Dielectric Characterization and Microwave Roasting of Molybdenite Concentrates at 915 MHz Frequency
2019-07-25YonglinJiangBingguoLiuPengLiuJinhuiPengandLiboZhang
Yonglin Jiang,Bingguo Liu* ,Peng Liu,Jinhui Pengand Libo Zhang
(1.National Local Joint Engineering Laboratory of Engineering Applications of Microwave Energy and Equipment Technology,Kunming 650093,Yunnan,China;2.Key Laboratory of Unconventional Metallurgy,Ministry of Education,Kunming University of Science and Technology,Kunming 650093,Yunnan,China;3.Faculty of Metallurgical and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,Yunnan,China)
Abstract:The magnetic hysteresis loop was measured to know the magnetic property of molybdenite concentrate.In order to evaluate its microwave absorption capacity,the dielectric properties of molybdenite concentrate was investigated using cavity perturbation method at 915 MHz dependent on densities and temperatures.The parameter data were fitted using regression fit and a model related to the same density and temperature ranges was developed.A nonlinear surface fitting was used to present visually the effect of dielectric parameters on the microwave penetrate depth of molybdnite concentrate.The crystal products of MoO3obtained from microwave roasting at different temperatures were examined by scanning electron microscopy(SEM)and X-ray diffraction(XRD).The results show both the dielectric constants and loss factors increase in the increase of apparent densities and temperatures with different growth rates in the experimental range.Due to the distinguished trend of dielectric performance dependent on temperatures,two parts in the heating scenario for the molybdenite concentrate samples were divided.The microwave penetration depth is inversely proportional to both apparent densities and temperatures.The nonlinear fitting surfaces indicate the increase of dielectric loss provides an enough decrease in microwave penetration depth.In contrast,the dielectric constant has a positive effect for it.Pure MoO3was produced at 800 ℃ by using microwave energy.This work can be helpful to design and simulate microwave system for efficient beneficiation of molybdenite concentrate and to prepare molybdenum products from this concentrate.
Keywords:dielectric properties;temperature;molybdenite concentrate;microwave heating
1 Introduction
Microwave heating can be more efficient and economic than conventional heating methods in which the heat is conducted from the surface into inner media of the samples beside an outer heat source,and be beneficial to save energy and time because the heat transferred from microwave energy is created directly among the molecular within a sample.Normally,microwave brings selective and rapid heating about the whole efficiency of 80% - 85%[1]compared to the conventional thermal system.Microwave heating under the commercial frequency of 915 MHz or 2 450 MHz is currentlyas a new technique which has been increasingly applied to metallurgy, drying[2],welding,mineral processing and coking coal desulfurization[3]. For instance, due to the nonhomogeneity and composition difference within coal,the microwave absorption capacity from different part of the coal is different,thus the heat generation level is different and the decomposition for small molecule species including sulfur first occurs.The microwave effects imposed in mined ore are similar to those of coals.
How strong asubstance can beheated by microwave depends on its dielectric properties that depend on the polarization[4-5]capacity of molecules and atoms in the substance.The higher the values of dielectric properties are,the strongerinteraction between dipoles and microwave is,which indicates an excellent heating performance under microwave radiation.Materials dependent microwave processing can be selected by dielectric properties held on normal conditions orhigh temperature.Moreover, when temperature becomes high,the dielectric properties will be changed[6].It is essential to evaluate what the temperature is when a material can reach through the dielectric dataathigh temperature in microwave incident,as well as how to adjust the sizeof microwave power in a continuous production.The microwave penetration depth deprived from the sample’s dielectric permittivity is also the basic information to determine if a uniform heating has been achieved during a microwave treat.In our work the cavity perturbation method was used for a measure of dielectric properties of the concentrate samples.The fundamental perception of this resonance method is that the presence of a small rod-shaped dielectric specimen in the resonant cavity or the change of the cavity’s itself volume will cause a shift of resonant frequency and an offset of the quality factor to finally determine the scattering parameters[7].
Molybdenite concentrate(about 90%MoS2)[8]is a feedstock for the molybdenum industry in the production of technical grade molybdenum-containing productssuch asmolybdenum,ferromolybdenum alloy, molybdenum oxide[9]and other pure molybdenum compounds. At present, the pyrometallurgical[10]processing of molybdenite concentrate has been commercially carried out in the multiple heart furnace,fluidized bed furnace and part of rotary kiln[11].While most of these processes still utilize a heating method in traditional types.Therefore,itis crucialto study the dielectric properties for molybdenite concentrate at room temperature and how they change as the temperature rises,aiming to help set up an appropriate microwave heating system for the molybdenite technology.In this work,the dielectric properties of molybdenite concentrate were measured and discussed under different apparent densities and temperatures.The productsobtained from roasting the molybdenite concentrate at different temperatures were characterized and compared.The research results indicate the high-temperature roasting and efficient mineral processing for molybdenite concentrate by using microwave energy are feasible.
2 Materials and Methods
2.1 Sample Preparation
The molybdenite concentrate as the raw material wasprovided by Jiangsu Hengxing Tungsten &Molybdenum Co., Ltd.ofChina.Thechemical composition of the concentrate is given in Table 1.Mineralogical analysis showed that two mass fractions of Mo and S contents are about 44.64%and 28.17%,respectively.In the molybdenite concentrate material the main impuritiesare silicon dioxide, copper,calcium oxide and lead.
The powderX-ray diffraction (XRD) was recorded in Fig.1.

Table 1 The main chemical composition of the molybdenite concentrate (wt%)

Fig.1 XRD pattern of the raw concentrate materials
Fig.2 shows that the grain sizes of molybdenite concentrate have a wide ranging from tens of microns up to about 100 μm and the concentrate particles possess layer structure or irregular block structure.It is possible to infer that impurity mixtures are scattered bonding with the concentrate.
2.2 Setup for Dielectric Measurements
The perturbation testdevice thatcarriesout dielectric mesurement is independently developed by the Key Laboratory of Unconventional Metallurgy,Kunming University of Science and Technology.The main system components are connected as shown in Fig.3.The test sample is filled into a quartz cube with certain volume and the quartz cube nozzle is stoppered by high-temperature cottons to avoid any oxidation for the sample and prevent it from coming out.The quartz cube is loaded by the quartz support.The cylindrical cavity is installed in the upper ofthe resistance furnace.The quartzsupport is transmitted in an alumina ceramic tube between the cylindrical cavity and resistance furnace via a pressure control.Before commencing each measurement,the sample is heated in the resistance furnace to arrive a specific temperature and then is rapidly pushed up into the dielectric resonator.The TE11mode wave at 915 MHz is fed into dielectric resonator by the coaxial probe and thus the perturbative effects forthesamples are realized.The vector network analyser(E5071C)is utilized to mesure and converse the scattering parameterswhich are recorded by a computer.Penetration depths were calculated from these resultant datas. Addtionally, room-temperature magnetic properties of the concentrate samples were measured by a Riken vibrating specimen magnetometer.

Fig.2 SEM micrographs of molybdenite concentrate specimen
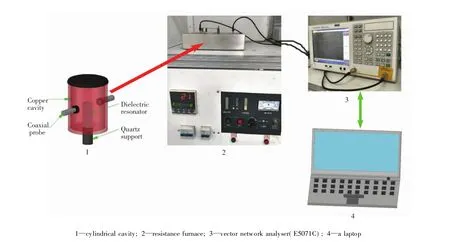
Fig.3 Schematic showing the developed system for the dielectric measurement(Linking an industrial chiller and an oil-free air compressor in operating)
2.3 Heating Equipment and Procedures
The microwave heating equipment(as shown in Fig.4)with continuous adjustable power of 0-3 kW and alternative microwave frequency of 915 MHz,2 450 MHz was developed by the Key Laboratory of Unconventional Metallurgy,Kunming University of Science and Technology,China.A mullite crucible with an inner radius of 4.5 cm and a height of 10 cm has good properties including wave transparency and hot tearresistance and is utilized to load the molybdenite concentrate samples.In an experiment,the samples being filled in the crucible were placed into the heating chamber,which is a single mode microwave resonant cavity.Microwave is radiated by three magnetrons from the left,below and right of the resonant cavity respectively.

Fig.4 Themicrowavehigh-temperaturematerial treatment system(drumming air into the heating chamber,linking cooling water and exhaust gas treatment device in an operation)
The temperature of the samples can be measured accurately by a thermocouple with shield sleeve,which has the controllable temperature range of 0-1 300 ℃.The thermocouple is inserted into the center of samples.A schematic for how to measure the temperature of the samples is shown in Fig.5.In the present study,the molybdenite concentrate sample mass of 25 g was used and the microwave power of 1 kW was given for each experimental run.After reaching the target temperature to hold 3 min,then the crucible was taken out promptly to cool the samplesto reach room temperature.Thereafter,white crystal products upon the samples were produced.

Fig.5 The schematic forloading samplesand measuring temperature
3 Results and Discussion
3.1 Hysteresis Performance Test
The magnetic property of molybdenite concentrate wasfirstinvestigated.Fig.6 shows the roomtemperature magnetic hysteresis(M-H)loop for the sample without any treatment.Where the value of magnetization saturation(Ms),remnant magnetization(Mr) and coercivity (Hc) ofthe samplesare,respectively,0.116 3 emu/g,0.017 6 emu/g and 17.5 KOe.Although the magnetization of molybdenite concentrate sample ata highermagnetic field increasesin association with a positive slope,it has exhibited a small numerical ferromagnetic behavior with coercive field in a range of-165 KOe<H < 165 KOe.The magnetic strength is lower compared with magnetic minerals[12], which might be governed by its composition feature and microstructure defects.While the substance magnetic properties are evidently related to the atomic layer structures and magnetocrystalline anisotropy[13],as well to the contributions to the microwave absorption capacity for an absorber.The test result divulges the magnetic separation technology is not applicable for molybdenite concentrate,while microwave technology can be utilized since the intrinsic magnetic property of molybdenite concentrate enhances its inner molecular polarization ability under microwave radiation.
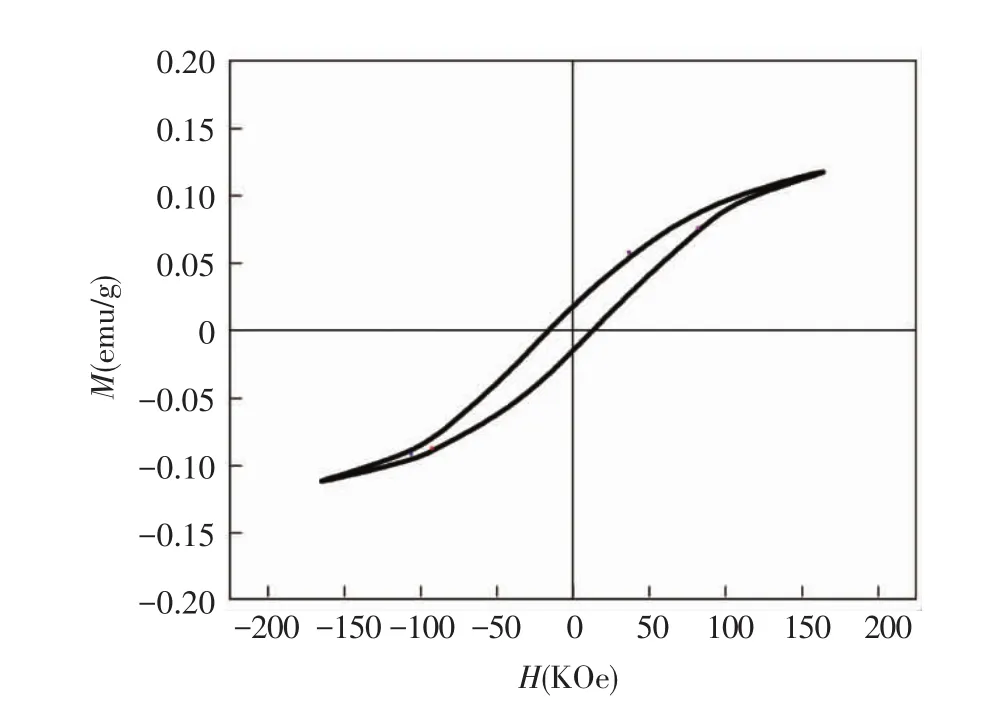
Fig.6 Room-temperature magnetic hysteresis loops of the samples
3.2 Dielectric Properties
The effects of apparent density on dielectric constants, dielectric loss, and loss tangentare respectively presented in Figs.7(a),(b)and(c).The dielectric properties( ε'r, ε″rand tan δ) of molybdenite concentrate samples have a good monotonically positive correlation with the increase of apparent density in an experimentalrange.The dielectric constant is high up to 5.04 at the value 1.4 g/cm3of apparent density.It is argued that a great number of voids among material particles full of air lead to a lower dielectric characteristic value when the molybdenite concentrate sample apparent density is relatively small.And when the apparent density is increasing,the airamong specimen particlesis continuous discharging.The increase in dielectric properties with the increase ofapparentdensity increases the microwave absorption ability of molybdenite concentrate,which may be the reason for more heat generation due to microwave attenuation.

Fig.7 The effect of density on properties of molybdenite concentrate
The complex permittivity(εr= ε'r+j ε″r)[14]has to be known precisely to expound the microwave absorption properties of an absorber.Where ε'ris termed as dielectric constant which indicates the ability of a material to store electromagnetic energy in the microwave field.The ε″ris called dielectric loss which assesses the ability of a dielectric material to attenuate microwave energy under same conditions.The loss tangent is defined as tan δ= ε″r/ ε'r,which naturally represents the convection efficiency of electromagnetic energy into heat in a material at a specific frequency.It is worth to note that the magnetic properties also contribute to the microwave absorption of molybdenite concentrate because the saturation magnetization,knowing from Section 3.1,provides a proper complementariness[15]for the electromagnetic field coupling between microwave and the dipoles within material.
In Fig.8 the dielectric constant ε'r,dielectric loss ε″rand loss tangent tan δ for molybdenite concentrate samples are plotted versus temperature ranging from 25℃ to 500℃ at the commercial frequency of 915 MHz.The dielectric constant increases in the increase of the entire test temperature with a relative slow trend before 300℃and a sharp rise in the later scenario.Both the dielectric loss ε″rand loss tangent tanδshow asimilartrendwithinthewhole experimentalrange,they are almostremaining constant before 300℃and then follow an upsurge up to 500℃.Taking into account the results above two temperature regions could be distinguished,viz.first(from room temperature to 300℃) and second(from 300℃ to 500℃).During the first stage,the slight fluctuations of the dielectric properties might be associated with the complete loss of volatile matters and the release of bounding or capillary water within the interiorstructure ofmolybdenite concentrate samples.For the second stage,increasing temperature under microwave radiation provides thermal energy to the dipoles,thus resulting in a stronger polarization(mainly from ionic displacement polarization,dipole steering polarization and space charge polarization)[16]and free electron conduction caused by the formation of polarization field,all which consequently lead to the increase in dielectric constant.That’s because under high temperature the intergranular softening and amorphous phases within a substance can lead to an increase in its electrical and ionic conductivity which is proportional to loss factors[17](i.e.ε″r= σ/2π ε0f and tan δ= σ/2πf ε0ε').Overall,with a certain range increasing the material’s density and temperature will lead to a superior interaction between the material and microwave,which is beneficial to microwave treating for the molybdenite concentrate.
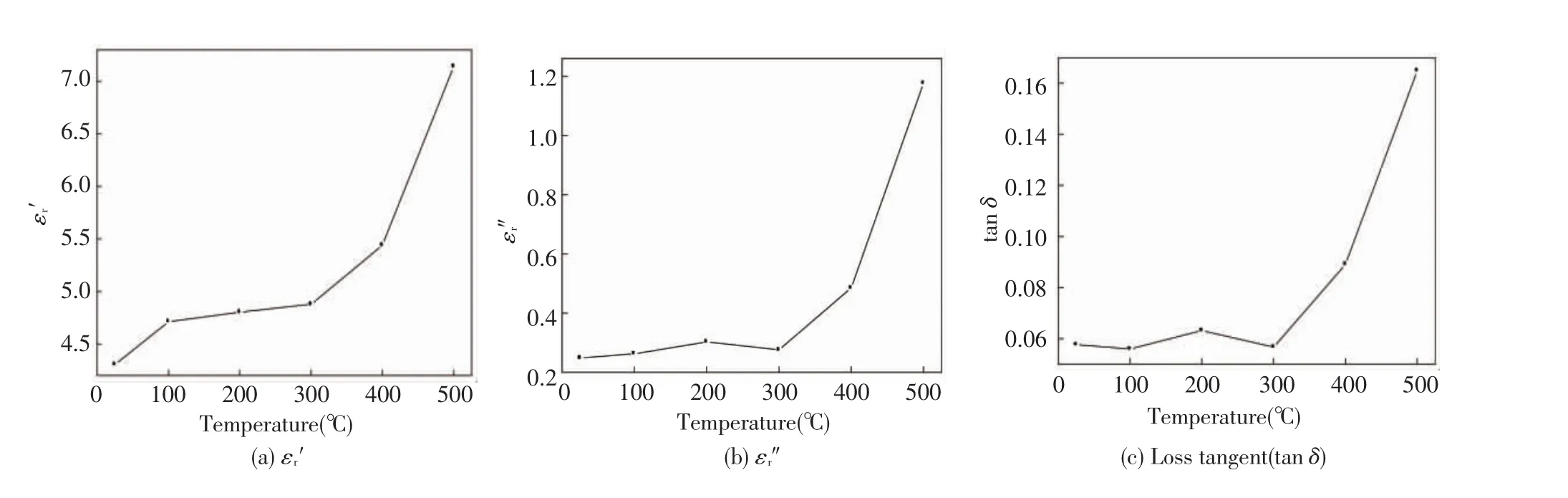
Fig.8 Effect of temperature(℃)on the electrical permittivity of molybdenite concentrate at 915 MHz frequency
3.3 Penetration Depth
The microwave penetration depth is defined as the depth in a material where the power carried by a forward-travelling electromagnetic wave at one specified frequency falls to 1/e of the value surrounding the outer surface of the material.The penetration depth can be calculated based on the following well-known equation[18]:
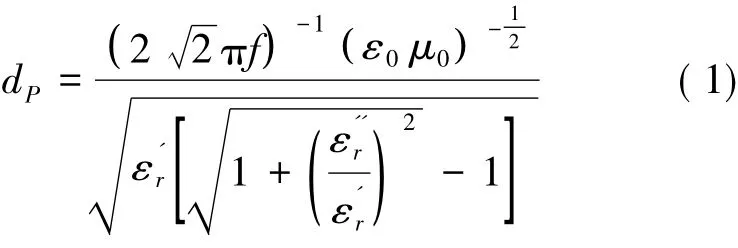
Here f, μ0and ε0are respectively the microwave frequency,permeability and permittivity of vacuum,and μ0or ε0is defined asμ0=1/ ε0( c2),cis electromagnetic wave propagation velocity in a vacuum.
Fig.9 shows the best fitted curves calculated from the experimental data points of molybdenite concentrate sample at 915 MHz.It is seen that penetration depth is inversely proportional to both the apparent density and temperature with two opposite slopes in the experimental range,and obviously the temperature has a greater influence on penetration depth than that of apparent density.The penetration depth is 4.215 cm and 8.511 cm at temperature of 500 ℃ and apparentdensity of1.402 g/cm3,respectively.In general,the larger the penetration depth is,the more uniform the temperature distributions would be[19].Along with the attenuation of electromagnetic field strength and power,the microwave energy was converted into heat,which would determine the microwave penetration depth.Thus,the molybdenite concentrate sample might be transparent to the microwaves under lower apparent density if the penetration depth is larger than the sample size[20].On the other side,the microwave penetration depth was related to the structure transformation within molybdenite concentrate material as the temperature increased.Combining the discussion in Section 3.1.2, the dielectric characterizations values increase in the increase of both apparent density and temperature,thus resulting in a shorter microwave penetration depth and a strongermicrowave absorption capability forthe materials.It is worth mentioning that in an actual launcher if the incident wave diverges(not normally transmitted) the power length deposition will be shorter than these in Eq.(1),this was substantiated by a full-wave simulation in 915 MHz microwaves transmitted into a concrete sample[21].

Fig.9 The experimental values and fitted curves of penetration depth
A regression method nonlinear was utilized to manage the experimentaldata,and the best-fit universalequation for theresulting curve in an exponential one was obtained as follows:
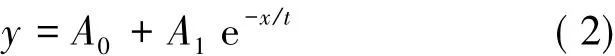
where y is microwave penetration depth,x is the lone independent variable(represented as apparent density or temperature),e is natural base and Ai(i=0,1),t are the regression coefficients.The regression coefficients(A0,A1and t)for different experimental stages(lone independent measurement about apparent density or temperature as a variable)of molybdenite concentrate microwave radiating are provided in Table 2.The relationship described in Eq.(2)shows that the penetration depth has an exponential decay law as the function of apparent density and temperature.The coefficient of determination(R2)in the range of 0.9 and 1 shows the good fitness of the model.Therefore,the developed penetration depth model is closely in keeping with the measured values.The decreased penetration depth means an enhanced attenuation for microwave that can be quicker converted into heat.Thisshould be considered in various applications of microwave including in molybdenum industries.
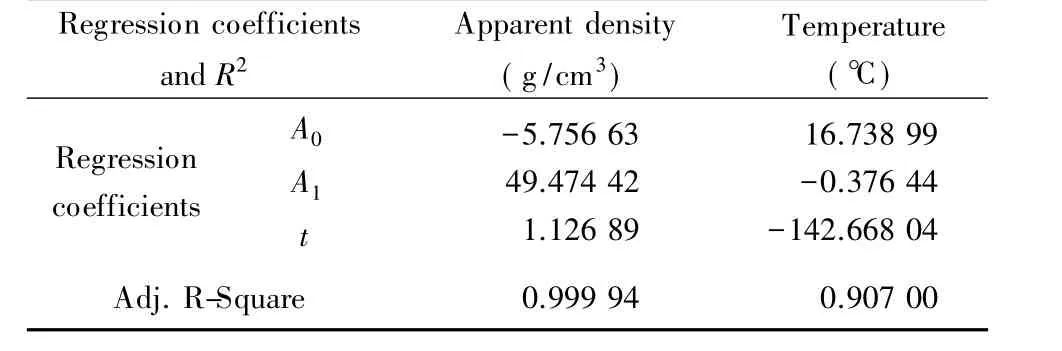
Table 2 Regression coefficients and R2for microwave penetration depth modal for molybdenite concentrate
In order to clearly show the variation of penetration depth of molybdenite concentrate samples with the continuity changes in complex permittivity at the selected frequencies,the plot of nonlinear surface fitting is presented in Fig.10.

Fig.10 The penetration depth nonlinear fitting surfaces(penetration depth trend of molybdenite concentrate changes as the complex permittivity change in a certain range)
This surface helps to better understand how dpis linked to the dielectric properties at the commercial frequency.It was further observed that,the increase in the dielectric loss contributes to a significant decrease in the penetration depth,mainly associated with the generation ofheat(microwave power consumption),whereas the dielectric constant has a weak positive effect.The distinction as compared to one-dimensional fitting curves shown in Fig.9 is that the contour surfaces represente the microwave penetration depth of molybdenite concentrate specimens possess a substantially consistent trend,which exactly demonstrates the interior factor for determining penetration depth is dielectric properties in a material.It also can be literally interpreted as that,within an effective radiation area more microwave energy being stored while a dielectric material interact with microwaves is dissipated and absorbed by the material,thus resulting in reduced penetration depth.
3.4 Characterization of Roasting Products
3.4.1 X-ray diffractrograms
The diffraction spectrum of products obtained at three different roasting temperatures are shown in Fig.11.The main diffraction peaks of(0 2 0),(0 4 0),(0 6 0)and(0 10 0)planes at 12.76,25.7,38.98 and 67.53 of double Bragg angle are in well agreement with that of α-MoO3(JCPDS Card No.05-0508).Obviously, the intensity ofthese peaks increases in the increase of temperature.At 650 ℃ in the product,there are still some weak miscellaneous peaks which correspond respectively to unoxidized MoS2,intermediate forms of MoO2,or other forms of molybdenum oxides.NopeaksofMoS2are identified at 700℃and the pure MoO3is obtained at 800℃.Because the melting point of MoO3is 795℃,the oxidation reaction for the MoS2is completed and even the liquid phase of MoO3crystals begins to form at 800℃,other original impurities are either sintered at the bottom of crucible or released out away from the surface under a formed vapor pressure[22].

Fig.11 XRD powder patterns of products obtained from the roasting test for molybdenite concentrate
3.4.2 SEM Morphologies
Further characterization for the products without any preparation was performed by SEM.The results are shown in Fig.12.
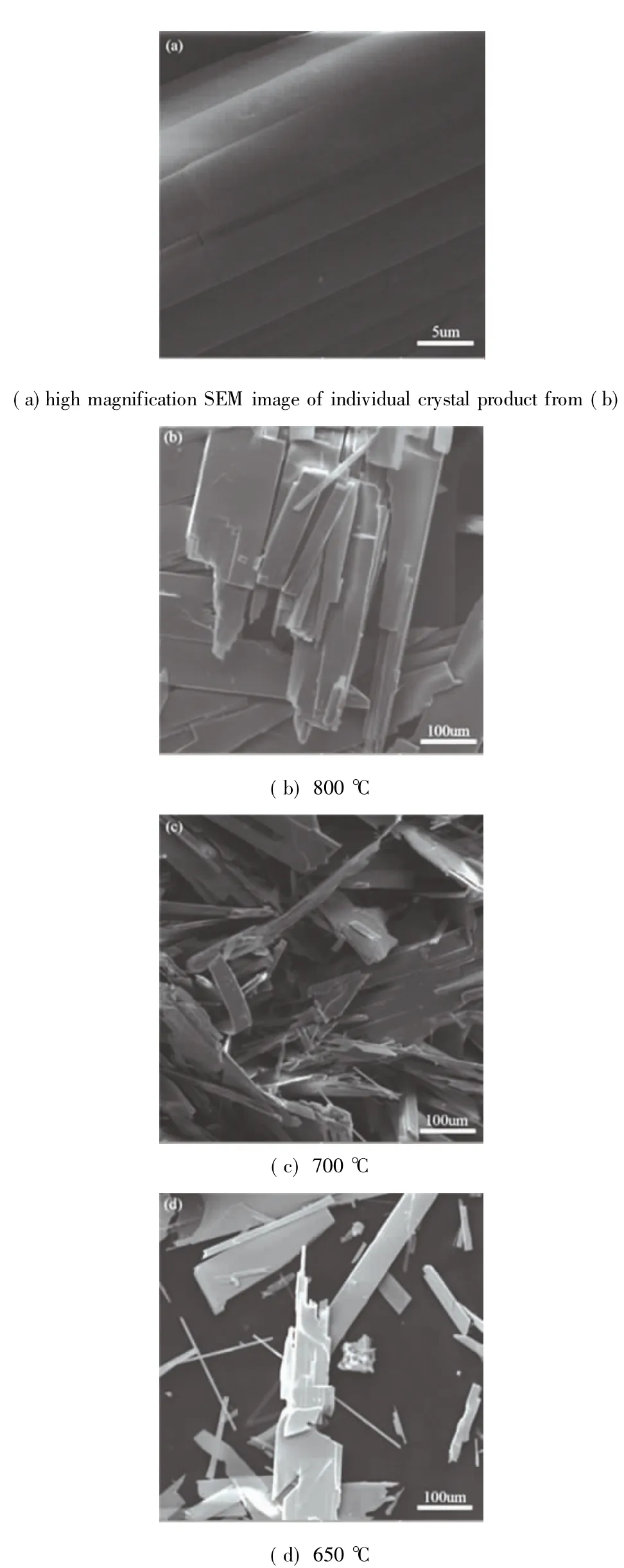
Fig.12 SEM images of products obtained from different temperatures
The regular and compact block crystals were produced in Fig.12(b).The Fig.12(a)is the close viewing of individual crystal of(b),it can be seen the surface is smooth and homogeneous.While at 700 ℃,as shown in Fig.12(c),a large number of strip and sheet crystals were generated and lots of cracks can be seen easily,which may be due to the diffusion[23]of the incoming O2and the outflow SO2.Finally at 650 ℃ shown as Fig.12(d),the formed crystals are in a small amount and there are still unoxidized particles,in this stage the formation of MoO3justbegun,within itmany intermediate products were contained,manifesting as its XRD analysis.Emphatically,the good products bear out the microwave heating(at 915 MHz frequency)can be applied in the ore-dressing and refinementfor molybdenite concentrate.
4 Conclusions
The magnetic hysteresis loop,dielectric property and microwavepenetration depth ofmolybdenite concentrate were investigated.The crystal products obtained under microwave condition were characterized by scanning electron microscope and X-ray diffraction.On the basis of this work the following conclusions can be drawn:
1)With the applied magnetic field of 156 KOe the saturation magnetization value isjust 0.116 3 emu/g,which is too low comparing the magnetic minerals.Magnetic separation technology is not applicable for molybdenite concentrate while the inherent magnetic intensity can contribute to its microwave absorption capacity.
2)The dielectric constant,dielectric loss and loss tangent of molybdenite concentrate with an even trend increase from 3.35 to 5.04,from 0.22 to 0.51 and from 0.065 to 0.102 respectively for the density range from 0.91 to 1.4 g/cm3.The three parameters also increase from 4.3 to 7.14,from 0.25 to 1.18 and from 0.058 to 0.17 respectively as the temperature rise from 25℃ up to 500℃,but with big different rise rates before and after 300℃.Correspondingly,the penetration depth decreases from 16.28 cm to 8.51 cm and from 16.3 cm to 4.44 cm under the same density and temperature range conditions.Additionally,the penetration depth increasesdramatically with the decrease of dielectric loss factors,but slightly with the increase of dielectric constants.These results data suggest molybdenite concentrate has a good microwave absorption capacity.
3) Pure α-MoO3crystal can be produced at 800℃ from molybdenite concentrate by using microwave energy.This research may be useful for designing and modeling ofapplicationssuch as microwave drying,microwave heating,microwave dressing and microwave metallurgy for molybdenite concentrate.
杂志排行
Journal of Harbin Institute of Technology(New Series)的其它文章
- High-Resolution ISAR Imaging Based on Sparse Iterative Covariance-Based Estimation
- X-ray Computed Tomography Characterization of 3D Tufted Twill Textile Composite for Aerostructures
- Measurement Uncertainty of Blade Surface by Coordinates Measuring Machines in Rotation and Parallel Sampling Modes
- Exponential Stabilization of Euler-Bernoulli Beam with Input Time-Delay in the Boundary Control
- Support Vector Regression for Bus Travel Time Prediction Using Wavelet Transform
- Model Reference Adaptive Control for Switched Systems with Closed-loop Reference Model Under Arbitrary Switching
