Assessing significant fibrosis using imaging-based elastography in chronic hepatitis B patients: Pilot study
2019-07-12HeeSunParkWonHyeokChoeHyeSeungHanMiHyeYuYoungJunKimSungIlJungJeongHanKimSoYoungKwon
Hee Sun Park, Won Hyeok Choe, Hye Seung Han, Mi Hye Yu, Young Jun Kim, Sung Il Jung, Jeong Han Kim,So Young Kwon
Abstract BACKGROUND Accurate detection of significant fibrosis (fibrosis stage 2 or higher on the METAVIR scale) is important especially for chronic hepatitis B (CHB) patients with high viral loads but with normal or mildly elevated alanine aminotransferase (ALT) levels because the presence of significant fibrosis is accepted as the indication for antiviral treatment. Liver biopsy is the reference standard for diagnosing significant fibrosis, but it is an invasive procedure.Consequently, noninvasive imaging-based measurements, such as magnetic resonance elastography (MRE) or two-dimensional shear-wave elastography (2DSWE), have been proposed for the quantitative assessment of liver fibrosis.AIM To explore MRE and 2D-SWE to identify fibrosis stage, and to compare their performance with that of serum-based indices.METHODS The study enrolled 63 treatment-naïve CHB patients with high viral loads but with normal or mildly elevated ALT levels who underwent liver biopsy before a decision was made to initiate antiviral therapy. MRE and 2D-SWE were performed, and serum-based indices, such as FIB-4 and aspartate transaminase to platelet ratio index (APRI), were calculated. The diagnostic performances of MRE, 2D-SWE, FIB-4, and APRI for assessing significant fibrosis (≥ F2) and cirrhosis (F4) were evaluated with liver histology as the reference standard, using additional data are available.STROBE statement: The authors have read and checked the STROBE checklist.Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0)license, which permits others to distribute, remix, adapt, build upon this work non-commercially,and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:http://creativecommons.org/licen ses/by-nc/4.0/Manuscript source: Unsolicited manuscript Received: April 19, 2019 Peer-review started: April 19, 2019 First decision: May 9, 2019 Revised: May 20, 2019 Accepted: June 8, 2019 Article in press: June 8, 2019 Published online: July 7, 2019 P-Reviewer: Cheungpasitporn W,Ierardi E, Mihaila RG, Tamori A,Yao D S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ receiver operating characteristic analyses.RESULTS The liver fibrosis stage was F0/F1 in 19, F2 in 14, F3 in 14, and F4 in 16 patients,respectively. MRE significantly discriminated F2 from F0/1 (P = 0.022), whereas 2D-SWE showed a broad overlap in distinguishing those stages. MRE showed a higher correlation coefficient value with fibrosis stage than 2D-SWE with fibrosis stage (0.869 vs 0.649, Spearman test; P < 0.001). Multivariate linear regression analyses showed that fibrosis stage was the only factor affecting the values of MRE (P < 0.001), whereas body mass index (P = 0.042) and fibrosis stage (P <0.001) were independent factors affecting 2D-SWE values. MRE performance for diagnosing significant fibrosis was better [area under the curve (AUC) = 0.906,positive predictive value (PPV) 97.3%, negative predictive value (NPV) 69.2%]than that of FIB-4 (AUC = 0.697, P = 0.002) and APRI (AUC = 0.717, P = 0.010),whereas the performance of 2D-SWE (AUC = 0.843, PPV 86%, NPV 65%) was not significantly different from that of FIB-4 or APRI.CONCLUSION Compared to SWE, MRE might be more precise non-invasive assessment for depicting significant fibrosis and for making-decision to initiate antiviral-therapy in treatment-naïve CHB patients with normal or mildly-elevated ALT levels.
Key words: Antiviral therapy; Chronic hepatitis B; Liver fibrosis; Magnetic resonance elastography; Ultrasound elastography
INTRODUCTION
Hepatitis B virus (HBV) infection remains a major health problem, causing chronic liver disease. If left untreated, chronic HBV infection may potentially lead to complications such as cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC)[1]. Therefore, effective antiviral treatment in chronic hepatitis B (CHB)patients can reduce the disease progression towards HBV-related cirrhosis and the risk of HCC development[1,2].
Accurate staging of liver fibrosis in CHB patients is necessary not only for predicting the long-term clinical course but also for determining whether and when to begin antiviral therapy. Recent clinical guidelines have recommended that CHB patients with high serum HBV-DNA levels [hepatitis B e-antigen (HBeAg) positive patients with serum HBV-DNA levels > 20000 IU/mL or HBeAg-negative patients with serum HBV-DNA levels > 2000 IU/mL] and elevated alanine aminotransferase(ALT) levels of twice the upper limit of normal (ULN) or greater should be considered for antiviral treatment[3-5]. CHB patients with high viral loads and significant fibrosis(METAVIR scoring system ≥ F2) should also be considered for treatment even if the ALT level is normal or mildly elevated (less than 2 times) because long-term viral suppression reduces liver-related complications, such as decompensated cirrhosis or HCC, in these patients[3-5].
Liver biopsy is still considered the “gold standard” for the evaluation of significant fibrosis in CHB patients[6]. However, its utilization is often restricted because its invasiveness can cause lifethreatening complications[7]. Moreover, tissue obtained via biopsy represents approximately only 1/50000 of the liver volume, which may result in a sampling error and is associated with considerable interobserver variability in the microscopic evaluation. Furthermore, repeating the liver biopsy to monitor changes in liver fibrotic burden is generally not feasible in clinical practice[7,8]. To overcome these limitations of liver biopsy, noninvasive serum- and imaging-based measurements for staging liver fibrosis have been developed[9,10].
To date, noninvasive methods incorporating serum-based indices or imaging-based tests using elastography have been increasingly used to assess liver fibrosis[11]. A variety of serum-based indices have been evaluated to predict the degree of liver fibrosis[10,12]. Among those, aspartate transaminase (AST)-to-platelet ratio index (APRI)and fibrosis index based on four factors (FIB-4) are commonly used for identifying liver fibrosis and cirrhosis in CHB patients because they are easily calculated with routine laboratory tests, and they have successfully predicted liver fibrosis in large cohorts[13]. However, their main disadvantage is their low accuracy in detecting mild to intermediate stages of fibrosis[10,11,13]. Imaging-based methods of elastography estimate liver stiffness that is associated with the severity of fibrosis by applying mechanical waves and by measuring their propagation speed through tissue using imaging[14-16]. Elastographic modalities can be either ultrasound (US)-based or magnetic resonance imaging (MRI)-based. US-based elastography techniques include strain-based imaging, transient elastography (TE), and shear wave elastography(SWE)[17,18]. MRI measures tissue stiffness with magnetic resonance elastography(MRE)[19,20]. These techniques have been proven superior to conventional crosssectional imaging for the evaluation of fibrosis and cirrhosis, especially in the precirrhotic stages[19-22]. Several studies comparing the diagnostic performance of serumbased indices and imaging-based elastographies have been published[17,23], but little is known regarding their diagnostic performances that can be used to inform the applicability of these modalities to whether and when to initiate antiviral therapy in treatment-naive CHB patients with high viral loads but with normal or mildly elevated ALT levels.
Therefore, the objective of this study was to evaluate the liver stiffness values of MRE and two-dimensional SWE (2D-SWE) to assess liver fibrosis and to compare their diagnostic performances with those of FIB-4 and APRI for the prediction of significant fibrosis, which is an indicator for initiating antiviral therapy in treatmentnaïve CHB patients with high viral loads but with borderline-normal or mildly elevated ALT levels.
MATERIALS AND METHODS
Patients
Between March 2013 and February 2018, 67 treatment-naïve CHB patients with high viral loads but borderline-normal or mildly elevated ALT levels who underwent liver biopsy at Konkuk University Medical Center before a decision was made to initiate antiviral therapy were recruited. The following inclusion criteria were applied: (1)Hepatitis B surface antigen (HBsAg) positivity more than 6 months, HBeAg positive patients with > 20000 IU/mL, or HBeAg-negative patients with > 2000 IU/mL,normal ALT values (our laboratory reference value was 40 IU/L), or less than two times ULN; (2) Absence of any previous or concomitant anti-HBV therapy; (3) No liver comorbidity, including hepatitis C virus (HCV) coinfection, chronic ethanol consumption (more than 20 g of alcohol per day), HIV coinfection, or autoimmune hepatitis; (4) Availability of liver histologic assessment after liver biopsy, and time interval between liver biopsy and MRE/ 2D-SWE within 2 wk; and (5) Availability of both MRE and 2D-SWE, and time interval between MRE and 2D-SWE within 3 d.Patients who have clinical features or complications of liver cirrhosis, including ascites, medium/large gastroesophageal varices, or moderate to severe thrombocytopenia (platelet counts < 80000/μL), were excluded because they should be considered for antiviral treatment without requiring liver biopsy for confirmation of liver cirrhosis. Patients under 35 years of age were also excluded because they might stay in the immune-tolerant phase of chronic HBV infection. Our Institutional Review Board approved this study, waiving informed consent because of its retrospective nature.
MR elastography
All MR examinations were performed using a 3-T MR unit (Magnetom Skyra,Siemens Medical Solutions, Erlangen, Germany). Patients were asked to hold their breath at the end-expiratory period to obtain a consistent position of the liver for each phase offset. When the acquisition was completed, wave images were automatically processed by the MR scanner, and images depicting tissue stiffness (elastograms)were generated (Figure 1A-D). These quantitative images represented shear stiffness in units of kilopascals (kPa). In addition, the elastogram was reviewed automatically by the intrinsic software for artifacts, such as significant wave interference and oblique wave propagation. Elastograms with 95% confidence mapping were produced by excluding the artifact area. MRE technical failure was considered when the following occurred: (1) Wave images showed no wave propagation; (2) Anatomic images showed severe respiratory motion artifact along the z-axis; or (3) Substantial loss of signal in the liver parenchyma suggesting an iron overload was present[16].
The mean shear stiffness of the liver was calculated by placing a manually specified region of interest (ROI) into the stiffness map of MRE images. The stiffness value of the liver parenchyma was calculated as the mean value in four ROIs (mean area,4044.8 ± 1715.8 mm2) placed by one radiologist.
SWE technique
Measurements for 2D SWE were obtained by using an Aixplorer US system(SuperSonic Imagine, Aix-en-Provence, France) equipped with a broadband convex transducer (SC6-1). The operator was a single board-certified abdominal radiologist with more than 10 years of liver US experience and more than one year of clinical experience performing real-time elastography studies. SWE examinations were performed in the right lobe of the liver through the intercostal space. Liver stiffness measurements were obtained within an ROI of 10 mm2in diameter at the area where the elasticity image was most homogeneously displayed. SWE measurement failure was considered when little or no signal was obtained in the SWE box, and an appropriate color-coded elasticity map was not acquired. Five consecutive acquisitions were obtained in the same location of the liver for each patient. Each measurement was performed during a separate breath hold. The system calculated the mean, maximum, minimum, and standard deviation of the elasticity value of each measurement in kPa (Figure 1E). The mean value of five liver stiffness measurements was calculated.
FIB-4 and APRI formulae
The FIB-4 values were calculated automatically using the formula [age (years) × AST(U/L)]/{platelets (109/L) × [ALT (U/L)]1/2}[24], in which the age of the patient was the age at the time of the liver biopsy. The APRI values were calculated using the formula(AST/upper limit of normal)/[platelet count (109/L)] × 100[25]. Our laboratory reference value of AST was 40 IU/L.
Histopathologic analysis
Biopsy specimens were fixed in formalin and embedded in paraffin. Thereafter, 4-mm-thick slices were cut and stained with hematoxylin-eosin. All specimens were analyzed by a pathologist who was blinded to the MRE results, SWE results, and the clinical data and who had 10 years of clinical experience interpreting liver pathologic examinations. The fibrosis stage and the degree of inflammation in the liver were assessed based on the METAVIR scoring system as shown below: F0, no fibrosis; F1,portal fibrosis; F2, periportal fibrosis; F3, septal fibrosis; and F4, cirrhosis[26]. In this study, a fibrosis stage of F2 or higher was considered to indicate significant fibrosis.Inflammatory activity was graded as A0 to A3: A0, no activity; A1, mild activity; A2,moderate activity; A3, severe activity.
Statistical analysis
Quantitative variables were expressed as the mean ± standard deviation (SD), which were analyzed with a t-test or a Mann-Whitney U-test, and categorical variables were demonstrated with numbers and percentages and compared using the Chi-squared method or Fisher’s exact test, when appropriate. Correlations between noninvasive methods and liver histological fibrosis stages were assessed using the Spearman correlation test. The strength of the correlation coefficients was classified as follows:0.0-0.2, very weak; 0.2-0.4, weak; 0.4-0.7, moderate; 0.7-0.9, strong; and 0.9-1.0, very strong correlation. The difference between two dependent correlations was calculated by the Steiger test. Factors affecting liver stiffness values of the MRE or 2D-SWE were first analyzed with univariate testing, and those with P < 0.05 were subsequently included in a multivariate linear regression analysis. The diagnostic performance of noninvasive methods was assessed using receiver-operating characteristic (ROC)analysis; areas under the curve (AUCs) with 95% confidence intervals, sensitivity,specificity, and positive and negative predictive values were used for the classification of significant fibrosis (≥ F2) and cirrhosis (F4). AUCs were compared using the method of DeLong et al. A P value less than 0.05 was considered to indicate a significant difference. All statistical analyses were performed by using commercially available software programs (SPSS version 17, SPSS, Chicago, IL, United States;MedCalc, version 11.6, MedCalc Software, Mariakerke, Belgium).
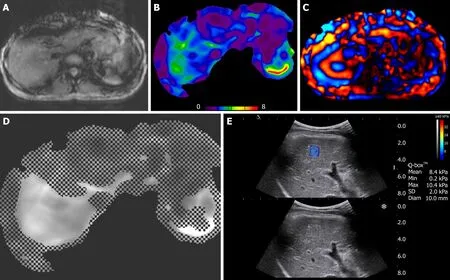
Figure 1 Images of magnetic resonance elastography (3A, 3B, 3C, 3D) and two-dimensional shear-wave elastography (3E) in 42-year old treatment-naïve chronic hepatitis B woman with fibrosis stage 3 on METAVIR score. A: anatomic image, B: elastography with color mapping, C: wave image, D: confidence map of an elastography in right lobe of the liver, and E: two-dimensional shear-wave elastography (2D-SWE) (top) and gray-scale (bottom) images of the right hepatic lobe.Her liver stiffness values of magnetic resonance elastography and 2D-SWE were 2.66 kPa and 8.4 kPa, respectively.
RESULTS
Patient characteristics
Among 67 participants, MRE failed to provide liver stiffness values in one patient because there were no visible waves on MRE images due to overweight (BMI = 27.9)(technical failure rate, 1.5%). With regard to 2D-SWE, a proper elasticity map was not ade-quately displayed in three patients due to overweight (n = 2), or uncontrolled respiration (n = 1), yielding a 4.5% technical failure rate.
Finally, a total of 63 patients who could be successfully measured using both MRE and 2D-SWE were evaluated in this study. All 63 patients were treatment naïve and included 37 men and 26 women, with a median (range) age of 50 (30-68) years. The mean (± SD) levels of serum ALT were 44 ± 20.8 U/L. The median HBV-DNA levels of 35 HBeAg-positive CHB patients and 28 HBeAg-negative patients were 6.93 ± 1.25 log10IU/mL and 4.35 ± 0.59 log10IU/mL, respectively. Histopathologically, 3, 16, 14,14, and 16 patients were diagnosed with fibrosis stage F0 to F4, respectively. The main characteristics of the patients are shown in Table 1.
Relationship between MRE, 2D-SWE, FIB-4, APRI and histological findings
The measurements of MRE, 2D-SWE, FIB-4 and APRI for different fibrosis stages are shown in Table 2. All measurements increased as the fibrosis score increased (MRE, F= 50.642,aP < 0.001; 2D-SWE, F = 16.063,bP < 0.001; FIB-4, F = 8.608,cP < 0.001; APRI,F = 4.165,dP = 0.010). Distributions of the liver stiffness values of MRE, 2D-SWE, and the FIB-4 and APRI scores in comparison with the different fibrosis stages using METAVIR scores as the reference methods are shown in Figure 2. MRE revealed a statistical significance in distinguishing between F0/1 and F2 fibrosis stages (eP =0.022), whereas 2D-SWE showed a broad overlap for those stages. Compared to MRE and 2D-SWE, large overlaps existed even with F4 fibrosis stage in FIB-4 and APRI,and they showed a wide range of readings (large SDs).
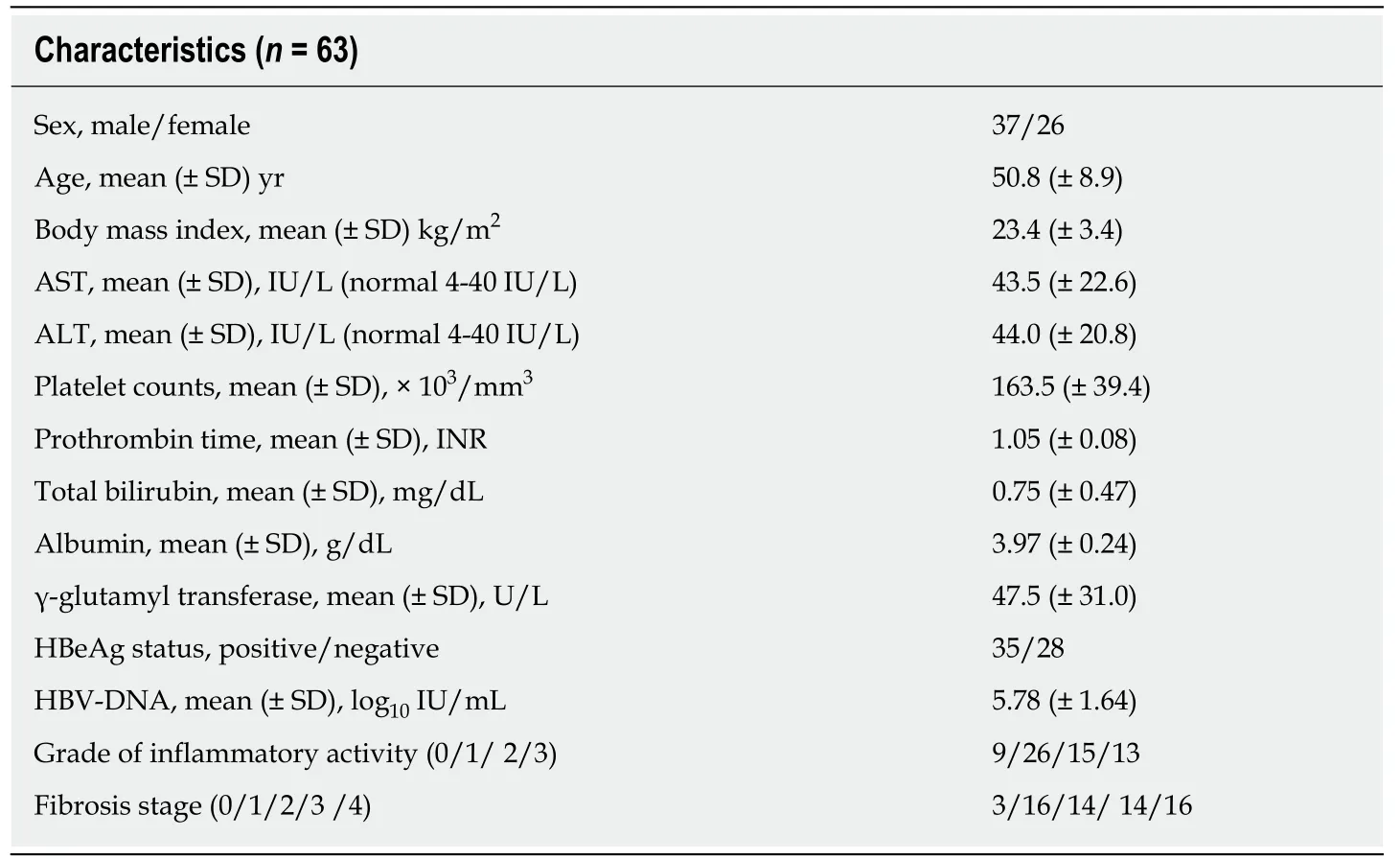
Table 1 The baseline characteristics of the enrolled treatment-naïve chronic hepatitis B patients with normal or minimally raised alanine aminotransferase levels
MRE showed strong correlations with fibrosis stage (MRE, r = 0.869,fP < 0.001;Spearman correlation), whereas 2D-SWE, FIB-4 and APRI scores showed a moderate correlation with fibrosis stage (SWE, r = 0.649,gP < 0.001; FIB-4, r = 0.517,hP < 0.001;APRI, r = 0.431,iP < 0.001: Spearman correlation). Using the Steiger test, the correlation coefficient between the liver stiffness values of MRE and liver fibrosis stage is significantly higher than that between the liver stiffness values of 2D-SWE and fibrosis stage (jP < 0.001). MRE and 2D-SWE measurements showed a moderate correlation with each other (MRE and 2D-SWE, r = 0.669,kP < 0.001), while there were moderate or weak correlations between radiology-based and serum-based measurements (MRE and FIB4, r = 0.465,lP < 0.001; MRE and APRI, r = 0.378,mP = 0.002;2D-SWE and FIB4, r = 0.553,nP < 0.001; 2D-SWE and APRI, r = 0.396,oP = 0.001:Spearman correlation).
Analyses of clinical parameters associated with liver stiffness values measured by MRE or 2D-SWE
We investigated the factors that affect liver stiffness values by MRE and 2D-SWE.These parameters include sex, age, body mass index (BMI), platelet counts, total bilirubin, albumin, AST, ALT, γ-GT, prothrombin time, HBeAg status, HBV-DNA levels, inflammatory grade, and liver fibrosis stage (Table 3). Concerning MRE, a univariate analysis revealed correlations between liver stiffness values of MRE and platelet counts, inflammatory grade, and liver fibrosis stage, and a multivariate analysis showed that only the liver fibrosis stage was an independent factor affecting liver stiffness values of MRE. Concerning 2D-SWE, a univariate analysis revealed correlations between liver stiffness values of 2D-SWE and BMI, platelet counts, inflammatory grade, and liver fibrosis stage, and a multivariate analysis showed that not only the liver fibrosis stage but also BMI were independent factors affecting liver stiffness values of 2D-SWE.
Comparing liver stiffness values measured by MRE or 2D-SWE from FIB-4 or APRI scores for the diagnosis of significant fibrosis (≥ F2) and cirrhosis (F4)
The areas under ROC curve (AUCs), cut-off values, sensitivity, specificity, positive predictive values, and negative predictive values for the diagnosis of significant fibrosis (≥ F2) and cirrhosis (F4) using radiology-based or serum-based measurement indices are shown in Table 4. The AUCs for MRE, 2D-SWE, FIB-4, and APRI scores were 0.906, 0.843, 0.697, and 0.717, respectively, for the diagnosis of significant fibrosis, and 0.894, 0.816, 0.786, and 0.701, respectively, for the diagnosis of cirrhosis.
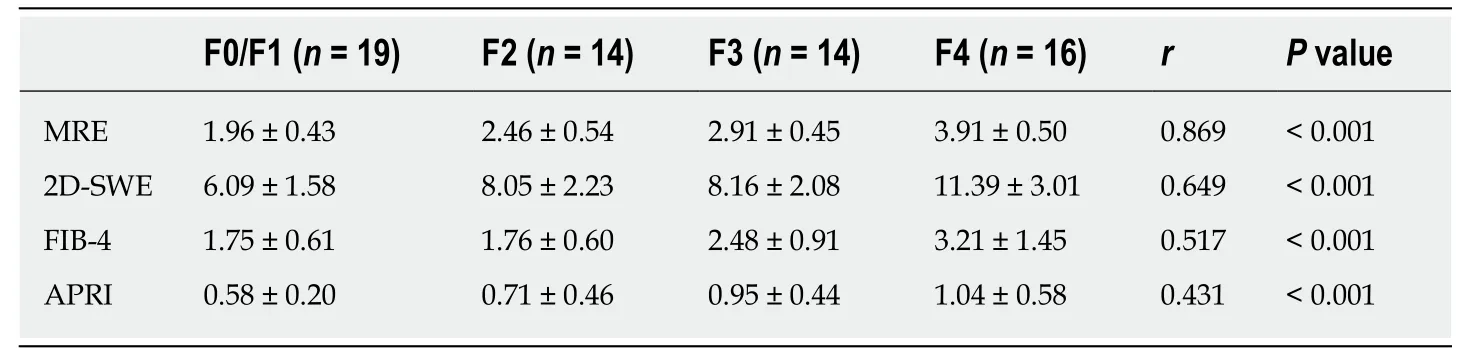
Table 2 Statistics of liver stiffness value measured by magnetic resonance elastography and two-dimensional shear wave elastography, fibrosis index based on four factors score, and aspartate transaminase-to-platelet ratio index score with the Spearman’s coefficients according to fibrosis stages
The AUCs of the MRE and 2D-SWE for the diagnosis of significant fibrosis were more than 0.80, with no statistically significant differences between indicators. The performance of MRE for the diagnosis of significant fibrosis was significantly better than that of serum-based measurements by pairwise comparison of the ROC curves(MRE vs FIB-4,pP = 0.002; MRE vs APRI,qP = 0.010, respectively). In addition, the performance of SWE was not significantly different compared to FIB-4 or APRI for the diagnosis of significant fibrosis (Figure 3A).
The AUCs of the radiology-based measurements for the diagnosis of cirrhosis were more than 0.80, and their performance was not significantly different from that of serum-based measurements for the identification of cirrhosis (F4) (Figure 3B).
DISCUSSION
The accurate diagnosis of significant fibrosis is of particular clinical value for treatment-naïve CHB patients with high viral loads but with normal or mildly elevated ALT levels because it is considered an indicator for antiviral treatment[3-5].Among 63 patients analyzed in our study, 44 (69.8%) patients should need to initiate antiviral therapy because they were diagnosed with significant fibrosis. If they did not undergo liver biopsy, they did not fulfill the indications for antiviral therapy.Therefore, a main application of our research is intended to reduce the need for invasive liver biopsy by assessing and comparing noninvasive measurements for a precise diagnosis of significant fibrosis and, consequently, to assist in making antiviral treatment decisions. Our results showed that MRE was able to better discriminate significant fibrosis from normal or mild fibrosis than 2D-SWE. Furthermore, MRE showed a higher correlation coefficient value with fibrosis stage than that between 2D-SWE and fibrosis stage. Moreover, the performance of MRE for diagnosing significant fibrosis was better than that of FIB-4 and APRI, whereas the performance of SWE was not significantly different from that of FIB-4 or APRI. Furthermore, liver fibrosis stage was the only independent factor affecting the liver stiffness values of MRE, whereas BMI as well as liver fibrosis stage can affect the liver stiffness values of 2D-SWE. In addition, technical failure rate was lower in MRE (n = 1, 1.5%) than in 2DSWE (n = 3, 4.5%). In our study, MRE could significantly discriminate between F0/1 and F2 fibrosis stage (P = 0.022), whereas 2D-SWE showed a broad overlap for those stages. The correlation coefficient between fibrosis stage and the liver stiffness values of MRE (r = 0.859) is higher than that between fibrosis stage and the values of 2DSWE, FIB-4, and APRI (r = 0.647, r = 0.498, r = 0.442, respectively). These data suggest that MRE has a better diagnostic performance in the identification of significant fibrosis than 2D-SWE as well as FIB-4 and APRI, and this is similar to a previous study comparing MR-based and US-based elastographies[17,19]. The possible reason may be that MRE can measure a larger volume of liver, and therefore potentially assesses the stiffness of nearly the entire liver, whereas SWE is able to analyze a smaller volume of liver[27,28]. Thus, MRE is more representative of liver parenchyma with less sampling variability[29,30].
The AUCs in our study showed that MRE has excellent diagnostic accuracy in the assessment of significant fibrosis. The AUC of MRE was numerically higher than that of 2D-SWE but the difference was statistically insignificant (0.906 vs 0.843). The statistical insignificance might be explained by the homogeneity of the patients in our study, as our study selected only CHB patients with normal or mildly elevated ALT levels, who are borderline in terms of a decision to initiate antiviral treatment,whereas the previous studies, which showed MRE has statistically significant higher accuracy than US-based elastography, enrolled participants with a wide range of ALT values[16,27,28,31,32]. Compared to serum-based indices, the diagnostic performance of MRE for diagnosing significant fibrosis is better than those of FIB-4 and APRI,whereas the performance of 2D-SWE is not significantly different from those of FIB-4 and APRI. These data suggested that among MRE and 2D-SWE, only MRE might help identify CHB patients who may benefit from treatment compared to serum based indices, such as FIB-4 or APRI.
We also investigated the confounding factors affecting liver stiffness values by MRE and 2D-SWE, including sex, age, BMI, platelet counts, total bilirubin, albumin,AST, ALT, γ-GT, prothrombin time, HBeAg status, HBV-DNA levels, inflammatory grade, and liver fibrosis stage. Except for liver fibrosis stage, the multivariate linear regression analysis revealed no associations between those factors and liver stiffness values of MRE. However, BMI and liver fibrosis stage were independent factors affecting liver stiffness values of 2D-SWE, and these data suggested that BMI might be a confounder that decreases liver stiffness values of 2D-SWE, potentially causing underestimation of the real liver fibrosis stage. The reason why BMI affect liver stiffness measurements of 2D-SWE is not clear. A possible explanation is that high BMI is the most common condition associated with hepatic steatosis, and several studies have shown that the liver stiffness value of US-based elastography is fundamentally influenced by hepatic liver fat content[33,34]. On the other hand, a few clinical studies revealed that hepatic steatosis did not affect liver stiffness values of MRE[35,36].
There are some limitations to the present study. First, the use of liver biopsy as the reference standard for assessing liver fibrosis has limitations associated with sampling errors, as well as intra- and interobserver variability, which are at least partly linked to the size of the biopsy. Second, despite MRE has the best effectiveness, it is much more expensive than 2D-SWE and is available only in tertiary centers. Third, as the sample size of this study is relatively small, the present results need to be validated independently in further studies.
In conclusion, MRE might be a non-invasive and more precise measurement for the assessment of significant fibrosis compared to 2D-SWE as well as serum-based indices in treatment-naive CHB patients with high viral loads but with normal or mildly elevated ALT levels who should be considered for initiation of antiviral therapy depending on the presence of significant fibrosis.
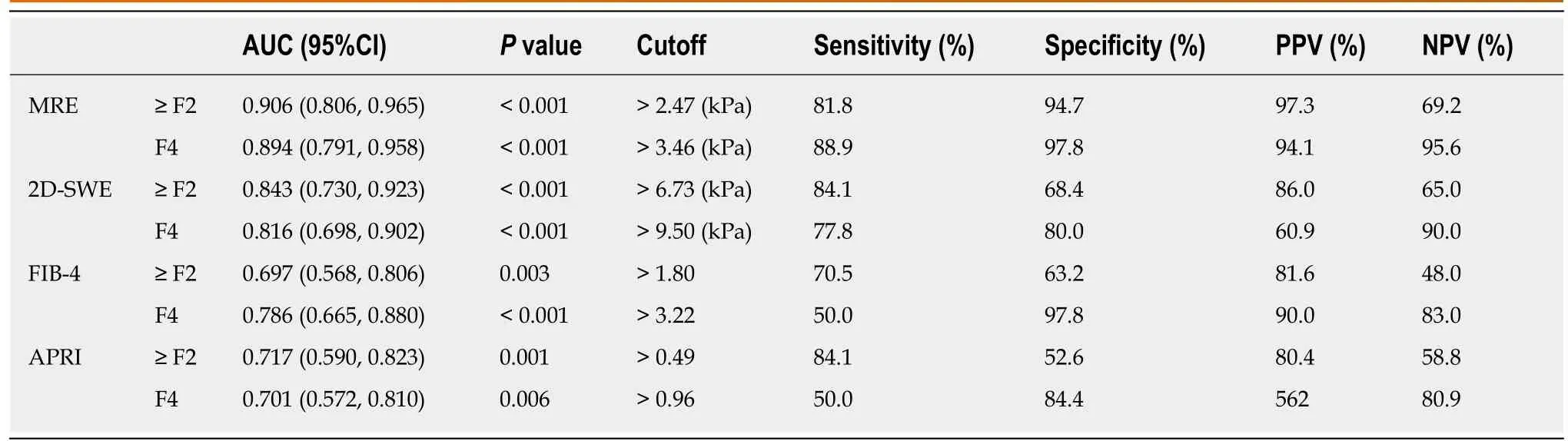
Table 4 Diagnostic performance of magnetic resonance elastography and two-dimensional shear wave elastography, fibrosis index based on four factors score, and aspartate transaminase-to-platelet ratio index for evaluation of significant fibrosis (≥ F2) and cirrhosis(F4)

Figure 3 Graphs showing area under the receiver operating characteristic curves of magnetic resonance elastography, two-dimensional shear-wave elastography, fibrosis index based on four factors, and aspartate transaminase to platelet ratio index for prediction of significant fibrosis (A) and cirrhosis(B) in trea-tment-naive chronic hepatitis B patients with normal or mildly elevated alanine aminotransferase. MRE: Magnetic resonance elastography; 2DSWE: Two-dimensional shear-wave elastography; APRI: Aspartate transaminase to platelet ratio index; FIB-4: Fibrosis index based on four factors.
ARTICLE HIGHLIGHTS
Research background
Accurate detection of significant fibrosis (fibrosis stage 2 or higher on the METAVIR scale) is important especially for chronic hepatitis B (CHB) patients with high viral loads but with normal or mildly elevated alanine aminotransferase (ALT) levels because the presence of significant fibrosis is accepted as the indication for antiviral treatment. Liver biopsy is the reference standard for diagnosing significant fibrosis, but it is an invasive procedure. Consequently, noninvasive imaging-based measurements, such as magnetic resonance elastography (MRE) or twodimensional shear-wave elastography (2D-SWE), have been proposed for the quantitative assessment of liver fibrosis.
Research motivation
Liver biopsy is still considered the “gold standard” for the evaluation of significant fibrosis in CHB patients. However, its utilization is often restricted because its invasiveness can cause life threatening complications. Moreover, tissue obtained via biopsy represents approximately only 1/50000 of the liver volume, which may result in a sampling error and is associated with considerable interobserver variability in the microscopic evaluation. Furthermore, repeating the liver biopsy to monitor changes in liver fibrotic burden is generally not feasible in clinical practice.
Research objectives
The objective of this study was to evaluate the liver stiffness values of MRE and two-dimensional SWE (2D-SWE) to assess liver fibrosis and to compare their diagnostic performances with those of FIB-4 and APRI for the prediction of significant fibrosis, which is an indicator for initiating antiviral therapy in treatment-naïve CHB patients with high viral loads but with borderlinenormal or mildly elevated ALT levels.
Research methods
The study enrolled 63 treatment-naïve CHB patients with high viral loads but with normal or mildly elevated ALT levels who underwent liver biopsy before a decision was made to initiate antiviral therapy. MRE and 2D-SWE were performed, and serum-based indices, such as FIB-4 and APRI, were calculated. The diagnostic performances of MRE, 2D-SWE, FIB-4, and APRI for assessing significant fibrosis (≥ F2) and cirrhosis (F4) were evaluated with liver histology as the reference standard, using receiver operating characteristic analyses.
Research results
The liver fibrosis stage was F0/F1 in 19, F2 in 14, F3 in 14, and F4 in 16 patients, respectively.MRE significantly discriminated F2 from F0/1 (P = 0.022), whereas 2D-SWE showed a broad overlap in distinguishing those stages. MRE showed a higher correlation coefficient value with fibrosis stage than 2D-SWE with fibrosis stage (0.859 vs 0.647, Spearman test; P < 0.001).Multiple-regression analyses showed that fibrosis stage was the only factor affecting the values of MRE (P < 0.001), whereas body mass index (P = 0.042) and fibrosis stage (P < 0.001) were independent factors affecting 2D-SWE values. The MRE performance for diagnosing significant fibrosis was better than FIB-4 (P = 0.002) and APRI (P = 0.010), whereas the performance of 2DSWE was not significantly different from that of FIB-4 or APRI.
Research conclusions
MR elastography might be a non-invasive and more precise measurement for the assessment of significant fibrosis compared to 2D-SWE as well as serum-based indices in treatment-naïve CHB patients with high viral loads but with normal or mildly elevated ALT levels who should be considered for initiation of antiviral therapy depending on the presence of significant fibrosis.
Research perspectives
There are some limitations to the present study. First, the use of liver biopsy as the reference standard for assessing liver fibrosis has limitations associated with sampling errors, as well as intra and interobserver variability, which are at least partly linked to the size of the biopsy.Second, despite MRE has the best effectiveness, it is much more expensive than 2D-SWE and is available only in tertiary centers. Third, as the sample size of this study is relatively small, the pre-sent results need to be validated independently in further studies.
杂志排行
World Journal of Gastroenterology的其它文章
- Retraction Note: Construction of Gpm6a/ReelinGFPCreERT2 by BAC recombination using a specific gene in hepatic mesothelial or stellate cells
- Botulinum toxin injections after surgery for Hirschsprung disease:Systematic review and meta-analysis
- Gut microbiota contributes to the distinction between two traditional Chinese medicine syndromes of ulcerative colitis
- Nuclear magnetic resonance-based metabolomics and metabolic pathway networks from patient-matched esophageal carcinoma,adjacent noncancerous tissues and urine
- Silicone-covered biodegradable magnesium stent for treating benign esophageal stricture in a rabbit model
- Sporamin suppresses growth of xenografted colorectal carcinoma in athymic BALB/c mice by inhibiting liver β-catenin and vascular endothelial growth factor expression
