2(C-11)。以上数据与Kim et al.(2016)的报道一致,故鉴定化合物8为大黄素。
化合物9 无色针状晶体,ESI-MS(m/z):477[M+Na]+,分子式为C30H46O3。1H-NMR(500 MHz,CDCl3) δ:5.30(1H,t,J=3.7 Hz,H-12),4.14(1H,d,J=5.4 Hz,H-22),3.21(1H,m,H-3),1.20(3H,s,H-30),1.07(3H,s,H-27),0.99(3H,s,H-23),0.94(3H,s,H-25),0.93(3H,s,H-26),0.86(3H,s,H-28),0.78(3H,s,H-24);13C-NMR(125 MHz,CDCl3) δ:38.6(C-1),27.2(C-2),78.9(C-3),38.8(C-4),55.2(C-5),18.3(C-6),33.1(C-7),42.5(C-8),47.5(C-9),37.0(C-10),23.5(C-11),124.7(C-12), 140.2(C-13),39.3(C-14),25.2(C-15),24.3(C-16),35.3(C-17),43.4(C-18),39.8(C-19),39.5(C-20), 33.9(C-21),83.2(C-22),28.1(C-23),15.7(C-24),15.7(C-25),17.0(C-26),24.1(C-27),25.0(C-28), 182.5(C-29),21.0(C-30)。以上数据与Wang KW & Wang SW(2014)的报道一致,故鉴定化合物9为雷公藤内酯甲。
化合物10 无定形粉末,ESI-MS(m/z):353[M+Na]+,分子式为C20H26O4。1H-NMR(500 MHz,CDCl3) δ:6.35(1H,d,J=1.0 Hz,H-12),4.03(1H,d,J=11.4 Hz,H-19),3.44(1H,d,J=11.4 Hz,H-19), 2.98(1H,sept,d,J=6.9,1.2 Hz,H-15),2.82(1H,m,H-7),2.78(1H,m,H-1),2.67(1H,m,H-2), 2.46(1H,ddd,J=15.7,9.0,5.9 Hz,H-2),2.27(1H,m,H-7),1.99(1H,dd,J=13.0,2.2 Hz,H-5), 1.86(1H,m,H-6),1.79(1H,m,H-7),1.44(1H,m,H-1),1.32(3H,s,18-Me),1.26(3H,s,20-Me), 1.09(6H,dd,J=6.9,1.7 Hz,16,17-Me);13C-NMR(125 MHz,CDCl3) δ:34.4(C-1),34.2(C-2),220.6(C-3),50.2(C-4),51.6(C-5),17.8(C-6),25.4(C-7),142.5(C-8),147.5(C-9),37.0(C-10),187.3(C-11),131.8(C-12),153.3(C-13),187.5(C-14),26.3(C-15),21.3(C-16),21.2(C-17),22.5(C-18), 65.5(C-19),21.1(C-20)。以上数据与张彦文等(2007)的报道一致,故鉴定化合物10为雷藤二萜醌 B。
化合物11 无色针状晶体,ESI-MS(m/z):329[M+Na]+,分子式为C20H34O2。1H-NMR(600 MHz,CDCl3) δ:1.82(1H,m,H-13),1.55(2H,s,H-15),1.38(3H,s,H-17),1.03(3H,s,H-20),0.97(1H,d,J=6.2 Hz,H-9),0.95(3H,s,H-18),0.91(1H,t,J=11.2 Hz,H-5);13C-NMR(150 MHz,CDCl3) δ:40.4(C-1),18.3(C-2),35.7(C-3),39.2(C-4),56.8(C-5),20.7(C-6),42.4(C-7),45.3(C-8),57.0(C-9),38.7(C-10),18.0(C-11),26.8(C-12),49.0(C-13),37.5(C-14),57.9(C-15),79.3(C-16),24.5(C-17),27.1(C-18),65.5(C-19),18.3(C-20)。以上数据与Rocha et al.(2009)的报道一致,故鉴定化合物11为ent-kauran-16β,19-diol。
3.2 化合物抗菌活性结果
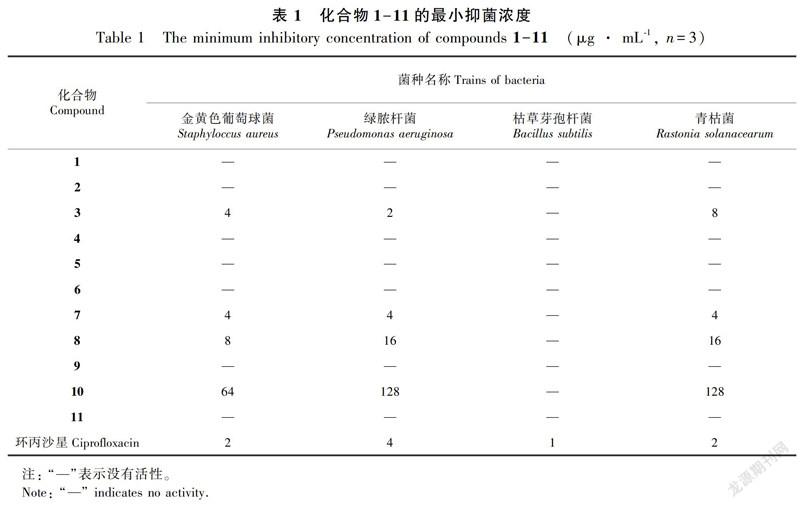
以金黄色葡萄球菌、绿脓杆菌、青枯菌、枯草芽孢杆菌等4种菌株对分离鉴定的11个化合物进行抗菌活性筛选,结果表明化合物3、7、8和10对金黄色葡萄球菌、绿脓杆菌、青枯菌有抑制作用,尤其是化合物3、7和8对金黄色葡萄球菌、绿脓杆菌、青枯菌有较强的抗菌作用,MIC值为2~16 μg ·mL-1(表1)。
4 结论
昆明山海棠和雷公藤是雷公藤属植物中的两个种,二者药理作用很相似,均具有显著的抗炎、抗肿瘤等活性,但其毒性要低于雷公藤。目前,针对雷公藤的相关研究及文献报道非常多,相比之下,对昆明山海棠的化学成分及其生物活性研究就显得比较少。通过对黔产昆明山海棠化学成分及其抑菌活性的研究,共分离鉴定化合物11个,其中化合物6、11为首次从该种植物中分离得到,且化合物11为首次从雷公藤属植物中分离得到。抑菌活性结果显示,化合物3、7、8和10对金黄色葡萄球菌、绿脓杆菌、青枯菌有抑制作用,并首次发现了化合物3、7对绿脓杆菌和青枯菌具有明显的抑制作用。从抑菌结果可以推测昆明山海棠的抑菌活性成分主要为萜类化合物,后續还需对分离得到的其他同类化合物进行抑菌活性测定,并对其构效关系进行研究,为设计和改造具有更高活性的化合物提供思路。另外,针对化合物3、7的毒性、其对绿脓杆菌抗药性方面的研究,以及对青枯菌的抑菌作用机理也有待进一步探究。
参考文献:
AGETA H, ARAI Y, SUZUKI H, et al., 1995. NMR spectra of triterpenoids. Ⅲ. oleanenes and migrated oleanenes[J]. Chem Pharm Bull, 43(2):198-203.
CARVALHO LM, SEITA J, 1993. A new oleanolic acid derivative from securinega tinctoria[J]. Planta Med, 59(4):369-372.
Editorial Board of the Chinese herbology of the State Administration of Traditional Chinese Medicine, 1999. The Chinese herbology[M]. Shanghai:Shanghai Science and Technology Press:200-205.[国家中医药管理局《中华本草》编委会,1999.中华本草[M]. 上海:上海科学技术出版社:200-205.]
KIM MO, PARK YS, NHO YH, et al., 2016. Emodin isolated from Polygoni Multiflori Ramulus inhibits melanogenesis through the Liver X receptor-mediated pathway[J]. Chem-Biol Interact, 250:78-84.
KUTNEY JP, HAN K, 1996. Studies with plant-cell cultures of the chinese herbal plant, Tripterygium wilfordii. Isolation and characterization of diterpenes[J]. Recl Trav Chim Pays-Bas, 115:77-93.
LI WH, CHANG ST, CHANG SC, et al., 2008. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark[J]. Nat Prod Res, 22(12):1085-1093.
NI HY, ZHANG CH, SONG WJ, et al., 2014. Chemical constituents of root bark of Celastrus orbiculatus Thunb[J]. Chin Pharmacol J, 49(21):1889-1891.[倪慧艳, 张朝晖, 宋文静, 等, 2014. 南蛇藤化学成分研究[J]. 中国药学杂志, 49(21):1889-1891.]
ROCHA D, TAKAHASHI JA, BOAVENTURA MAD, 2009. Di- and Tri-hydroxylated kaurane derivatives from microbial transformation of ent-kaur-16-en-19-ol by Cephalosporium aphidicola and their allelopathic activity on Lactuca sativa[J]. Ecl Quím, So Paulo, 34(1):57-62.
SUN H, FANG WS, HU C, 2008. An efficient semi-synthesis and structure revision of a cytotoxic triterpenoid 25-acetoxy-3α-hydroxyolean-12-en-28-oic acid from Liquidamber styraciflua[J]. J Asian Natl Prod Res, 10(4):271-276.
SUN HX, YE YP, YANG K, 2002. Studies on the chemical constituents in Radix Astilbes Chinensis[J]. Chin J Chin Mater Med,(10):34-37.[孙红祥, 叶益萍, 杨可, 2002. 落新妇化学成分研究[J]. 中国中药杂志,(10):34-37.]
WANG KW, WANG SW, 2014. Chemical constituents of Euonymus bockii[J]. Chem Nat Compd, 50(5):948-949.
ZHANG YW, FAN YS, WANG XD, et al., 2007. Diterpenoids possessed immunosuppressive activity from Tripterygium hypoglaucum[J]. Chin Trad Herb Drugs,(4):493-496.[张彦文, 范云双, 王晓东, 等, 2007. 昆明山海棠中具有免疫抑制活性的二萜化合物[J]. 中草药,(4):493-496.]
ZHEN QS, YE S, WEI ZJ, 1995. Recent progress in research on Tripterygium:A male anti fertility plant[J]. Contraception, 51(2):121-129.
ZHOU JW, PAN RN, LIU LW, 1991. Preliminary report on the anti-fertility effect of T. hypoglaucum Lévl. Hutchins. on male rats[J]. Med Pharm Yunnan, 12(4):232-235.[周激文, 潘汝能, 刘黎闻, 1991. 昆明山海棠对雄性大鼠的抗生育作用初试报告[J]. 云南医药, 12(4):232-235.]
