Spinal cord organoids add an extra dimension to traditional motor neuron cultures
2019-07-17Winanto,Zi-JianKhong,Jin-HuiHor等
Since Lancaster et al. (2013) first described the formation of self-organizing cerebral organoids for modeling neurodevelopmental disorders, it became evident that three-dimensional (3D) neural organoid cultures are more superior systems for modeling neurodevelopment and neurodegeneration in human. The use of a spinning bioreactor to grow organoids allows better nutrient absorption and enhances formation of neuroepithelial-like zones, making it a great tool to study neurodevelopment and neurodegeneration. Neural organoids are 3D cell culture systems formed by proliferating, differentiating, migrating and self-organizing pools of neural progenitors. They mimic brain structures in their cell type composition,cytoarchitecture, and to some extent maturity and functionality(Lancaster et al., 2013).
Because of these unique properties, neural organoids have been used extensively to study diseases associated with neurodevelopment and neurodegeneration. As neural organoids recapitulate early stages of neurogenesis, neural organoids have be applied to model microcephaly, a neurodevelopment disorder, demonstrating that cerebral organoids from microcephalic patients have fewer proliferating progenitor cells and impaired neuron differentiation,shedding light into the underlying mechanism of microcephaly(Lancaster et al., 2013). Forebrain organoids were also used to understand the association between Zika virus infection and the destruction of neural progenitor pools (Garcez et al., 2016). The self-organization properties of brain organoids allow one to model neuropsychiatric disorders where circuit formation and refinement are impaired, allowing identification of underlying molecular and cellular mechanisms (Mariani et al., 2015).
Neural organoids have also shown potential in mimicking maturation and late-onset phenotypes that are undetectable in a traditional two-dimensional culture system. Neuromelanin, the adult substantia nigra-specific pigment, was observed in dopaminergic neurons within a midbrain-like organoids and not in two-dimensional cultures, demonstrating that the 3D environment in the organoid aids in the maturation of the neurons (Jo et al., 2016). Studies have also demonstrated that cerebral and midbrain organoids can be used to study Alzheimer's disease and Parkinson's disease,respectively. For instance, 3D cell cultures were able to recapitulate extracellular amyloid aggregation, and demonstrate an accumulation of hyperphosphorylated tau proteins and higher neuronal apoptosis rates in Alzheimer's disease cerebral organoids (Choi et al., 2014), modeling the neurodegeneration phenotype. Therefore,brain organoids are of immense interest for modeling human neurodevelopment and neurodegeneration because of their ability to recapitulate fundamental pathogenic features and mechanisms.
As part of the central nervous system, the spinal cord is a highly complex organ that controls locomotion and is patterned along the dorsoventral axis and the rostrocaudal axis. Within the spinal cord,motor neurons are located at the ventral horns along the rostrocaudal axis and are of particular interest because motor neuron degeneration results in a number of debilitating and often fatal disorders such as spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS). By recapitulating motor neuron development,efficient protocols directing pluripotent stem cells towards spinal motor neurons have been reproducibly achieved by first caudalizing early neural cultures with retinoic acid, followed by exposure to the ventralizing signal Sonic Hedgehog. This results in a fairly homogeneous monolayer culture of motor neurons, mostly belonging to the hindbrain or cervical subtype, but does not mimic diverse sub-populations of motor neurons found along the rostrocaudal axis within the spinal cord. This lack of rostral-caudal patterning poses a difficulty in modeling lower motor neuron diseases such as SMA where lumbar motoneurons are affected. In order to generate more ventral motor neurons belonging to the lumbar subtypes, the morphogen growth and differentiation 11 (GDF11) is often added to the cultures. However, cultures exposed to GDF11 are also fairly homogeneous for lumbar markers, which again do not represent the rostro-caudal patterning in the spinal cord.
Other than motor neurons, the spinal cord also consists of other neuronal populations such as the interneurons that provide inhibitory and excitatory signals to the motor neurons, sensory neurons and neuroglial. It has been found that these cell types play a role in disease pathogenesis and progression in motor neuron diseases. For instance, SMA interneurons have been found to have a smaller soma size and reduced VGLUT1 synapses was observed in the motor neurons of a SMA mouse model, hypothesizing that interneurons may play a role in SMA pathology (Thirumalai et al.,2013). However, in the traditional two-dimensional cultures, spinal interneurons are largely absent and hence not able to mimic the complex cellular interactions that exist between various spinal cell types that contribute to a functional spinal cord unit.
Recently, our group presented an approach to generate a 3D spinal cord organoid from human induced pluripotent stem cells(iPSCs) (Hor et al., 2018). Adopting the culture system used by Lancaster et al. (2013), we encapsulated retinoic acid-treated and caudalized embryoid bodies within Matrigel droplets that were allowed to expand and grow in spinner flasks. Remarkably, the resultant organoids resemble the ventral spinal cord in multiple ways: First, we are able to derive different spinal cell types including limb-innervating motor neurons, excitatory V2a interneurons,inhibitory Renshaw interneurons and spinal astrocytes in our spinal cord organoids. Second, these organoids were patterned along the rostrocaudal axis, where we observed HOXB4+brachial and HOXC8+thoracic spinal cell type, in the absence of exogenous GDF11. This suggests that the 3D microenvironment in these organoids creates the morphogen gradient, allowing the differentiation of different spinal cell types, mimicking the ventral spinal cord(Figure 1). The presence of both brachial and thoracic cell types within the same organoid suggests that cell-cell interaction in a three-dimensional environment plays an important role in allowing the expression of lower Hox genes, creating the rostrocaudal axis within it. In addition, we were able to observed larger motor neurons at later time points of the differentiation that are ChAT positive, indicating that these spinal cord organoids are able to generate mature motor neurons for downstream studies. Most importantly,these organoid-specific characteristics were not observed in traditional monolayer cultures where a uniform exposure to patterning factors results in a relatively homogeneous culture.
The diversity in spinal cell types observed in our spinal cord organoids allows us to study motor neuron diseases such as SMA and ALS. For instance, our group generated spinal cord organoids derived from iPSCs of SMA patients and showed no defects in generating motor neurons but observed an accelerated motor neuron death phenotype, consistent with our current understanding of the disease as a degenerative, rather than developmental disorder(Rodriguez-Muela et al., 2017). Other than studying the underlying pathogenic mechanism of motor neuron diseases, spinal cord organoids are an ideal platform for therapeutic discovery before moving to in vivo model. For instance, we were able to use SMA spinal cord organoids for drug screening and study the drug effects on neuronal survival and cell-to-cell interactions in a 3D setting(Hor et al., 2018). The spinal cord also plays an important role in controlling locomotion. In motor neuron diseases, the motor neurons in the spinal cord denervated from the muscle and eventually died, leading to muscle atrophy and affects locomotion in these patients. Motor neurons derived from the spinal cord organoid we generated were able to cause myotube contraction through the formation of functional neuromuscular junctions, demonstrating the functionality of these motor neurons and thus providing a potential neuromuscular junction model in vitro and platform for therapeutic testing for patients with spinal cord injury.
While cell-type heterogeneity in organoid cultures poses challenges in population-level analyses, the spatial and temporal organization of different spinal cord cell types recapitulate development, neuronal networks and disease pathogenesis. Deep sequencing at the single cell level is also emerging as a valuable technique to provide resolution in characterizing cell-type specific changes as well as whether the organoid-derived cell types are similar to that of their in vivo counterpart. As an example, Quadrato et al. (2017) found through single cell RNA-seq that human brain photosensitive organoids comprise an extensive diversity of neural cell types, each containing subclasses of forebrain or retina cells. Hence, single cell technologies together with our ventral spinal cord organoids will help to shed light on cell type specification, diversity and disease-specific programs in our ventral spinal cord organoids. Using such technology,the diversity of cell types in the spinal cord organoids represents an excellent model to uncover the mysteries underlying selective motor neuron vulnerability in SMA and ALS (Figure 1).
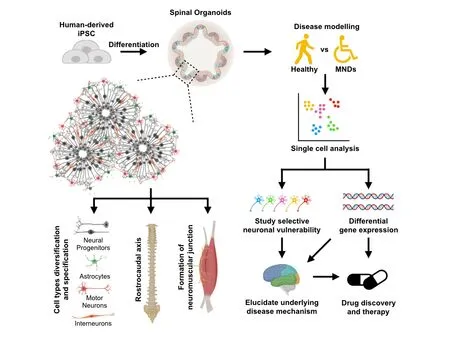
Figure 1 Features and applications of spinal cord organoids.Spinal cord organoids can be generated from human-derived iPSCs via small molecules to direct differentiation, forming self-organizing pools of neural progenitors in a three-dimensional environment. Spinal cord organoids can generate a diversity of spinal cell types that are specific to the spinal cord. In addition, the spinal cord organoids pattern closely to the spinal cord rostrocaudal axis and are functionally capable to form neuromuscular junction, demonstrating its potential in modeling motor neuron diseases. With cell-type heterogeneity arising from the three-dimensional environment, single cell analysis can be useful to provide resolution to cell-type specific changes and uncover underlying disease mechanism on selective motor neuron vulnerability in spinal muscular atrophy and amyotrophic lateral sclerosis. Hence, spinal cord organoids serve as a unique platform to model motor neuron diseases and potentially an important tool for drug discovery and therapy. iPSCs: Induced pluripotent stem cells; MNDs: motor neuron disorders.
Although spinal cord organoids recapitulate major features of spinal cord development and disease phenotypes, a number of improvements have to be made in order for them to mimic the in vivo spinal cord more closely. Currently, we are unable to generate both ventral and dorsal structures within a single spinal cord organoid.Moving forward, microfluidic devices that can maintain distinct gradients of morphogens over time can overcome this problem (Lim et al., 2019). Ogura et al. (2018) reported the formation of separate dorsal and ventral spinal cord organoids by modulating concentrations of bone morphogenetic protein 4 and Sonic Hedgehog,respectively. These dorsal and ventral spinal cord organoids may be manually fused together to form a functional sensory-motor circuit. Previous evidence indicated that in a fused organoid model,cortical interneurons migrate from medial ganglionic eminence organoids to functionally integrate into cerebral organoids (Xiang et al., 2017). We anticipate that similar strategies to fuse ventral and dorsal spinal cord organoids can assemble the sensory-motor circuitry that is critical for investigation on motor-sensory disorders.
Winanto, Zi-Jian Khong, Jin-Hui Hor, Shi-Yan Ng*
Institute of Molecular and Cell Biology, A*STAR Research Entities,Singapore, Singapore (Winanto)
School of Biological Sciences, Nanyang Technological University,Singapore, Singapore (Khong ZJ)
Department of Biological Sciences, National University of Singapore, Singapore, Singapore (Hor JH)
Yong Loo Lin School of Medicine (Physiology), National University of Singapore, National Neuroscience Institute, Singapore,Singapore; The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong Province, China (Ng SY)
*Correspondence to: Shi-Yan Ng, PhD, syng@imcb.a-star.edu.sg.orcid: 0000-0003-3418-2757 (Shi-Yan Ng)
Received:January 11, 2019
Accepted:February 19, 2019
doi: 10.4103/1673-5374.255966
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Chong Gao, University of Hong Kong, China.
杂志排行
中国神经再生研究(英文版)的其它文章
- Novel miRNA, miR-sc14, promotes Schwann cell proliferation and migration
- Knowledge domain and emerging trends in Alzheimer's disease: a scientometric review based on CiteSpace analysis
- Lesions of mediodorsal thalamic nucleus reverse abnormal firing of the medial prefrontal cortex neurons in parkinsonian rats
- Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease
- Identification of microRNAs and messenger RNAs involved in human umbilical cord mesenchymal stem cell treatment of ischemic cerebral infarction using integrated bioinformatics analysis
- miR-15b-5p targeting amyloid precursor protein is involved in the anti-amyloid eflect of curcumin in swAPP695-HEK293 cells