Optimal timing and route of nutritional support after esophagectomy: A review of the literature
2019-09-02RichardZhengCourtneyDevinMichaelPucciAdamBergerErnestRosatoFrancescoPalazzo
Richard Zheng, Courtney L Devin, Michael J Pucci, Adam C Berger, Ernest L Rosato, Francesco Palazzo
Abstract Some controversy surrounds the postoperative feeding regimen utilized in patients who undergo esophagectomy. Variation in practices during the perioperative period exists including the type of nutrition started, the delivery route, and its timing. Adequate nutrition is essential for this patient population as these patients often present with weight loss and have altered eating patterns after surgery, which can affect their ability to regain or maintain weight. Methods of feeding after an esophagectomy include total parenteral nutrition,nasoduodenal/nasojejunal tube feeding, jejunostomy tube feeding, and oral feeding. Recent evidence suggests that early oral feeding is associated with shorter LOS, faster return of bowel function, and improved quality of life.Enhanced recovery pathways after surgery pathways after esophagectomy with a component of early oral feeding also seem to be safe, feasible, and cost-effective,albeit with limited data. However, data on anastomotic leaks is mixed, and some studies suggest that the incidence of leaks may be higher with early oral feeding.This risk of anastomotic leak with early feeding may be heavily modulated by surgical approach. No definitive data is currently available to definitively answer this question, and further studies should look at how these early feeding regimens vary by surgical technique. This review aims to discuss the existing literature on the optimal route and timing of feeding after esophagectomy.
Key words: Esophagectomy; Oral feeding; Early feeding; Delayed feeding; Enteral nutrition; Esophageal cancer; Jejunostomy tube; Postoperative complications
INTRODUCTION
Esophagectomy is one of the most complex gastrointestinal operations with historic complication rates ranging from 20% to 80%[1,2]. Anastomotic leaks, one of the most feared and life-threatening complications, have a reported incidence of 5% to 40%.
Given these data, several aspects of the operative technique and perioperative care have been focus of continued study in an attempt to improve outcomes. Critical to the success of any operation is the optimization of the patient's nutritional status. With regards to esophageal cancer, malnutrition and cancer cachexia are particularly prevalent and should be addressed in the perioperative period to improve outcomes[3,4].
Some controversy exists for certain aspects of postoperative management of patients who undergo an esophagectomy. Among these, much variation exists with regards to nutritional support, particularly regarding the optimal timing and delivery route of nutrition. Some institutions have embraced the practice of enhanced recovery after surgery (ERAS) programs, with variation in practice related to early introduction of oral intake, enteral feeding, and timely removal of nasogastric tube and drains[5].Others follow a more traditional approach of postoperative management with long periods of nil-per-os (NPO)[6]. Proponents of this traditional approach feel that delaying oral feeding protects the anastomosis and may avoid pulmonary complications secondary to aspiration. Means of providing nutrition during this period have included total parenteral nutrition (TPN), nasoduodenal/nasojejunal tube feeding, and jejunal tube feeding.
General concepts and historical perspective
Although many methods of nutritional support have been utilized, enteral feeding via jejunostomy tube (j-tube) has become the standard of care in patients undergoing esophagectomy[7,8]. Gerndt and Orringer first documented the routine use of the Witzel tube jejunostomy in 1994 as a means of providing enteral nutrition after esophagectomy, reporting a low rate of major complications (2.1%) comparable to that of needle catheter jejunostomy[9]. Since then, intraoperative placement of a Witzel jejunostomy at the time of esophagectomy has become the preferred method of enteral access in the immediate postoperative period. Enteral nutrition supplementation, however, does not prevent weight loss entirely. Donohoe et al[10]performed a prospective cohort study to analyze the impact of supplemental home enteral nutrition post esophagectomy on nutrition parameters and patient satisfaction;they found patients had substantial weight loss [41% lost more than 10% body mass index (BMI)] despite also having high satisfaction scores and compliance rates (96%).Weijs et al[7]found that weight loss following esophagectomy occurred after tube feeds were discontinued, and that discharging patients home with tube feeding did not reduce length of stay or readmissions.
Weight loss after esophagectomy appears to be universal despite dedicated diet support and prolonged home enteral supplementation. Seventeen percent to eightytwo percent of patients will experience weight loss in the first postoperative month[7].In addition, 5%-12% of these patients suffer the majority of their weight loss within the first six months postoperatively and 27%-95% of patients do not return to their baseline weight[11]. A population-based study in Sweden reported that 63.7% of patients had lost more than 10% of their preoperative BMI six months after their esophagectomy due to appetite loss, eating difficulties, and odynophagia[12]. It is likely that the original disease process and method of surgical treatment have a significant impact on a patient's nutritional status independent of other adjustable risk factors.An adequate postoperative feeding regimen is essential for maintaining weight and adequate nutrition parameters.
TIMING OF FEEDING - EARLY VS DELAYED
Enteral feeding after esophagectomy can be provided in the form of artificial nutritional supplementation (i.e., tube feeds) or direct oral feeding. There is no consensus among surgeons with respect to the timing at which enteral feeding - oral or artificial - should be initiated. Enteral supplementation via feeding jejunostomy is largely well-tolerated and can decrease morbidities associated with malnutrition, such as surgical site infections[4]. However, feeding through a jejunostomy tube is not without risks; minor complications, like dislodgement or occlusion, are common, and can cause difficulties with maintaining prolonged enteral feeding. More serious complications, such as torsion or obstruction, are rare but can cause serious delays in nutritional support. Historically, reluctance to start an oral diet after major gastrointestinal surgery generally has not been evidence-based, but instead based on fears regarding anastomotic leakage, aspiration, and inadequate nutritional intake with oral feeding[6]. More recent evidence suggests no advantage to a lengthy NPO period, and early initiation of feeding (within 24 h) after gastrointestinal resections of any kind may confer a mortality benefit[13]. As outcomes improve and more minimally invasive esophagectomies (MIE) are performed, surgeons have begun to challenge the practice of artificial enteral feeding after esophagectomy by starting oral feeding early in the postoperative course. Definitions of “early” and “late” feeding are also not universally agreed upon, but early feeding informally refers to any nutrition started in the first 24-48 h after esophagectomy, whereas delayed feeding is started anywhere from five days to several weeks postoperatively. The following section provides a review of the most relevant studies regarding early vs delayed oral and enteral feeding.
The concept and practice of early artificial enteral feeding
Enteral nutrition given through a j-tube enters the jejunum well downstream of the esophago-gastric anastomosis and may be started shortly after esophagectomy. A number of studies show that early artificial enteral nutrition has a clear association with improved functional and nutritional outcomes with decreased rates of infectious and other complications in patients who undergo esophagectomy. These studies,however, are limited by their inconsistent definition of “early” feeding and the variety of surgical approaches used in each study (Table 1).
Wang et al[14]looked at esophageal cancer patients to evaluate early enteral nutrition(within 48 h postoperatively) compared to later feeding (more than 72 h). Patients who started j-tube feeds within 48 h after undergoing an esophagectomy had the lowest thoracic drainage volume, the earliest first fecal passage, and the lowest length of hospital stay and costs compared to those who started after 72 h. In addition, the incidence of pneumonia was the highest in the late feeding group and the nutrition parameters were significantly worse. This suggests that enteral feeding early in the first 48 h is associated with better outcomes.
A retrospective review of transthoracic esophagectomies from 1996-2010 found that patients with enteral nutrition started on postoperative day three via j-tube had earlier return of bowel function (ROBF), shorter duration of systemic inflammatory response,and no significant difference in infectious complications such as pneumonia, wound infection, and sepsis compared to j-tube feeding after the third postoperative day[15].The frequency of anastomotic dehiscence was higher in the early enteral feed group,which the authors attribute to the higher rates of neoadjuvant chemotherapy in this patient cohort. Early enteral nutrition reduces the rate of life-threatening complications after thoracic esophagectomy in patients with esophageal cancer[16].
In support of early direct oral feeding after esophagectomy
Despite the many advantages of early artificial enteral feeding, surgeons have been more hesitant to start oral feeding early after esophageal resections and for these reasons studies on this topic have been historically limited and often included a variety of gastrointestinal procedures, such as colorectal[17]and gastric surgery[18].More recently, surgeons have dared to carry out randomized clinical trials in which early oral feeding (EOF) without artificial enteral supplementation is compared to jtube feeding or delayed oral feeding regimens. In 2008, Lassen et al[19]published a study in which patients undergoing major upper gastrointestinal surgeries were randomized to either NPO with j-tube feeding or EOF starting on the first postoperative day. Esophagectomies were a small percentage of this cohort (n =8/447) and were not specifically parsed out in subgroup analysis. They found that LOS and ROBF were both significantly shorter in the EOF group without a significant difference in leakage rates or complications. A 2015 randomized, controlled trial by Mahmoodzadeh et al[20]comparing early oral feeding on POD1 to delaying oral feeding after ROBF in patients undergoing transthoracic esophagectomy or total/partial gastrectomy also found that EOF alone was associated with shorter LOS,faster ROBF, and fewer readmissions. An observational cohort study from 2018 evaluating the timing of oral feeding after upper gastrointestinal surgery (including 24 esophagectomies) also found a significant decrease in LOS when patients were fed orally on POD1 vs POD3[21].
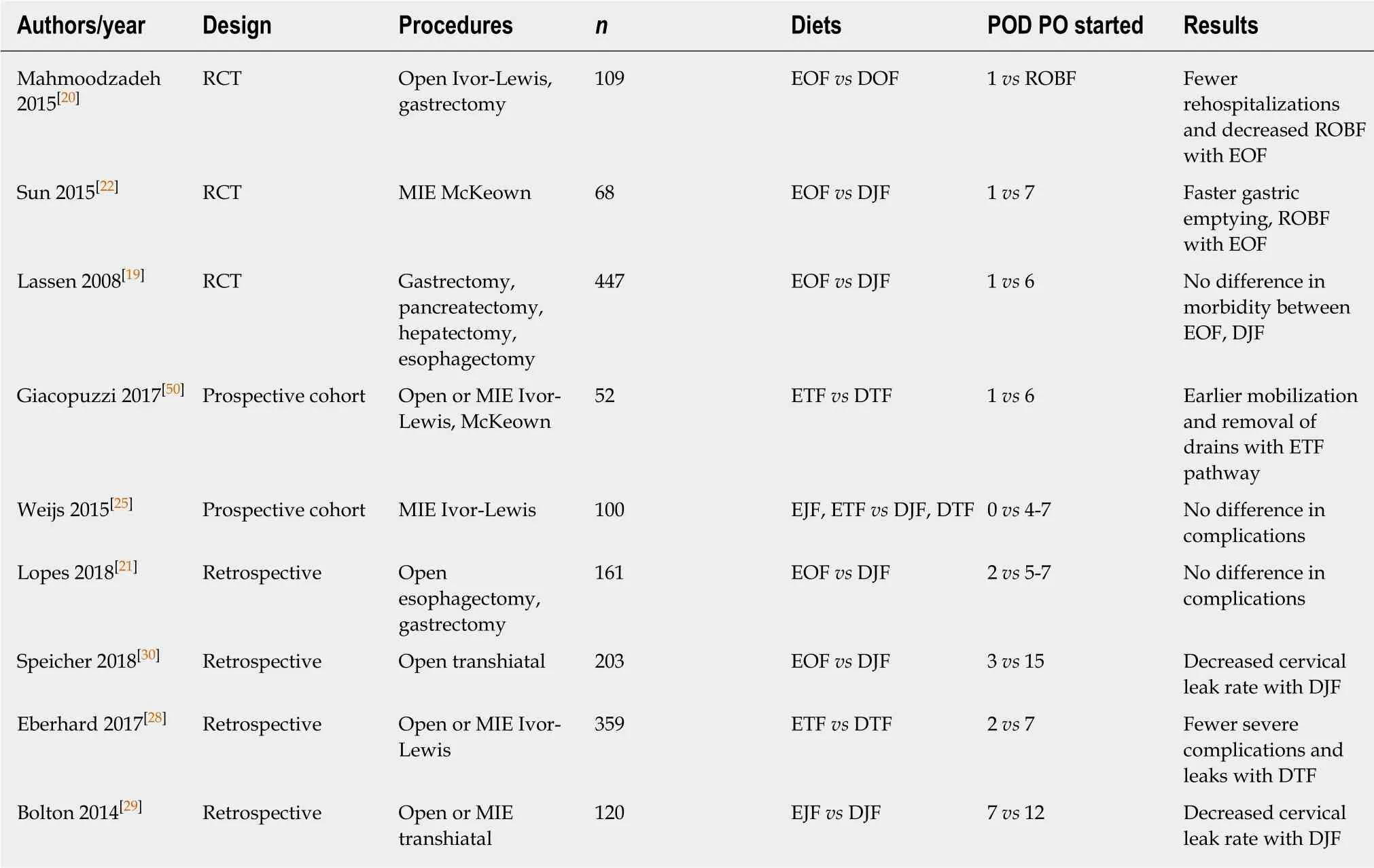
Table 1 Literature review of comparative trials on oral feeding after esophagectomy
Studies that have focused on EOF after esophagectomy alone have largely confirmed that there is a significant benefit with regards to return of bowel function and hospital stay. A prospective cohort study from Sun et al[22]in 2015 comparing EOF(POD1) to late feeding (POD7) after minimally-invasive McKeown esophagectomy confirmed that EOF patients had shorter ROBF and improved short-term quality of life metrics without any meaningful increase in complications. Like Mahmoodzadeh and Lassen, their EOF group was not supplemented with supplemental artificial enteral nutrition. In the long term, patients that started EOF had similar changes in weight after one year and required fewer procedural interventions related to nutrition(i.e., prolonged tube feeding, restarting tube feeding, placement of a catheter for TPN)than patients with delayed oral feeding[23]. Additionally, early oral feeding after esophagectomy also decreases the stress response after minimally-invasive McKeown esophagectomy as measured by levels of circulating inflammatory cytokines[24].
Risks of early direct oral feeding
Despite these reported benefits, early direct oral feeding after esophagectomy is not without risks. Some of the concerns that initially deterred surgeons from reliance on early postoperative feeding - namely, increased anastomotic leak rates and inadequate nutritional intake - appear to be viable concerns based on the results of several studies. A multicenter, prospective trial by Weijs et al[25]of 50 minimally invasive thoracoabdominal (Ivor-Lewis) esophagectomies compared EOF (clear liquids on POD1) to starting clear liquid intake on POD4-7; they found that patients in the EOF arm were only able to achieve 58% of goal caloric intake with oral feeding alone. In general, nutritional goals are rarely met in the immediate postoperative period after esophagectomy[26]. Furthermore, weight loss at six months occurs in the vast majority of patients undergoing esophagectomy, even in those who are supplemented with jejunostomy tube feeding[27]. It is unclear whether oral feeding can truly affect this in the long-term.
Some retrospective studies have found a significantly increased leakage rate in EOF patients. A retrospective study of 359 esophagectomies by Eberhard et al[28]compared EOF (POD1) to delayed feeding (POD7) and found that leakage rates were lowest in patients with delayed feeding who were transitioned to a blended diet (2% vs 9%; P =0.043). Patients in the delayed feeding group also had a lower incidence of pulmonary complications (31% vs 39%, P = 0.001). Most of the procedures were open Ivor Lewis esophagectomies, and patients in the delayed group had significantly fewer comorbidities as measured by mean Charlson Comorbidity Index score (16 vs 26, P =0.027). A retrospective review of leak rates found that patients undergoing cervical esophagectomy had a significantly decreased rate of anastomotic leak (3% vs 23%)with delayed oral feeding (POD12) vs early feeding (POD3-7)[29]. Similarly, a separate retrospective review of 203 patients undergoing open transhiatal esophagectomy also showed the cervical anastomotic leak rate was 4.2% in the delayed cohort (oral feeding on POD15) compared to 14.5% in the EOF group (POD3)[30]. Among patients with anastomotic leaks, most were managed with bedside drainage and NPO, but a minority required operative or endoscopic intervention. In all of these studies,artificial enteral feeding was administered while feeds were delayed.
These findings are in contrast to those of other prospective studies by Berkelmans et al[23]and Sun et al[22], which both failed to find significant differences in the incidence of either anastomotic leakage or pneumonia. This may be due to the retrospective nature of these studies and, as Jules Lin points out in his commentary on the Speicher et al.study, the problematic inclusion of historical controls[31]. However, the Weijs study also uses a retrospective cohort as a control group. Instead, the differences in anastomotic leak rates may plausibly be related to two confounding factors: Location of anastomosis (cervical vs intrathoracic) and surgical approach (open vs minimally invasive). In the studies by Eberhard, Speicher, and Bolton, which found an increased leakage rate in the EOF group, all esophagectomies were performed via an open approach, whereas Berkelmans et al[23]and Sun et al[22]only included minimally invasive McKeown and Ivor Lewis esophagectomies, respectively. Furthermore, the two trials with the most profound differences in leak rates between EOF and delayed feeding (Bolton and Speicher) were both limited to cervical anastomoses, which are significantly more prone to anastomotic leak than transthoracic anastomoses. Finally,the literature surrounding early oral feeding is largely lacking in studies with large sample sizes or studies focusing specifically on esophagectomy, limiting our ability to develop firm recommendations about the practice of oral feeding at this time.
Several ERAS pathways have been developed for post-esophagectomy management with early oral feeding as a central component. Overall, ERAS protocols after esophagectomy appear to be a feasible way of improving general functional recovery without an increase in non-surgical morbidity. A systematic review of postesophagectomy ERAS pathways included thirteen studies in its meta-analysis and found that ERAS pathways were associated with a shorter LOS and decreased pulmonary complications without a significant increase in readmissions[32]. A similar review of the same studies also singled out early oral feeding as one of several key components associated with reduction in LOS in a multivariable analysis that included other factors such as early mobilization, artificial enteral feeding, and early removal of drains[33]. An ERAS pathway with early oral feeding (clears on POD3)without concurrent tube feeding has also been associated with significant cost-savings when compared to conventional management[34]. From these limited non-randomized studies, it appears that ERAS pathways are practical and safe in selected patients;however, not all of these pathways include early oral feeding, and most still initiate oral intake between POD3 and 5. Furthermore, there exists significant variability among these ERAS pathways with regards to the type of esophagectomy performed and to the degree to which oral feeding was supplemented by tube feeding; the vast majority (12/13) of these institutional ERAS pathways included a mix of transthoracic, transhiatal, and McKeown approaches. Moreover, 11/13 studies included in this same review supplemented their early oral feeding with concurrent artificial enteral feeding[32]. The precise impact of postoperative diet alone on the outcomes measure remains to be understood. Conclusions about the efficacy of these non-homogenous, single-center pathways is limited; more standardized and rigorous study of ERAS pathways with early feeding is required.
METHODS OF ARTIFICIAL FEEDING AFTER ESOPHAGECTOMY
TPN
TPN is provided through a central venous catheter. In a meta-analysis, TPN was shown to have no effect on mortality and no significant reduction in complication rates in surgical patients when compared to an oral diet[33]. However, one of the early prospective, randomized trials showed the incidence of severe complications was higher in patients receiving TPN compared to enteral nutrition (EN), with a higher rate of sepsis requiring intervention, venous thrombosis, electrolyte imbalance, and liver failure[34]. Overall, prospective trials have shown the use of TPN after esophagectomy to be associated with increased infectious and thrombotic complications while also achieving inferior nutritional status and higher costs compared to enteral feeding[35,36]. TPN has largely been relegated to situations in which enteral nutrition is contraindicated.
Nasoduodenal/nasojejunal tube feeding
Enteral feeding administered via a nasoduodenal (ND) or nasojejunal (NJ) route allows for the associated benefits of enteral feeding when compared to TPN and may be considered less invasive than a surgically placed feeding tube. A meta-analysis of ten studies on enteral feeding showed postoperative enteral nutrition via ND or NJ feeds during the first seven postoperative days may decrease pulmonary complications and lower the anastomotic leak rates while also maintaining a higher albumin level and likely better nutritional status when compared to parenteral nutrition[37]. While an advantage is evident over TPN, the most common complication of ND/NJ enteral access is dislocation of the feeding tube, which occurs in 20%-35%of patients[7]. This results in interrupted feeds while awaiting replacement with subsequent delayed nutrition.
Jejunostomy tube feeding
Early postoperative enteral nutrition improves gut oxygenation and reduces costs in patients who undergo upper gastrointestinal tract surgery for cancer when compared with TPN[36]. In a large retrospective cohort study looking at Chinese patients with esophageal cancer, the authors found that early EN reduced the postoperative length of stay and subsequently, hospital charges with no significant difference in anastomotic leaks or clinical outcomes when compared to TPN[38]. As such, enteral feeding is definitively the standard of care in most feeding protocols after esophagectomy.
Using the Surveillance Epidemiology and End Results-Medicare (SEER) database,Lorimer et al[39]found that in patients who underwent esophagectomy, mortality was lower and short-term survival was improved at 90 d with an overall shorter length of hospital stay in patients with a j-tube. Enteral feeding via jejunostomy appears to be associated with improved patient outcomes and allows for early establishment of enteral nutrition for eventual transition to an oral diet, which can be managed in an outpatient setting. Furthermore, patients undergoing neoadjuvant treatment for locally advanced esophageal cancer are at particularly high risk for malnutrition, and thus may have j-tubes placed prior to initiating their neoadjuvant regimen[40]. A retrospective review of a prospective database by Dalton et al[41]showed that patients with feeding jejunostomies placed prior to undergoing neoadjuvant therapy have a significantly lower incidence of pneumonia within 30 d of esophagectomy.
Despite the many benefits of enteral nutrition, tube-related complications do occur and can impact a patient's recovery after surgery. The incidence of jejunostomyrelated complications is approximately 30%. Minor complications - leakage,dislocation, superficial infection, tube occlusion - make up the vast majority of occurrences[9]. Severe complications are exceedingly rare but can be life-threatening,including small bowel necrosis, intestinal torsion, and intussusception[42-44]. Patients who suffer more serious complications related to their jejunostomy tubes often are unable to tolerate enteral feeding; nearly 20% of patients with feeding tubes still required some period of TPN administration, with 7% directly attributable to tube feeding intolerance or complications[45].
In looking at return visits to the emergency department in the first year after esophagectomy, Kidane et al. found that feeding tube-related problems were the most common cause of returning to the ED (39%)[46]. Alvarez-Sarrado et al[47]performed a retrospective study comparing outcomes in esophagectomy patients with feeding jejunostomy compared to TPN and noted that the overall rate of j-tube complications in this study was 51%. Although this rate appears especially high, 88% of these complications were mild, and only 4% of patients had severe complications.Additionally, 70% of patients were no longer using a j-tube for enteral feeds after 30 d.
Selective feeding jejunostomy placement
Such complications and the lack of long-term use of j-tubes for feeding have led Álvarez-Sarrado et al[47]to recommend that jejunostomy placement should not be routine and instead, considered only in select patients. They recommended considering feeding jejunostomy at time of surgery in patients who are malnourished or have severe dysphagia preoperatively. Selective placement of feeding jejunostomy was also supported by a Japanese study led by Akiyama et al[48]who compared the operative outcomes of patients who received postoperative enteral nutrition via feeding jejunostomy with concurrent peripheral parenteral nutrition (PPN) and those who received only parenteral nutrition. Interestingly, the authors found no significant differences in the rate of infectious complications, postoperative length of stay,readmissions within 30 d, or pneumonia within 6 months between the two groups.Similarly, a retrospective analysis of patients undergoing minimally invasive Ivor-Lewis esophagectomy found that patients without j-tubes (in whom oral feeding was delayed until POD5) had similar levels of weight loss and complication rates when compared to those with j-tubes[49]. Given that they identified no increase in postoperative complications in patients who did not receive enteral nutrition via feeding jejunostomy, these authors have suggested that j-tube placement may not be necessary for all patients undergoing esophagectomy.
Patients who have an uncomplicated postoperative course and are able to tolerate oral feeding during the initial perioperative period may find the associated risk of feeding jejunostomy complications to be too high. However, for those patients who are already at higher preoperative risk for complications - those with preoperative dysphagia, the frail or elderly, the malnourished, and those with severe pulmonary or systemic comorbidities - feeding jejunostomy will allow for a safer and more durable option of delivering nutrition than TPN (Table 2). Compliance with early feeding is difficult; 32%-58% of patients in early feeding programs struggle to meet goal caloric intake with oral feeding alone[25,50]. Furthermore, for patients that are intraoperatively unstable or develop postoperative complications (such vocal cord palsy, respiratory issues, or a significant anastomotic leak), a feeding tube is an invaluable means of providing nutrition during an otherwise lengthy NPO period.
Studies that question the benefits of routine feeding jejunostomy placement have largely been retrospective in nature. Prospective and randomized studies are needed prior to determination of the selective use of feeding jejunostomies. The NUTRIENT II trial is one multicenter, randomized controlled trial which is currently open and aims to compare functional recovery with regards to timing of oral feeding between patients undergoing minimally invasive esophagectomy. More high-level evidence such as this is needed to elucidate the optimal timing of oral feeding.
CONCLUSION
Although enteral feeding after esophagectomy is now a widely-accepted standard of care, the timing and method of delivering enteral nutrition remains in question.Recent evidence suggests that early oral feeding is associated with shorter LOS, faster ROBF, and improved quality of life. However, data on anastomotic leaks is mixed,and some studies suggest that the incidence of leaks may be higher with early oral feeding. This risk of anastomotic leak with early feeding may be heavily modulated by surgical approach. No definitive data is currently available to definitively answer this question, and further studies should look at how these early feeding regimens vary by surgical technique. Although some randomized controlled trials of early oral feeding after esophagectomy exist, they are lacking in size and standardization of esophagectomy techniques between these trials remains difficult. Pathways that successfully incorporate early oral feeding may allow for the selective placement of jtubes and avoidance of common j-tube related complications, but for patients who develop serious complications that prevent oral intake, the presence of a j-tube remains a necessary back-up plan. However, there may be certain patients who do not need j-tubes during esophagectomy and may suffer more harm than benefit from having one. It is clear that a one-size-fits-all approach may not be the right way to think about dietary management after esophagectomy. As we continue to push the boundaries of care with early oral feeding, we must continuously remind ourselves that we can and must continue to improve. We have come very far in reducing the trauma associated with such a historically morbid procedure, but we still have a long way to go.
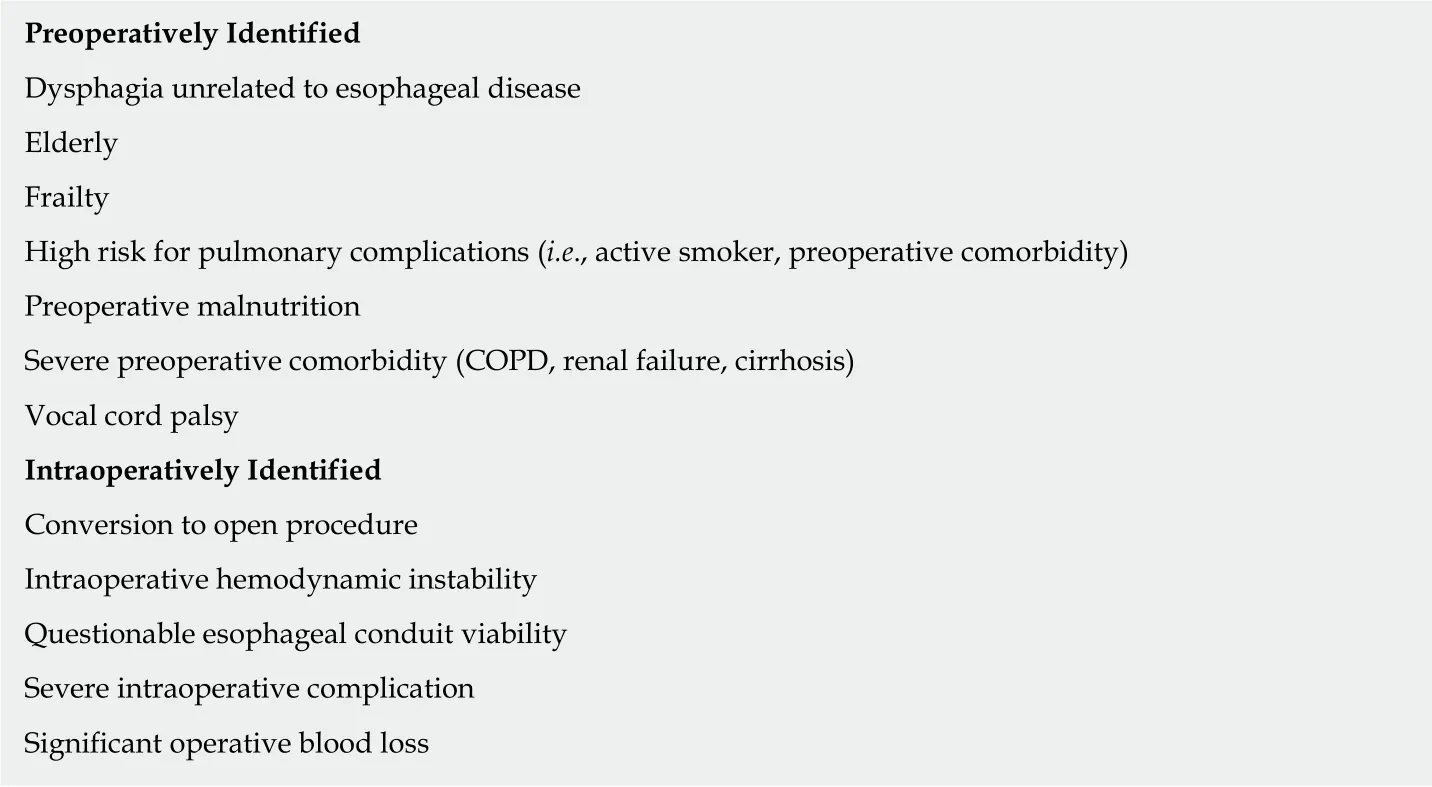
Table 2 Recommended indications for jejunostomy tube placement during esophagectomy
杂志排行
World Journal of Gastroenterology的其它文章
- Methionine adenosyltransferases in liver cancer
- lleal-anal pouches: A review of its history, indications, and complications
- Common features between neoplastic and preneoplastic lesions of the biliary tract and the pancreas
- Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers
- Systemic inflammation in colorectal cancer: Underlying factors,effects, and prognostic significance
- Overview and comparison of guidelines for management of pancreatic cystic neoplasms
