Silencing Huwe1 reduces apoptosis of cortical neurons exposed to oxygen-glucose deprivation and reperfusion
2019-07-18GuoQianHeWenMingXuHuiJuanLiaoChuanJiangChangQingLiWeiZhang
Guo-Qian He, Wen-Ming Xu, Hui-Juan Liao, Chuan Jiang, Chang-Qing Li, Wei Zhang
1 Department of Pediatrics, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, Sichuan Province, China
2 Joint Laboratory of Reproductive Medicine, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University),Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, Sichuan Province, China
3 Department of Neurology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
4 Department of Medical Oncology, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Cancer Hospital Affiliated to School of Medicine,University of Electronic Science and Technology of China, Chengdu, Sichuan Province, China
Abstract HECT, UBA and WWE domain-containing 1 (Huwe1), an E3 ubiquitin ligase involved in the ubiquitin-proteasome system, is widely expressed in brain tissue. Huwe1 is involved in the turnover of numerous substrates, including p53, Mcl-1, Cdc6 and N-myc, thereby playing a critical role in apoptosis and neurogenesis. However, the role of Huwe1 in brain ischemia and reperfusion injury remains unclear. Therefore, in this study, we investigated the role of Huwe1 in an in vitro model of ischemia and reperfusion injury. At 3 days in vitro, primary cortical neurons were transduced with a control or shRNA-Huwe1 lentiviral vector to silence expression of Huwe1. At 7 days in vitro, the cells were exposed to oxygen-glucose deprivation for 3 hours and reperfusion for 24 hours. To examine the role of the c-Jun N-terminal kinase (JNK)/p38 pathway, cortical neurons were pretreated with a JNK inhibitor (SP600125) or a p38MAPK inhibitor (SB203508) for 30 minutes at 7 days in vitro, followed by ischemia and reperfusion. Neuronal apoptosis was assessed by TUNEL assay. Protein expression levels of JNK and p38MAPK and of apoptosis-related proteins (p53, Gadd45a, cleaved caspase-3, Bax and Bcl-2) were measured by western blot assay. Immunofluorescence labeling for cleaved caspase-3 was performed. We observed a significant increase in neuronal apoptosis and Huwe1 expression after ischemia and reperfusion. Treatment with the shRNA-Huwe1 lentiviral vector markedly decreased Huwe1 levels, and significantly decreased the number of TUNEL-positive cells after ischemia and reperfusion. The silencing vector also downregulated the pro-apoptotic proteins Bax and cleaved caspase-3, and upregulated the anti-apoptotic proteins Gadd45a and Bcl-2. Silencing Huwe1 also significantly reduced p-JNK levels and increased p-p38 levels. Our findings show that downregulating Huwe1 affects the JNK and p38MAPK signaling pathways as well as the expression of apoptosis-related genes to provide neuroprotection during ischemia and reperfusion. All animal experiments and procedures were approved by the Animal Ethics Committee of Sichuan University, China in January 2018 (approval No. 2018013).
Key Words: nerve regeneration; ischemic stroke; oxygen-glucose deprivation and reperfusion; ischemia/reperfusion; cortical neuron; ubiquitin proteasome system; Huwe1; apoptosis; therapeutic targets; cell culture; cell death; neural regeneration
Graphical Abstract
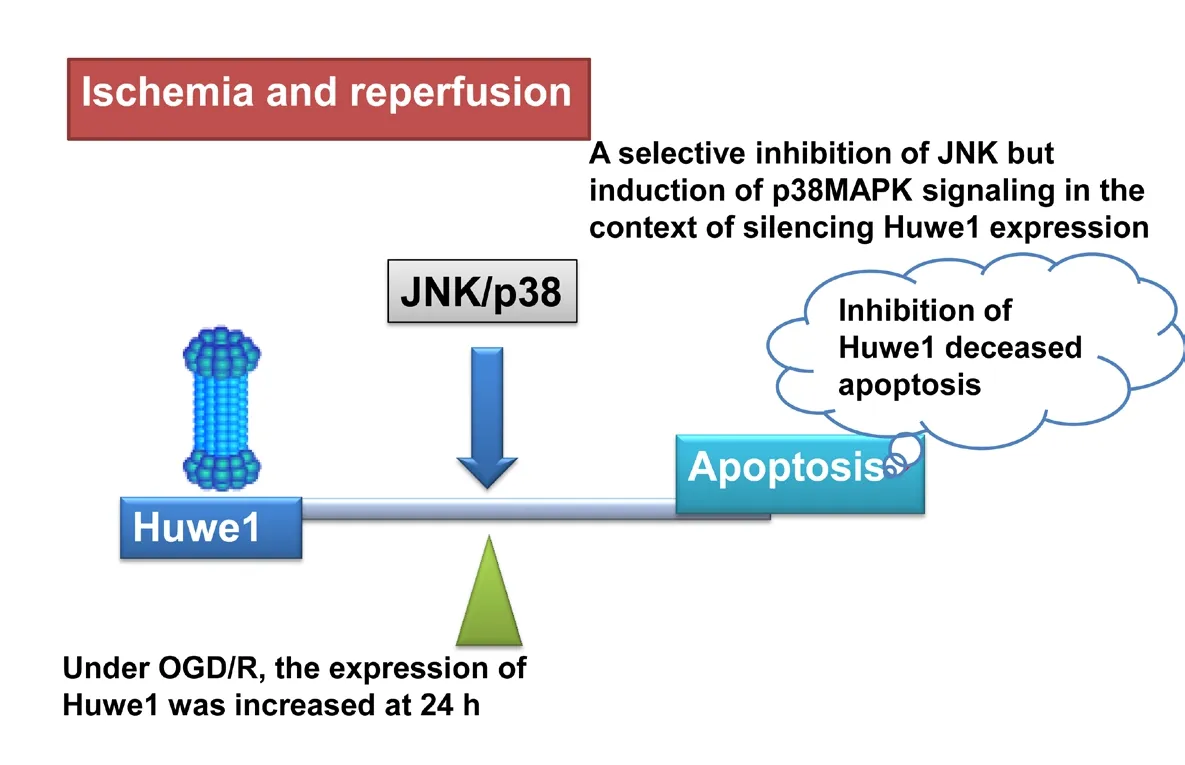
Huwe1 affects apoptosis through c-Jun N-terminal kinase (JNK)/p38 pathway under oxygen-glucose deprivation and reperfusion (OGD/R)
Introduction
Cerebral ischemic stroke is a major cause of morbidity and mortality worldwide. Currently, the best treatment for ischemic stroke is thrombolytic therapy. However, it is not always effective and tissue damage is usually inevitable(Eltzschig and Eckle, 2011; Kalogeris et al., 2016; Wu et al., 2017). Cerebral ischemia/reperfusion (IR) injury is a complex process, involving oxidative damage and neuronal apoptosis (Eltzschig and Eckle, 2011; Thornton et al., 2017).Apoptosis (type I programmed cell death) of neurons occurs post-ischemia (Nakka et al., 2008; Thornton et al., 2017;Qiao et al., 2018). Numerous studies have shown that inhibiting apoptosis reduces IR injury (Rami and Kogel, 2008;Delgado et al., 2014; Zhang et al., 2016; Liu et al., 2018; Xia et al., 2018; Zeng et al., 2018).
HECT, UBA and WWE domain containing 1 (Huwe1), a 500-kDa E3 ubiquitin ligase, regulates the turnover of numerous disparate substrates, including p53, Mcl-1, Cdc6 and N-myc, and plays a key role in cell cycle arrest, cell survival,apoptosis and repair (Zhao et al., 2008; D'Arca et al., 2010;2013; Wang et al., 2014a; Zhou et al., 2014; Lagunas-Martinez et al., 2017; Yanku et al., 2018). Numerous studies show that the biological effects of Huwe1 are complex. Some studies (Chen et al., 2006; Yang et al., 2018) showed that Huwe1 has an anti-apoptotic effect via p53 ubiquitination and degradation. However, many studies suggest that Huwe1 has a pro-apoptotic function via Mcl-1 ubiquitination and degradation (Zhong et al., 2005; Zhao et al., 2008, 2009). It is very likely that the role of Huwe1 is cell-type and context-specific(Lee et al., 2016). Huwe1 is ubiquitously expressed in the nervous system and has major roles in neuronal plasticity,neurogenesis, regeneration and neurological disease (Zhao et al., 2008, 2009; Zhou et al., 2014). Our previous study showed that the expression of Huwe1 increased under oxygen-glucose deprivation and reperfusion (OGD/R) (He et al., 2015). However, the function of Huwe1 in ischemic stroke remains unclear. In this study, we investigated the role of Huwe1 in an in vitro model of OGD/R, and we examined the underlying mechanisms of action.
Materials and Methods Cell culture
Primary cerebral cortical neuron cultures were prepared from embryonic day 17 fetuses from healthy Sprague-Dawley specific-pathogen-free female rats (180-200 g; SCXK(Chuan) 2008-24; Chengdu Da Shuo Laboratory Animal Co., Ltd., Chengdu, China), using a standardized protocol as described previously (Xu et al., 2012). All protocols were in accordance with the Care and Use of Laboratory Animals and the China Council on Animal Care and the National Institutes of Health guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1985), and were approved by the Animal Ethics Committee of Sichuan University, China in January 2018 (approval No. 2018013).
Neurons were resuspended in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, New York, NY, USA, #10099-141), and then filtered through a 70-µm cell strainer (BD Falcon, Franklin Lakes, NJ, USA, #352350). The cells were maintained in Neurobasal medium (Gibco, #12348-017), with 2% B27 supplement (Gibco, #17504-044), penicillin/streptomycin(100 U/mL) and 0.25% GlutaMax (Gibco, #35050-061), and then seeded onto 6-well culture plates at a density of 1.5 ×106cells per well. The 6-well plates were pre-coated with poly-D-lysine (Sigma-Aldrich, St. Louis, MO, USA, #P0899).The cells were cultured in an incubator (5% CO2/95% air) at 37°C. Anti-MAP2 (Proteintech, Rosemont, IL, USA, #17490-1-AP) and anti-GFAP (a marker for astrocytes; Proteintech,#60190-1-Ig) antibodies were used to identify neurons(MAP2-positive/GFAP-negative) by immunofluorescence microscopy. The percentage of neurons in the cultures was over 90%.
Cell treatment
Experiments were conducted using 10 groups. In the control group, cells were untreated. In the reperfusion (R) 24 hour(h) group, cells were subjected to OGD for 3 h and reperfusion for 24 h (OGD/R). In the sh-Huwe1 + R 24 h group,cells were treated with shRNA-Huwe1 lentivirus and then exposed to OGD/R. In the V-ctrl + R 24 h group, cells were treated with lentivirus containing a scrambled sequence and then exposed to OGD/R. In the dimethyl sulfoxide (DMSO)+ R 24 h group, cells were treated with DSMO and then exposed to OGD/R. In the SP + R 24 h group, cells were treated with the c-Jun N-terminal kinase (JNK) inhibitor SP600125 and then exposed to OGD/R. In the SB + R 24 h group,cells were treated with the p38 inhibitor SB203580 and then exposed to OGD/R. In the sh-Huwe1 + SP + R 24 h group,cells were treated with shRNA-Huwe1 lentivirus and JNK inhibitor and then exposed to OGD/R. In the sh-Huwe1 +SB + R 24 h group, cells were treated with shRNA-Huwe1 lentivirus and p38 inhibitor and then exposed to OGD/R. In the V-ctrl + DMSO + R 24 h group, cells were treated with lentivirus containing the scrambled sequence and DMSO and then exposed to OGD/R. Experiments were performed in triplicate and six times in each group.
In this study, OGD/R was used to mimic cerebral IR injury, as described previously (Gertz et al., 2012; Xu et al., 2012). At 7 days in vitro, the neurons were cultured in glucose-free Dulbecco's modified Eagle's medium (Gibco,#11966-025) in an atmosphere of 5% CO2and 0.3% O2at 37°C for 3 h to mimic ischemic insult (OGD). OGD was terminated by replacing the glucose-free medium with complete neurobasal medium, and the cultures were returned to the normoxic incubator for 24, 48 or 72 h to mimic reperfusion. In the control group, the cells were treated similarly,but were not subjected to OGD/R.
Lentivirus construction and transduction
ShRNA Huwe1 lentiviral vector (pGIPZ system) was obtained from Open Biosystems (Probe sequence targeted to the coding sequence: 5′-TCT AGT AGC CAA ATT GGA G-3′). As previously described, a third generation pseudotype lentivirus expressing shRNA and green fluorescent protein (GFP) was generated. pCMVdr-8.91 and pMD2G were used for packaging (Ding and Kilpatrick, 2013; He et al., 2015). Briefly, HEK293FT cells plated to 70% confluency were cotransfected using Lipofectamine 3000 for lentivirus production. After transfection for 48 or 72 h, the medium was collected and passed through a 0.45-µm filter. The lentivirus was concentrated by ultracentrifugation at 4000 × g for 2.5 h, resuspended in phosphate-buffered saline (pH 7.2),and stored at -80°C. Successful transduction by the lentivirus was assessed by western blot assay and quantitative real time PCR for Huwe1.
The cells were cultured in a normoxic chamber at 37°C. At 3 days in vitro, half of the culture medium was set aside and replaced with medium containing 100 µL shRNA-Huwe1 lentivirus and 5 µg/mL polybrene. The cells were incubated for 24 h, and then the previously set aside medium was returned to the well. Cultures were incubated for a further 4 days in a normoxic chamber. The percentage of GFP-positive neurons was over 90% before exposure to OGD. Western blot assay and PCR for Huwe1 were performed to confirm knockdown of Huwe1. Control cells were infected with lentivirus containing a scrambled sequence-GFP construct.
Drug treatment
The mitogen-activated protein kinase (MAPK) signaling pathways play a key role in apoptosis and take part in IR injury (Yu et al., 2015). The main MAPK transducers are JNK and p38MAPK (D'Arca et al., 2010; Guo et al., 2017; Xu et al., 2017). To examine the role of the JNK/p38 pathway in IR injury, cells were pretreated with a JNK inhibitor or a p38 MAPK inhibitor. The JNK inhibitor (SP600125, #S5567) and the p38 MAPK inhibitor (SB203580, #S8307) (both from Sigma-Aldrich) were dissolved in DMSO. SP600125 (final concentration of 10 mM) or SB203508 (final concentration of 20 mM) was added to the medium 30 minutes before and throughout exposure to OGD/R. DMSO-treated cells were used as the control group.
TUNEL assay
To assess apoptosis, TUNEL assay (Roche, Penzberg, Germany, #11684817910) was performed according to the manufacturer's instructions. Briefly, cells on coverslips were treated with Triton X-100 (0.1%) for 10 minutes, and then incubated with the TUNEL reaction mixture in the dark for 1 h at 37°C. Cells were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 minutes at room temperature. Images were captured on a fluorescence inverted microscope (Nikon, Tokyo, Japan). Ten fields were selected randomly, and TUNEL-positive cells and total cells were counted at 400× magnification. The apoptotic index was calculated as TUNEL-positive cells/total cells.
Western blot assay
Total cell lysates were prepared from cells using cold radioimmunoprecipitation assay lysis buffer. Cellular proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (10% or 15%) and blotted onto polyvinylidene fluoride membranes. The membranes were incubated with primary antibodies at 4°C overnight. The primary monoclonal antibodies included Huwe1 (rabbit, 1:1000;Lifespan Biosciences, Seattle, WA, #LS-B1359), cleaved caspase-3 (rabbit, 1:1000; Cell Signaling Technology, Beverly, MA, USA, #9664P), Bcl-2 (rabbit, 1:500; Proteintech,#12789-1-AP), Bax (rabbit, 1:500; Proteintech, #50599-2-Ig), Gadd45a (rabbit, 1:600; Proteintech, #13747-1-AP),phospho-Thr180/Tyr182 p38 (rabbit, 1:1000; Cell Signaling Technology, #4511), p38 (rabbit, 1:1000; Cell Signaling Technology, #8690), p53 (rabbit, 1:1000; Abcam, Cambridge, UK,#ab183544), JNK (rabbit, 1:1000; Cell Signaling Technology,#9252), phospho-Thr183/Tyr185 JNK (rabbit, 1:1000; Cell Signaling Technology, #4671), and mouse anti-β-tubulin(1:10,000; Zhengneng Biotechnology, Chengdu, China). The membrane was then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h. The blots were detected with an enhanced chemiluminescence detection reagent (Millipore, Billerica,MA, USA) on films. The optical density of the signals was analyzed with ImageJ software (NIH, Bethesda, MD, USA).Expression was normalized against β-tubulin.
Immunocytochemistry
Cells on coverslips were fixed in paraformaldehyde (4%)at room temperature for 10 minutes, treated with 0.1%Triton X-100 for 15 minutes, and blocked with 3% bovine serum albumin for 30 minutes. Cells were incubated with anti-cleaved caspase-3 antibody (rabbit monoclonal, 1:1000;Cell Signaling Technology, #9664) at 4°C overnight. The slips were washed with phosphate-buffered saline, incubated with the secondary antibody (Alexa 594-labeled goat F(ab′)2 anti-mouse IgG, 1:500; Invitrogen, Carlsbad, CA, USA) at room temperature for 2 h, and stained with DAPI to detect nuclei. Images were taken on an inverted fluorescence microscope (Nikon) and were analyzed with Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA).
Quantitative real-time polymerase chain reaction
SYBR green-based real-time polymerase chain reaction(PCR) was used to measure mRNA levels of Huwe1 (Long et al., 2018). Total RNA was extracted from cortical neurons with Trizol reagent (Invitrogen), according to the manufacturer's instructions. For real-time PCR analysis, the RNA was reverse transcribed into cDNA using Taq-Man Reverse Transcription reagents (Applied Biosystems, Foster City, CA, USA). The final real-time PCR mixture contained forward and reverse primers, the reaction solution containing the cDNA template, and SYBR Green I Master Mix(Invitrogen). Reactions were performed on an ABI Prism 7500 PCR system (Life Sciences, Carlsbad, CA, USA). The housekeeping gene GAPDH was used for normalization.Fold change in gene expression was calculated using the 2-ΔΔCtmethod (Long et al., 2018). ΔCT = CT (gene) - CT(B2m). The primer sequences of Huwe1 and GAPDH are listed in Table 1.
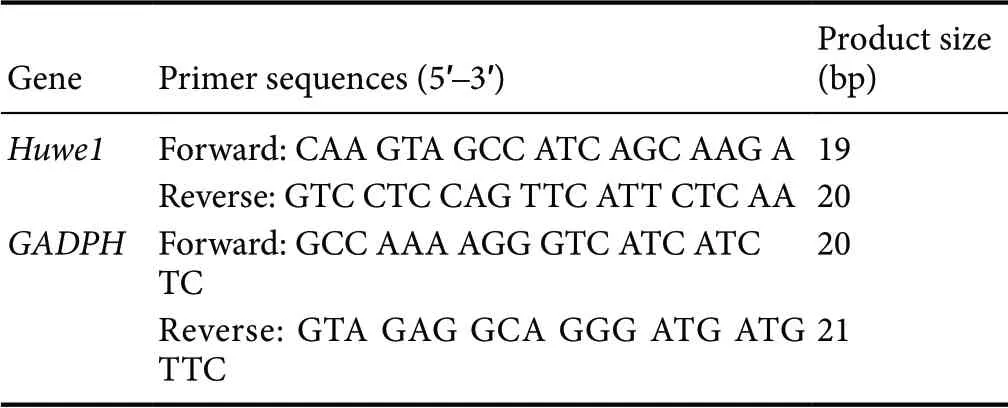
Table 1 Primer sequences used for quantitative real-time polymerase chain reaction
Statistical analysis
All values are presented as the mean ± SEM, and were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA).Data were analyzed by one-way analysis of variance followed by the Tukey's post hoc test for comparisons among three or more groups. A value of P < 0.05 was considered statistically significant.
Results
OGD/R induces cortical neuron apoptosis
The proportion of neurons (MAP2-positive/GFAP-negative)was higher than 90% (data not shown). At 7 days in vitro,cortical neurons were exposed to OGD for 3 h and reperfusion for 24, 48 or 72 h. Our previous study showed that cortical neuronal viability decreased progressively from 24 to 72 h after reperfusion (He et al., 2015). In this study, apoptosis was detected using TUNEL at the different time points after OGD/R. As shown in Figure 1A and B, OGD/R increased the percentage of TUNEL-positive cells after OGD for 3 h compared with the control group (P < 0.05). The percentage of TUNEL-positive cells was significantly increased at 24, 48 and 72 h of reperfusion compared with control (P < 0.05).Neuronal apoptosis increased progressively as the length of the reperfusion period increased from 24 to 72 h. Apoptosis in cerebral ischemia occurs in a caspase-dependent or independent manner. In the intrinsic signaling cascade of apoptosis, cleaved caspase-3 induces neuronal apoptosis, and elevated levels of this caspase may indicate an increase in apoptosis (Xia et al., 2018). Caspase-3 activation is a critical event in neuronal apoptosis following global and focal ischemia in the brain. As shown in Figure 1C, the expression of cleaved caspase-3 was significantly increased at 24 h of reperfusion. Therefore, we chose this time point for subsequent experiments.
Huwe1 downregulation decreases the expression of neuronal apoptosis-related proteins after OGD/R
Our previous study showed that OGD/R induced Huwe1 expression after 24 h of reperfusion (He et al., 2015). In the current study, OGD/R also upregulated protein and mRNA levels of Huwe1 at 24 h of reperfusion compared with the control group (Figure 2A and B). To examine the role of Huwe1 in apoptosis during OGD/R, cortical neurons were pre-treated with shRNA-Huwe1 lentivirus at 3 days in vitro,and then exposed to OGD for 3 h and reperfusion for 24 h at 7 days in vitro. Treatment with the silencing vector significantly decreased the protein and mRNA levels of Huwe1 at 24 h compared with treatment with the scrambled control virus (P < 0.05; Figure 2A and B). Quantitative real-time PCR showed that the knockdown efficiency of lentivirus shRNA-Huwe1 was > 90%.
Apoptosis is a complex process involving many proteins,including p53, Gadd45a, Bcl-2, Bax and caspase-3. Huwe1 silencing induced p53 expression at 24 h of reperfusion compared with the V-ctrl + R 24 h group (P < 0.05; Figure 2A and D). Gadd45a was the first stress gene discovered to be transcriptionally regulated by p53. Treatment with shRNA-Huwe1 lentivirus also increased the protein levels of Gadd45a. During IR injury, Bcl-2 is an anti-apoptotic protein. Bax, a pro-apoptotic protein, belongs to the Bcl-2 family of proteins. Treatment with shRNA-Huwe1 increased Bcl-2 levels but decreased Bax levels at 24 h of reperfusion compared with the V-ctrl + R 24 h group (P < 0.05). Treatment with shRNA-Huwe1 also decreased cleaved caspase-3 levels at 24 h of reperfusion compared with the V-ctrl + R 24 h group (P < 0.05).
Silencing Huwe1 affects the JNK/p38 pathway after OGD/R
During cerebral IR, MAPK signal transduction pathways play a key role in apoptosis. The main MAPK transduction pathways are the extracellular signal-regulated JNK pathway and the p38MAPK pathway (Nozaki et al., 2001). Tyrosine kinase-mediated phosphorylation of Huwe1 promotes tumor necrosis factor-induced JNK activation (Lee et al., 2016).
We examined whether Huwe1 affects apoptosis through the JNK and p38MAPK pathways. As shown in Figure 3A,OGD/R decreased the phosphorylation of JNK and p38.Treatment with shRNA-Huwe1 significantly decreased the p-JNK/JNK ratio at 24 h of reperfusion compared with the V-ctrl + R 24 h group (P < 0.05). Treatment with shRNA-Huwe1 also increased p-p38 levels at 24 h of reperfusion compared with the V-ctrl + R 24 h group (P < 0.05); however, total p38 content was not changed (P > 0.05). Compared with the DMSO + R 24 h group, p-JNK levels in cells treated with the JNK inhibitor SP600125 significantly decreased (P<0.05), but total JNK levels did not change (P > 0.05; Figure 3C). As shown in Figure 4, compared with the DMSO +R 24 h group, the JNK inhibitor also decreased the protein levels of p53, Bax and cleaved caspase-3 at 24 h of reperfusion (P < 0.05). In contrast, Bcl-2 expression in the JNK inhibitor groups was increased significantly at 24 h compared with the DMSO + R 24 h group (P > 0.05). Co-treatment with shRNA-Huwe1 and JNK inhibitor also increased the protein expression of Bcl-2 but decreased the levels of Bax and cleaved caspase-3 at 24 h compared with the V-ctrl +DMSO + R 24 h group (P < 0.05). As shown in Figure 3D,the p38 inhibitor SB203580 decreased p-p38 levels (P < 0.05),while the protein levels of p38 remained unchanged (P >0.05). Compared with the DMSO + R 24 h group, treatment with the p38 inhibitor upregulated p53, Bax and cleaved caspase-3, and downregulated Bcl-2 at 24 h (P < 0.05).However, co-treatment with shRNA-Huwe1 and the p38 inhibitor did not affect the expression of apoptosis-related proteins at 24 h compared with the V-ctrl + DMSO + R 24 h group (P > 0.05). These results indicate that Huwe1 activates the JNK pathway but inhibits the p38MAPK pathway in ischemic cortical neurons.
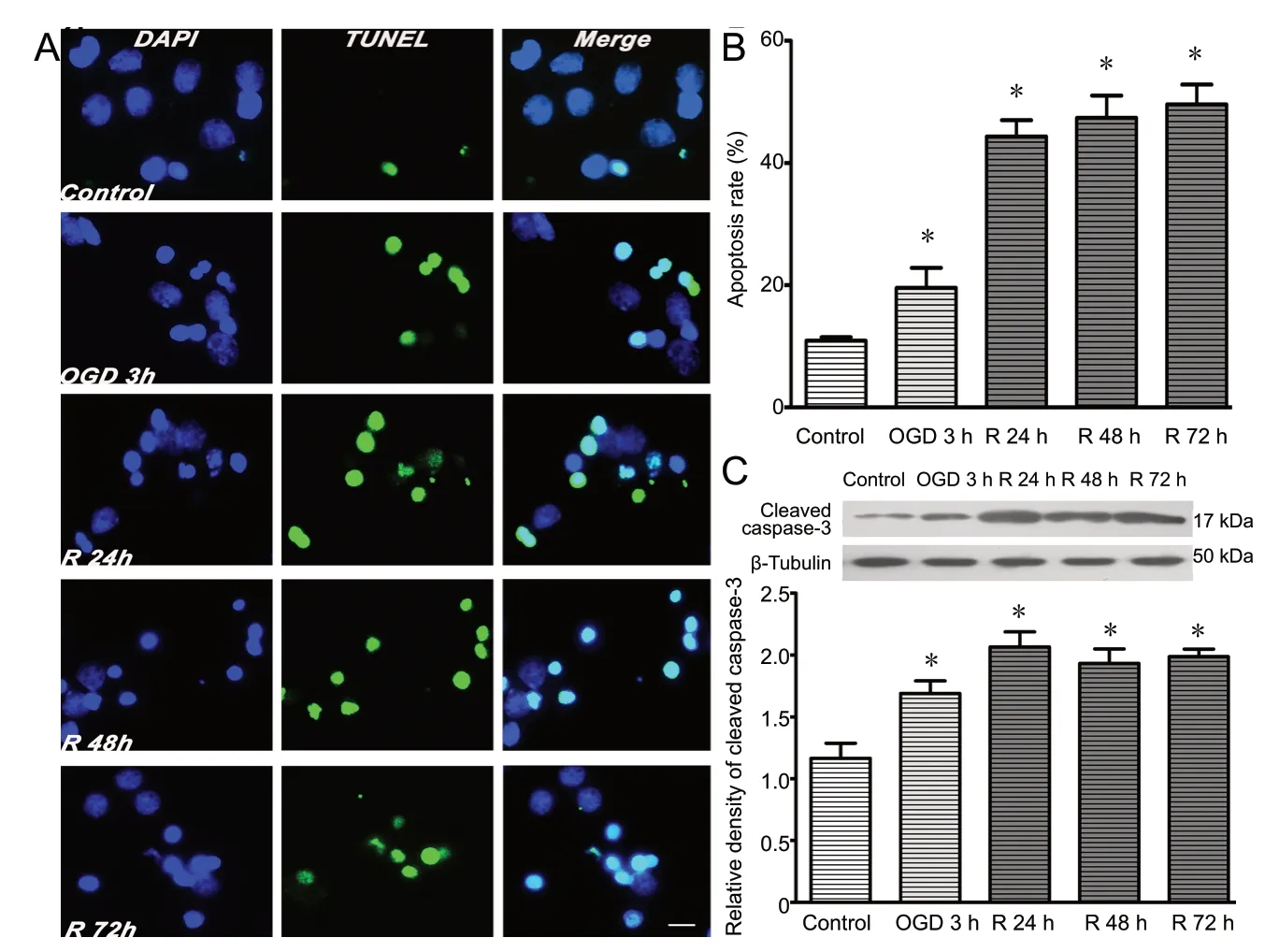
Figure 1 OGD/R induces apoptosis of cortical neurons.Primary cortical neurons were subjected to OGD for 3 h and reperfusion for 24, 48 or 72 h. (A)OGD/R induced apoptosis, as assessed by TUNEL assay. Apoptotic (TUNEL-positive) neurons are labeled green. Nuclei are labeled with DAPI (blue).Scale bar: 10 µm. (B) Quantitative analysis of the TUNEL assay. (C) Protein levels of cleaved caspase-3 were normalized to β-tubulin. Data are expressed as the mean ± SD (n = 5; one-way analysis of variance followed by the Tukey's post hoc test). Experiments were performed at least three times. *P < 0.05, vs. control group (no treatment).OGD/R: Oxygen-glucose deprivation and reperfusion; Huwe1: HECT, UBA and WWE domain containing 1; TUNEL: terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling; DAPI: 4′,6-diamidino-2-phenylindole; R:reperfusion; h: hours.
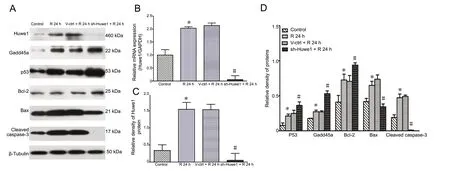
Figure 2 Silencing of Huwe1 affects the expression of apoptosis-related proteins in neurons exposed to OGD/R.Primary cortical neurons were pre-treated with shRNA-Huwe1 lentivirus, followed by OGD for 3 h and reperfusion for 24 h. (A) Treatment with shRNA-Huwe1 decreased the expression of Huwe1 at reperfusion for 24 h. Inhibition of Huwe1 induced the expression of p53 and Bcl-2, but decreased the levels of Bax and cleaved caspase-3. (B) Quantitative real-time PCR assay results. GAPDH was used as an internal control. (C, D) Western blot assay results, with expression normalized to β-tubulin. Data are expressed as the mean ± SD (n = 4; one-way analysis of variance followed by the Tukey's post hoc test). Experiments were performed at least three times. *P < 0.05, vs. control group (no treatment); #P < 0.05, vs. V-ctrl + R 24 h group (treatment with lentiviral scrambled control). Control group: Untreated cells; R 24 h group: OGD for 3 h and reperfusion for 24 h; V-ctrl+ R 24 h group: lentivirus containing a scrambled sequence, exposure to OGD/R; sh-Huwe1 + R 24 h group: shRNA-Huwe1 lentivirus, exposure to OGD/R. OGD/R: Oxygen-glucose deprivation and reperfusion; Huwe1: HECT, UBA and WWE domain containing 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; R: reperfusion; h: hours.
Role of Huwe1 and JNK/p38 signaling in apoptosis during OGD/R
As shown in Figure 5A and B, shRNA-Huwe1 significantly decreased neuronal apoptosis at reperfusion for 24 h after OGD compared with the V-ctrl + R 24 h group (P < 0.05).Treatment with JNK inhibitor also decreased the ratio of TUNEL-positive cells at 24 h of reperfusion compared with the DMSO + R 24 h group (P < 0.05). ShRNA-Huwe1 combined with JNK inhibitor significantly decreased the number of TUNEL-positive cells at 24 h compared with the V-ctrl +DMSO + R 24 h group (P < 0.05). Treatment with p38 inhibitor significantly increased the ratio of TUNEL-positive cells at 24 h compared with the DMSO + R 24 h group (P < 0.05).There were no differences between sh-Huwe1 + SB + R 24 h group and the V-ctrl + DMSO + R 24 h group (P < 0.05).These results indicate that Huwe1 induces the JNK pathway
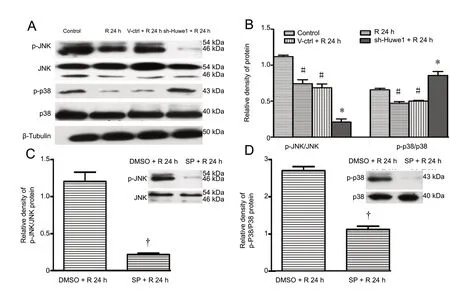
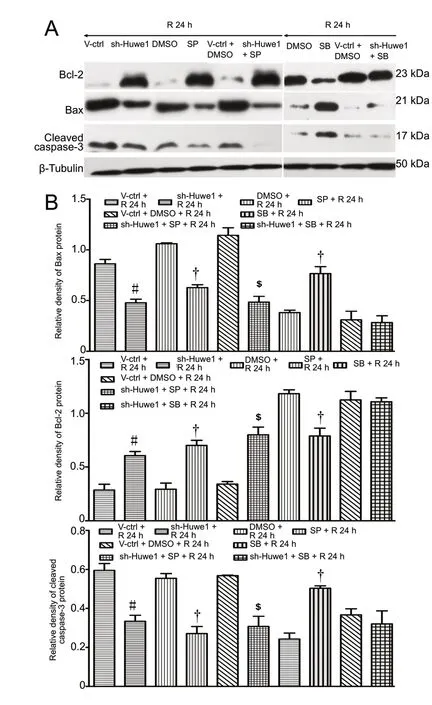
Figure 4 Silencing of Huwe1 and the JNK/p38 pathway modulates key apoptotic proteins under OGD/R.Primary cortical neurons were treated with shRNA-Huwe1 lentivirus at 3 days in vitro, followed by OGD 3 h and reperfusion for 24 h. The JNK inhibitor SP600125 or the p38 inhibitor SB203580 was used. (A) Treatment with shRNA-Huwe1 and JNK inhibitor or p38 inhibitor modulated the expression of apoptosis-related proteins(p53, Bcl-2, Bax, cleaved caspase-3). (B) Expression on western blots was normalized to β-tubulin. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by the Tukey's post hoc test). Experiments were performed at least three times. #P < 0.05, vs. V-ctrl + R 24 h group (treatment with lentiviral scrambled control); †P < 0.05, vs. DMSO group; $P < 0.05, vs. V-ctrl + DMSO + R 24 h group (treatment with lentiviral scrambled control + DMSO). V-ctrl + R 24 h group:Lentivirus containing a scrambled sequence, exposure to OGD/R; sh-Huwe1 + R 24 h group: shRNA-Huwe1 lentivirus, exposure to OGD/R; SP + R 24 h group: JNK inhibitor SP600125, exposure to OGD/R; V-ctrl + DMSO + R 24 h group: lentivirus containing the scrambled sequence, DMSO, exposure to OGD/R; DMSO + R 24 h group: DSMO, exposure to OGD/R; SB + R 24 h group: p38 inhibitor SB203580,exposure to OGD/R; sh-Huwe1 + SP + R 24 h group: shRNA-Huwe1 lentivirus,JNK inhibitor, exposure to OGD/R; sh-Huwe1 + SB + R 24 h group: shRNA-Huwe1 lentivirus, p38 inhibitor, exposure to OGD/R. OGD/R: Oxygen-glucose deprivation and reperfusion; JNK: c-Jun N-terminal kinase; Huwe1: HECT, UBA and WWE domain containing 1; DMSO: dimethyl sulfoxide; SP: JNK inhibitor SP600125; SB: p38 inhibitor SB203580; R: reperfusion; h: hours.but inhibits the p38MAPK pathway in ischemic cortical neurons. Thus, JNK/p38 signaling may mediate the effect of Huwe1 on apoptosis during OGD/R.
Immunofluorescence for cleaved caspase-3
Caspase-3 activation is a key event in neuronal death following focal brain ischemia (Wu et al., 2014; Simsek et al.,2016; Xia et al., 2018). Cleaved caspase-3 was at basal levels in normal cortical neurons. The immunofluorescence results paralleled the western blot results. As shown in Figure 6, under OGD/R, the immunoreactivity of cleaved caspase-3 was mainly around the nucleus and increased at 24 h. Treatment with shRNA-Huwe1 or JNK inhibitor or SP600125 + shRNA-Huwe1 decreased cleaved caspase-3 levels at 24 h. Treatment with p38 inhibitor remarkably increased the cleaved caspase-3 levels at 24 h. shRNA-Huwe1 combined with p38 inhibitor diminished the increase in cleaved caspase-3 induced by OGD/R .
Discussion
After brain IR, the affected neurons undergo apoptosis (Rami and Kogel, 2008). Therefore, strategies to inhibit apoptosis during IR are needed to rescue these ischemic cells.
The ubiquitin-proteasome system, which degrades cellular proteins, plays a complex and key role, both directly and indirectly, in cerebral IR (Yun and Lee, 2003; Wojcik and Di Napoli, 2004; Baptista et al., 2012; Ge et al., 2012; Caldeira et al., 2014; Fan et al., 2016). Previous studies show that there is cross-talk between the ubiquitin-proteasome system and the apoptotic machinery. The ubiquitin-proteasome system regulates apoptosis via the direct and indirect modulation of proteins associated with cell death (Neutzner et al., 2012;Delgado et al., 2014). The ubiquitin-proteasome system is catalyzed by a cascade of three enzymes, called E1, E2 and E3. E3 is responsible for targeting ubiquitination of substrate proteins. Among the various E3s, Huwe1 is highly associated with apoptosis by functional enrichment. Under different pathophysiological conditions, Huwe1 plays a pro-apoptotic or anti-apoptotic function by targeting different substrates,leading to different outcomes (D'Arca et al., 2010; Caldeira et al., 2014). Moreover, Huwe1 rescues neural progenitor cells from OGD-induced insults by inhibiting proliferation and inducing neuronal differentiation (Jiang et al., 2018).In this study, we found that silencing Huwe1 expression decreased cortical neuronal apoptosis under OGD/R. Huwe1 was first identified as an enzyme responsible for the degradation of p53 (Zhong et al., 2005; Zhang et al., 2011; Wang et al., 2014a; Xu et al., 2016). Indeed, we found that Huwe1 silencing increased p53 expression under OGD/R. There are several reports that under focal ischemia and hypoxia,p53 expression is enhanced in injured neurons before cell death. These studies suggest that p53 plays a role in ischemia-induced apoptosis (Nakka et al., 2008). These findings appear inconsistent with our present results. A previous study showed that p53 regulates Gadd45a transcription and protein expression in the striatum and cortex (Moskalev et al., 2012; Sultan and Sweatt, 2013). Gadd45 has a neuroprotective role (Chen et al., 1998; Moskalev et al., 2012; Schafer,2013; Sultan and Sweatt, 2013). Interestingly, we found here that Huwe1 silencing increased Gadd45a expression under OGD/R. Therefore, in the context of cerebral IR injury,Huwe1 may indirectly affect Gadd45a expression via the polyubiquitination of p53. This suggests that a Huwe1-p53-Gadd45a axis might regulate apoptosis during OGD/R injury. To test this concept, we will examine the effect of p53 and Huwe1 co-silencing in a future study.
The function and underlying mechanism of action of Huwe1 in apoptosis remain unclear. Apoptosis after cerebral IR is regulated by pro- and anti-apoptotic proteins such as the Bcl-2 family (Lalaoui et al., 2015; Yang et al., 2015; Yang and Yao, 2015). Changes in the balance of Bcl-2 and Bax may result in either inhibition or activation of apoptosis, and plays an important role in the pathogenesis of cerebral IR injury(Eltzschig and Eckle, 2011; Xi et al., 2011; Xia et al., 2018). In this study, silencing Huwe1 upregulated Bcl-2 and downregulated Bax in neurons exposed to OGD/R. It remains unclear how OGD/R induces these changes. It has been shown that the JNK and p38 pathways play a major role in ischemia-induced apoptosis through the regulation of the Bax/Bcl-2 ratio(Wang et al., 2014b; Kalogeris et al., 2016; Lee et al., 2016;Liu et al., 2018). Moreover, Huwe1 enhances tumor necrosis factor-induced JNK activation and cell death (Lee et al.,2016). In the present study, silencing Huwe1 also decreased the p-JNK/JNK ratio. It seems that silencing Huwe1 inhibits JNK activation. This result is consistent with previous studies. The role of JNK in apoptosis is complex, and it plays a pro-apoptotic, anti-apoptotic or no role in the process (Zhang et al., 2013; Zhan et al., 2015; Shvedova et al., 2018; Zhang et al., 2018). Here, we observed that in our model of IR injury,JNK participated in the apoptotic process. JNK inhibition decreased the levels of pro-apoptotic proteins. Co-treatment with shRNA-Huwe1 and a JNK pathway inhibitor also increased the Bcl-2/Bax ratio.
Huwe1 has multiple polyubiquitination substrates, including other members of the Bcl-2 family (Wojcik and Di Napoli, 2004; Zhong et al., 2005; Chen et al., 2006). However,it is still not clear whether Huwe1 affects Bcl-2 levels by polyubiquitination of Bcl-2 directly or indirectly through other substrates. It has been shown that p38 MAPK signaling either promotes apoptosis or enhances cell survival depending upon the cell type and stimulus (Whitmarsh, 2010; Liu et al.,2018; Xie et al., 2018). Gadd45a may play a role in p53-independent apoptosis via activation of p38MAPK signaling pathways (Fallsehr et al., 2005). In our study, inhibition of the p38 pathway decreased Bcl-2 expression and increased Bax expression in neurons exposed to OGD/R. Silencing Huwe1 expression increased the phosphorylation of p38. However,shRNA-Huwe1 combined with p38 inhibitor had no effect on apoptosis-related protein expression. Whether Huwe1 affects p38 level by regulating Gadd45a is not yet clear.
In conclusion, Huwe1 silencing protected cerebral cortical neurons from apoptosis during OGD/R in vitro, a model of cerebral IR injury. Huwe1 inhibited the JNK pathway but induced the p38MAPK pathway. Therefore, targeting Huwe1 and the MAPK pathways might have therapeutic potential in the treatment of cerebral IR injury.
Author contributions:Study design: GQH, WZ and WMX; experimental implementation: GQH, WZ and HJL; data analysis: CJ and WZ; paper writing: GQH and WZ; study review: WMX and CQL. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81771642 (to WMX); the New Bud Research Foundation of West China Second University Hospital of China (to GQH). The funding sources had no role in study conception and design,data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:All animal experiments and procedures were approved by the Animal Ethics Committee of Sichuan University, China in January 2018 (approval No. 2018013). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.Plagiarism check:Checked twice by iThenticate.
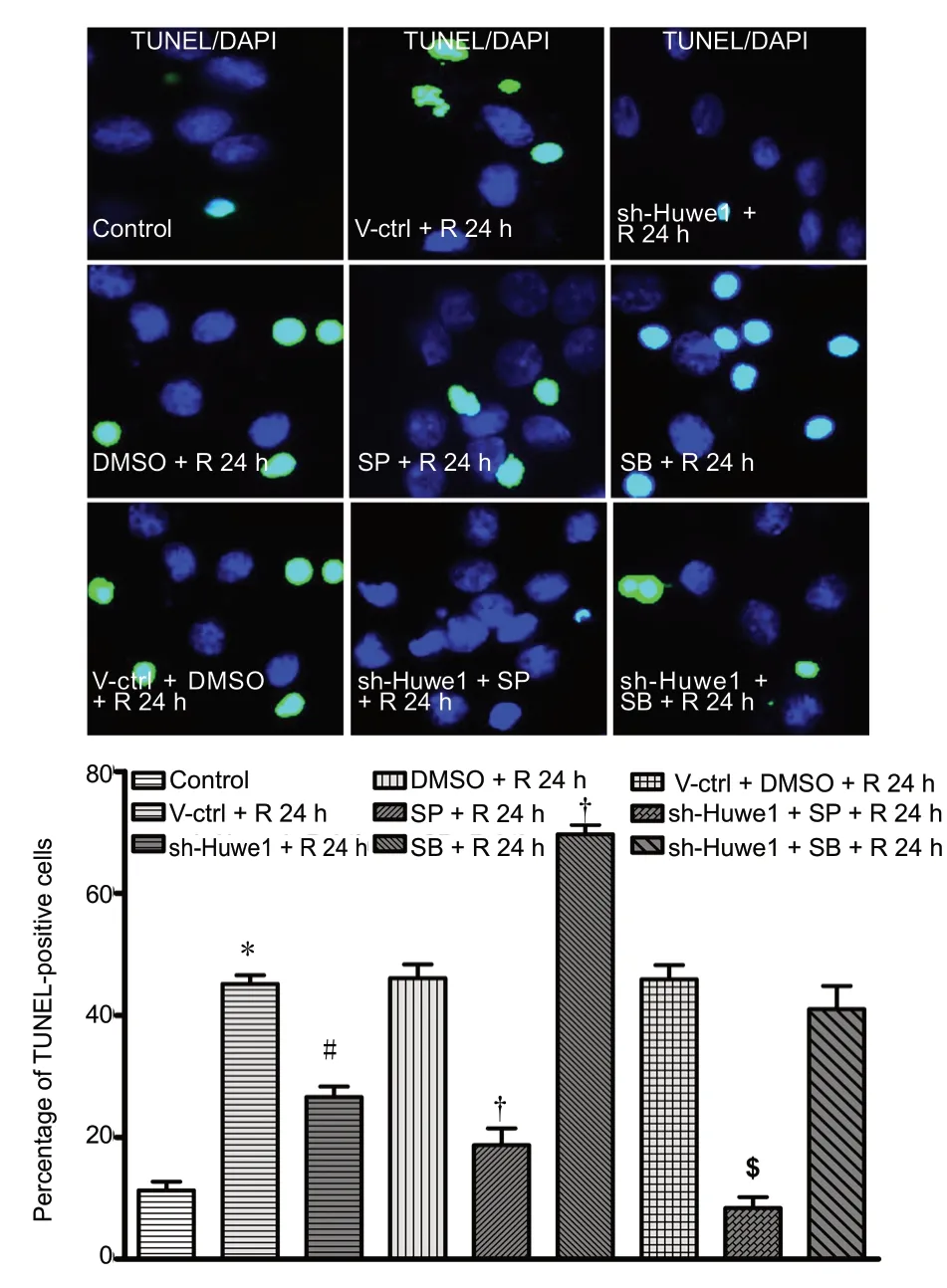
Figure 5 Silencing of Huwe1 and the JNK/p38 pathway affects the apoptosis rate of neurons, as assessed by TUNEL assay.Primary cortical neurons were pre-treated with shRNA-Huwe1 lentivirus, and then subjected to OGD for 3 h and reperfusion for 24 h.Apoptotic neurons were stained with TUNEL (green) by fluorescence assay. DAPI (blue) was used to label nuclei. Scale bar: 10 µm. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by the Tukey's post hoc test). *P < 0.05, vs. control group (no treatment); #P < 0.05, vs. V-ctrl + R 24 h group (treatment with lentiviral scrambled control); †P < 0.05, vs. DMSO + R 24 h group (treatment with DMSO); $P < 0.05, vs. V-ctrl + DMSO + R 24 h group (treatment with lentiviral scrambled control + DMSO). Control group: Untreated cells; DMSO + R 24 h group: DSMO, exposure to OGD/R; V-ctrl +DMSO + R 24 h group: lentivirus containing the scrambled sequence,DMSO, exposure to OGD/R; V-ctrl + R 24 h group: lentivirus containing a scrambled sequence, exposure to OGD/R; SP + R 24 h group:JNK inhibitor SP600125, exposure to OGD/R; sh-Huwe1 + SP + R 24 h group: shRNA-Huwe1 lentivirus, JNK inhibitor, exposure to OGD/R; sh-Huwe1 + R 24 h group: shRNA-Huwe1 lentivirus, exposure to OGD/R; SB + R 24 h group: p38 inhibitor SB203580, exposure to OGD/R; sh-Huwe1 + SB + R 24 h group: shRNA-Huwe1 lentivirus, p38 inhibitor, exposure to OGD/R. OGD/R: Oxygen-glucose deprivation and reperfusion; TUNEL: terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling; DAPI: 4′,6-diamidino-2-phenylindole; JNK: c-Jun N-terminal kinase; Huwe1: HECT, UBA and WWE domain containing 1; DMSO: dimethyl sulfoxide; SP: JNK inhibitor SP600125; SB: p38 inhibitor SB203580; R: reperfusion; h: hours.
Peer review: Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Dario Siniscalco, The Second University of Naples,Italy.
Additional file:Open peer review report 1.
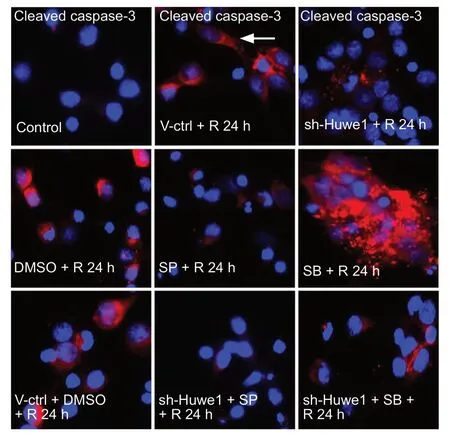
Figure 6 Immunofluorescence staining for cleaved caspase-3 in neurons exposed to OGD/R.Primary cortical neurons were treated with shRNA-Huwe1, and then exposed to OGD for 3 h and reperfusion for 24 h. The JNK inhibitor SP600125 or the p38 inhibitor SB203580 was used. Neurons were stained for cleaved caspase-3 (red) by immunofluorescence. DAPI (blue)was used to label nuclei. Immunoreactivity for cleaved caspase-3 was very low in control neurons. Immunoreactivity for cleaved caspase-3 was mainly around the nucleus (white arrowheads) in cortical neurons. Silencing Huwe1 or inhibiting JNK markedly decreased cleaved caspase-3 levels. Treatment with the p38 inhibitor increased the levels of cleaved caspase-3 compared with treatment with DMSO. Co-treatment with shRNA-Huwe1 and the JNK inhibitor decreased cleaved caspase-3 levels. Scale bar: 10 µm. Control group: Untreated cells; V-ctrl+ R 24 h group: lentivirus containing a scrambled sequence, exposure to OGD/R; sh-Huwe1 + R 24 h group: shRNA-Huwe1 lentivirus, exposure to OGD/R; DMSO + R 24 h group: DSMO, exposure to OGD/R; SP + R 24 h group: JNK inhibitor SP600125, exposure to OGD/R; SB+ R 24 h group: p38 inhibitor SB203580, exposure to OGD/R; V-ctrl +DMSO + R 24 h group: lentivirus containing the scrambled sequence,DMSO, exposure to OGD/R; sh-Huwe1 + SP + R 24 h group: shRNA-Huwe1 lentivirus, JNK inhibitor, exposure to OGD/R; sh-Huwe1+ SB + R 24 h group: shRNA-Huwe1 lentivirus, p38 inhibitor, exposure to OGD/R. OGD/R: Oxygen-glucose deprivation and reperfusion;TUNEL: terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labeling; DAPI: 4′,6-diamidino-2-phenylindole; JNK:c-Jun N-terminal kinase; Huwe1: HECT, UBA and WWE domain containing 1; DMSO: dimethyl sulfoxide; SP: JNK inhibitor SP600125; SB:p38 inhibitor SB203580; R: reperfusion; h: hours.
杂志排行
中国神经再生研究(英文版)的其它文章
- Etomidate affects the anti-oxidant pathway to protect retinal ganglion cells after optic nerve transection
- Pain inhibition through transplantation of fetal neuronal progenitors into the injured spinal cord in rats
- MicroRNA expression in the hippocampal CA1 region under deep hypothermic circulatory arrest
- Atsttrin reduces lipopolysaccharide-induced neuroinflammation by inhibiting the nuclear factor kappa B signaling pathway
- Association between PPARG genetic polymorphisms and ischemic stroke risk in a northern Chinese Han population: a case-control study
- Paired associative stimulation improves synaptic plasticity and functional outcomes after cerebral ischemia
