不同方向内含子对重组CHO细胞中神经生长因子表达的影响
2019-07-10董卫华李翠萍杨赟王天云王芳
董卫华,李翠萍,杨赟,王天云,王芳
不同方向内含子对重组CHO细胞中神经生长因子表达的影响
董卫华1,2,李翠萍1,杨赟1,王天云1,2,王芳1
1 新乡医学院 基础医学院 生物化学与分子生物学教研室,河南 新乡 453003 2 河南省分子诊断与医学检验技术协同创新中心,河南 新乡 453003
为了研究不同方向的嵌合体内含子对重组神经生长因子 (Nerve growth factor,NGF) 基因表达的影响,以人β-珠蛋白第一内含子5ʹ端剪接序列和人免疫球蛋白重链可变区内含子3ʹ端剪接序列组合而成的嵌合体内含子作为研究对象,在NGF基因5ʹ端插入不同方向的嵌合体内含子,构建含不同方向内含子的NGF基因表达载体。转染至CHO细胞后,G418筛选稳定转染的细胞,荧光定量PCR、ELISA和Western blotting检测不同载体NGF基因的表达情况。结果显示内含子可以大幅度提高NGF基因的表达,且正向内含子对NGF基因表达的增强作用无论是在mRNA水平还是在蛋白水平都要高于反向内含子。所以内含子能够提高外源NGF基因的表达,且内含子调控转基因表达具有方向性。
CHO细胞,基因表达,内含子,神经生长因子
The efficient expression of a transgene is a critical issue in the field of genetic engineering. However, transgene silencing and low level of expression due to the position effect is a widespread issue in transgenic animals and plants[1-3]. Construction of efficient expression vectors is an effective strategy for improving transgenic expression levels. Previous studies on this topic have focused on issues such as promoter selection, suitable enhancers, gene dosage, and optimization of vector-host combinations[4-8]. Introns are nucleotide sequences that are removed from the initial transcript during the post-transcriptional processing. The fact that introns could enhance gene expression was first reported by Callis in a study on transgenic maize[9]. Since then, many more introns have been confirmed to affect transgenic expression[10-13]. Different introns have different effects on gene expression, with some introns even reducing the gene expression levels[9]. The source of the introns, type of host cell, and the intron insertion site are some key factors that need to be considered if we aim to use introns for improving gene expression levels. Chimeric introns have exact splicing sites and have been confirmed to significantly promote the expression of the chloramphenicol acetyltransferase (CAT) gene in mice[14]. They have also been shown to increase the expression of GFP in 293T, COS and Chinese hamster ovary (CHO) cells[15]. Therefore, we chose to use chimeric introns in this study. The chimeric introns in this study consisted of the 5ʹ-donor site from the first intron of the human β-globin gene and the branch and 3ʹ-acceptor site from the intron of an immunoglobulin gene heavy chain variable region.
Nerve growth factor (NGF) is a member of the neurotrophic factor family. As a common humoral regulation factor used in the clinical field, NGF is widely distributed in the central and peripheral nervous systems. It maintains neuron growth, survival, and differentiation, and affects synaptic plasticity by promoting the development of the nervous system. Under pathological conditions, the purpose of protecting neurons can be achieved by inducing the expression of the nerve growth factor gene, which, in turn, inhibits neuronal death, removes free radicals, and promotes the recovery of neuronal function[16-17].
The mammalian cell expression system can provide post-translational machining process functions such as the direction of correct protein folding, and complex N-type glycosylation, and accurate O-type glycosylation. The expression products are closest to the natural protein molecules found in higher organisms in terms of the molecular structure, physical and chemical properties, and biological functions.
The most widely used mammalian cell expression systems are CHO cells, African green monkey kidney fibroblast COS cells, baby hamster kidney (BHK) cells, mouse thymoma NSO cells, and mouse myeloma SP2/0 cells[18-19]. Among these, CHO cells are the most commonly used expression systems[20-21]. Authors including Xu and Wang have been using CHO cells as a recombinant NGF gene expression system[22-23]. However, to our knowledge, no study has reported that chimeric introns can promote NGF gene expression in CHO cells.
In this study, a chimeric intron and NGF gene were cloned, and NGF expression vectors with different directions of chimeric introns were constructed. These vectors were then transfected into CHO cells and their effects on NGF gene expression were observed.
1 Materials and Methods
1.1 Reagents and cells
Restriction enzymes, includingⅠanddⅢ, were purchased from TaKaRa Company (Dalian, China). The Genome extraction kit, PCR mix, DNA gel extraction kit, and T4 DNA ligase were all purchased from TaKaRa Co. (Dalian, China). Agarose and G418 were purchased from Sigma-Aldrich Co. (St. Louis, Missouri, USA). Primer synthesis and sequencing of the chimeric intron and NGF gene were carried out by Invitrogen (Beijing, China). pCAT-3 vector was purchased from Promega (Madison, WI, USA). CHO cells used in this study were purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China). DMEM and serum were purchased from Gibco, Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Suo-hua transfection reagent was purchased from Xiamen Sunma Bioengineering Co. Ltd. (Xiamen, China). TRIzol reagents were purchased from Invitrogen (Beijing, China).
1.2 DNA cloning
Primers (Table 1) were designed based on the gene sequence of the nerve growth factor () (GenBank No. AB489186.1).Ⅰandd Ⅲ restriction sites were added at the ends of the primers, in order to subclone thegene. Human whole blood genome was extracted using a genome extraction kit, following the manufacturer’s instructions. Thegene was amplified using human genomic DNA as the PCR template. PCR results were visualized by 1% agarose gel electrophoresis. Target bands were recovered, ligated to T-vector, and sequenced.
Chimeric introns were amplified using pCAT-3 control as the PCR template. The PCR products were tested by 1% agarose gel electrophoresis. The target bands were recovered, ligated to the T-vector, and then sequenced.
1.3 Vector construction
Plasmid pCATG (constructed and preserved by our lab, pCAT-3 inserted G418 selection marker) was digested withⅠ andd Ⅲ. Thegene was connected to a large fragment of pCATG, and the new vector was named pNFG. The pNFG vector was digested withd Ⅲ, and the resultant fragments were recovered. The recovered pNFG fragments as well as the forward and reverse introns obtained from PCR amplification were treated with alkaline phosphatase. The forward and reverse introns were added to 5ʹ-upstream ofgene respectively. Vectors pNFGZ, containing the forward intron, and pNFGF, containing the reverse intron, were constructed (Fig. 1).
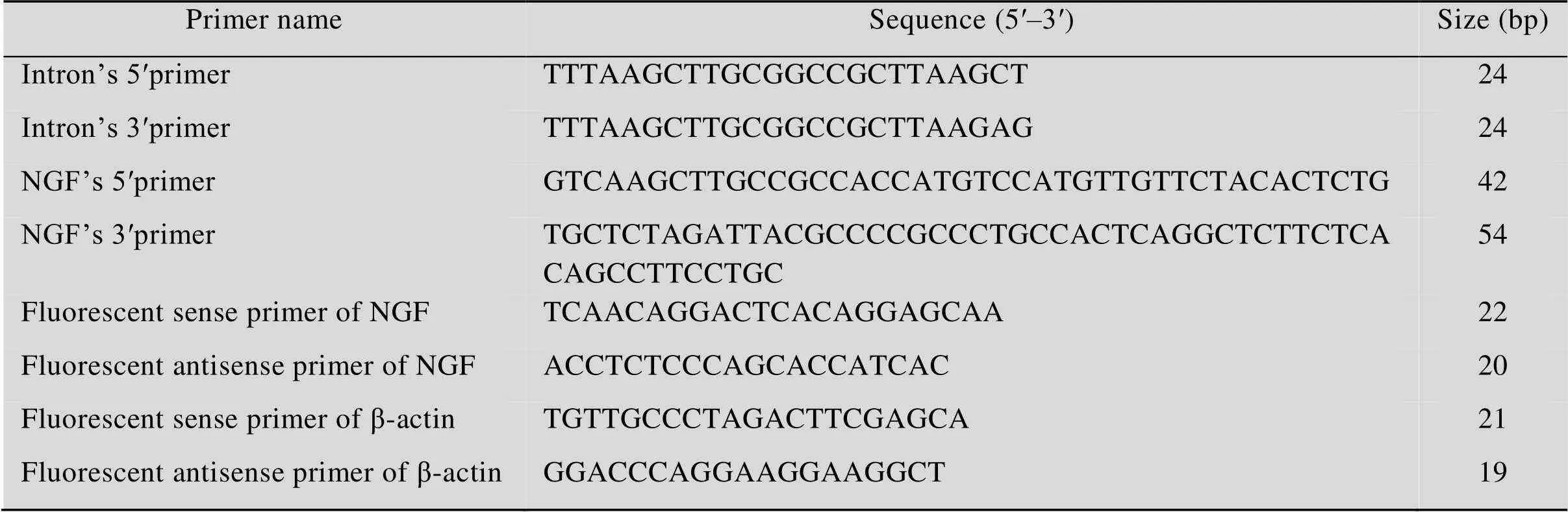
表1 本研究用到的引物
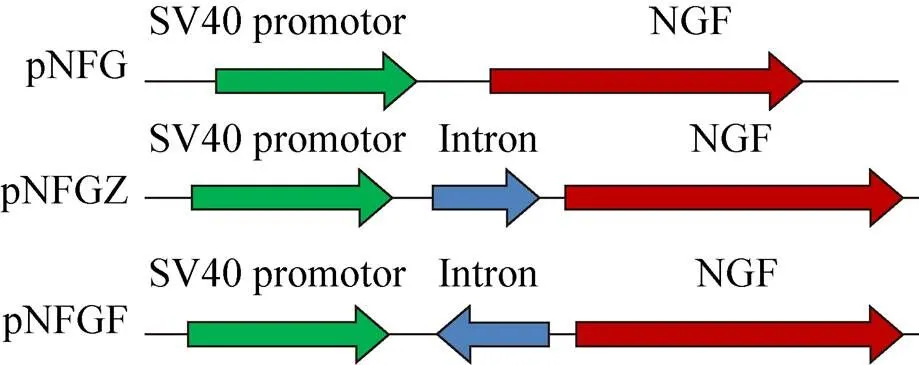
图1 本研究使用的质粒
1.4 Cell culture and transfection
CHO cells were cultured in DMEM containing 15% fetal bovine serum. When the culture attained the log phase, they were transferred to 24-well plates with 1×105cells per well. When the cell density reached 80%, three vectors, pNFG, pNFGZ and pNFGF, were transfected into CHO cells using Suo-hua transfection reagents. The manufacturer’s instructions were followed in all instances. After 24 h, G418 (800 µg/mL) was added to the DMEM, in order to screen stable transfected cells, until all control cells died. The cells were then cultured using 300 μg/mL G418 for 1 week. Single clones were selected and transferred to 96-well plates, followed by continuous culturing with 300 μg/mL G418 DMEM for 1–2 weeks. Single clones that grew well in 96-well plates were transferred to culture bottles, where they were further cultured for 1 week in DMEM with 300 μg/mL G418.
1.5 Quantitative PCR
CHO cells that were stably transfected for 1 week were washed twice with PBS, digested by 1% trypsin, and collected by centrifugation at 4 000 r/min for 5 min. Total RNA was extracted using Trizol, and the RNA content was measured by the UV method. The cDNA reverse transcription mixture comprised RNA (1 µg), buffer 2 µL, enzyme mix (0.5 µL), oligo dT (0.5 µL), and random 6-mers (0.5 µL), with ddH2O added to reach the reaction volume of 10 µL. The reaction temperature was 37 °C for 15 min, followed by 85 °C for 5 s.
The primer sequences used for quantitative PCR amplification of thegene and internal control β-actin are shown in Table 1. The PCR reaction mixture comprised SYBR 5 µL, P10.2 µL, P20.2 µL, cDNA 0.2 µL and ddH2O 4.45 µL. The reaction conditions were: 95 °C for 5 s, and 60 °C for 30 s, repeated for 40 cycles. The reverse transcription reagents and fluorescence quantitative PCR reagents were purchased from TaKaRa Co.
1.6 ELISA
Stably transfected CHO cells were washed twice with PBS and cultured in 3 mL of serum-free DMEM for 24 h. The expression of NGF protein per 106 cells in the supernatant was determined by ELISA (Shanghai Yi-qiao Biological Technology Co. Ltd., Shanghai, China). The number of stably transfected cells was determined simultaneously by cell counting.
1.7 Western blotting
The protein content was determined by the bicinchoninic acid (BCA) method. A 1-µg sample of protein was used for sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to PVDF membranes after separation. The membrane was incubated overnight at 4°C in a blocking buffer containing skimmed milk, and then incubated with the NGF antibody solution (1:500, Wuhan Boster Biological Technology Co. Ltd, Wuhan, China) at room temperature for 2 h. Finally, the membranes were incubated in horseradish peroxidase-labeled goat anti-rabbit IgG antibody solution (1:3 000) at room temperature for 1 h. The protein signals were detected using ECL luminescence reagents. The results of the Western blotting were visualized using the Tanon-5200 chemiluminescence imaging system (Shanghai, China).
1.8 Bioinformatic analysis
Specific transcription-factor-binding sites of forward and reverse chimeric introns were identified using Genomatix Online software MatInspector (http://www.genomatix.de/index.html).
1.9 Statistical analysis
2 Results
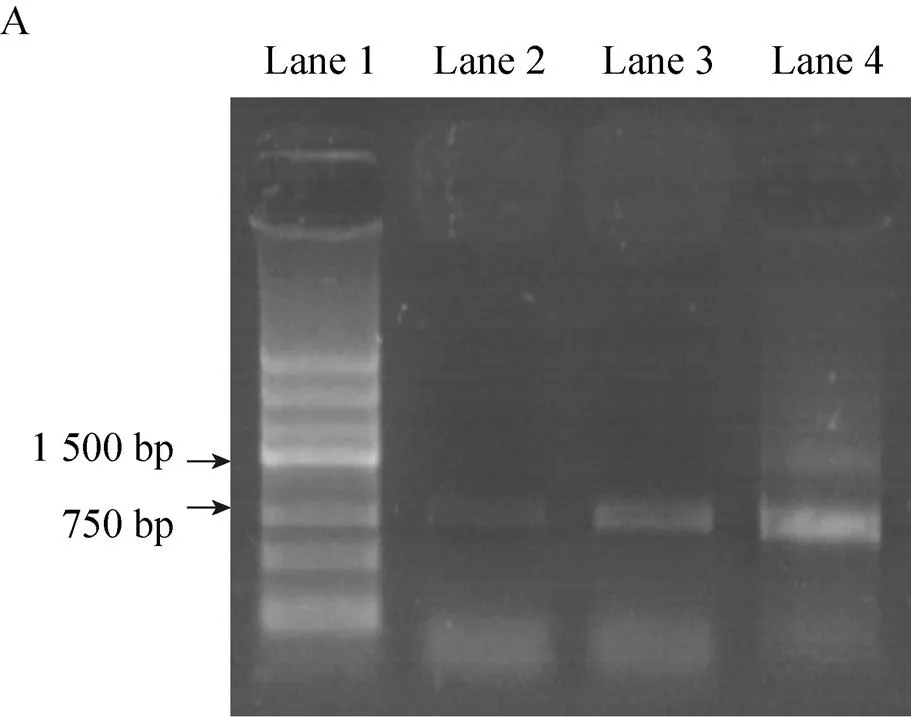
2.1 Identification of recombinant plasmids
Thegene was amplified by PCR, using pNFGZ, pNFGF and pNFG as templates. The presence of 770 bp bands established that thegene was cloned successfully into pNFG, pNFGZ and pNFGF vectors (Fig. 2A). pNFGZ and pNFGF were digested byd Ⅲ. The presence of 200 bp bands established that the introns had been cloned into pNFGZ and pNFGF (Fig. 2B). Sequencing further confirmed that all the vectors were constructed successfully.
2.2 mRNA level of the NGF gene
The results of fluorescence quantitative PCR are shown in Fig. 3. pNFG vector was able to correctly express the mRNA ofgene. The chimeric introns were able to enhance the mRNA level of thegene. The forward and reverse introns successfully promoted the transcription ofgene. The mRNA level in the pNFGF vector containing the reverse intron was 115-times higher than that in the pNFG vector without introns. Meanwhile, the mRNA level of pNFGZ containing the forward intron increased to 363-times that of the pNFG vector and 248-times that of the pNFGF vector containing the reverse intron. The difference in both cases was highly significant (<0.01).
2.3 NGF protein expression
Serum-free culture medium was collected after the stable transfected cells were cultured for 24 h. The expression levels of NGF protein were then detected by the ELISA (Fig. 4). The results demonstrated that the pNFG vector can correctly express the NGF protein. The NGF expression level was 2.9 ng/mL/106cells. Vectors containing the chimeric introns were able to appropriately increase the NGF protein level. This implies that both forward and reverse introns can enhance the expression ofgene to NGF protein. The NGF protein level of pNFGF vector containing the reverse intron increased to 1.75-times that of the pNFG vector without introns, while the protein level of pNFGZ was 1.52-times that of pNFGF.
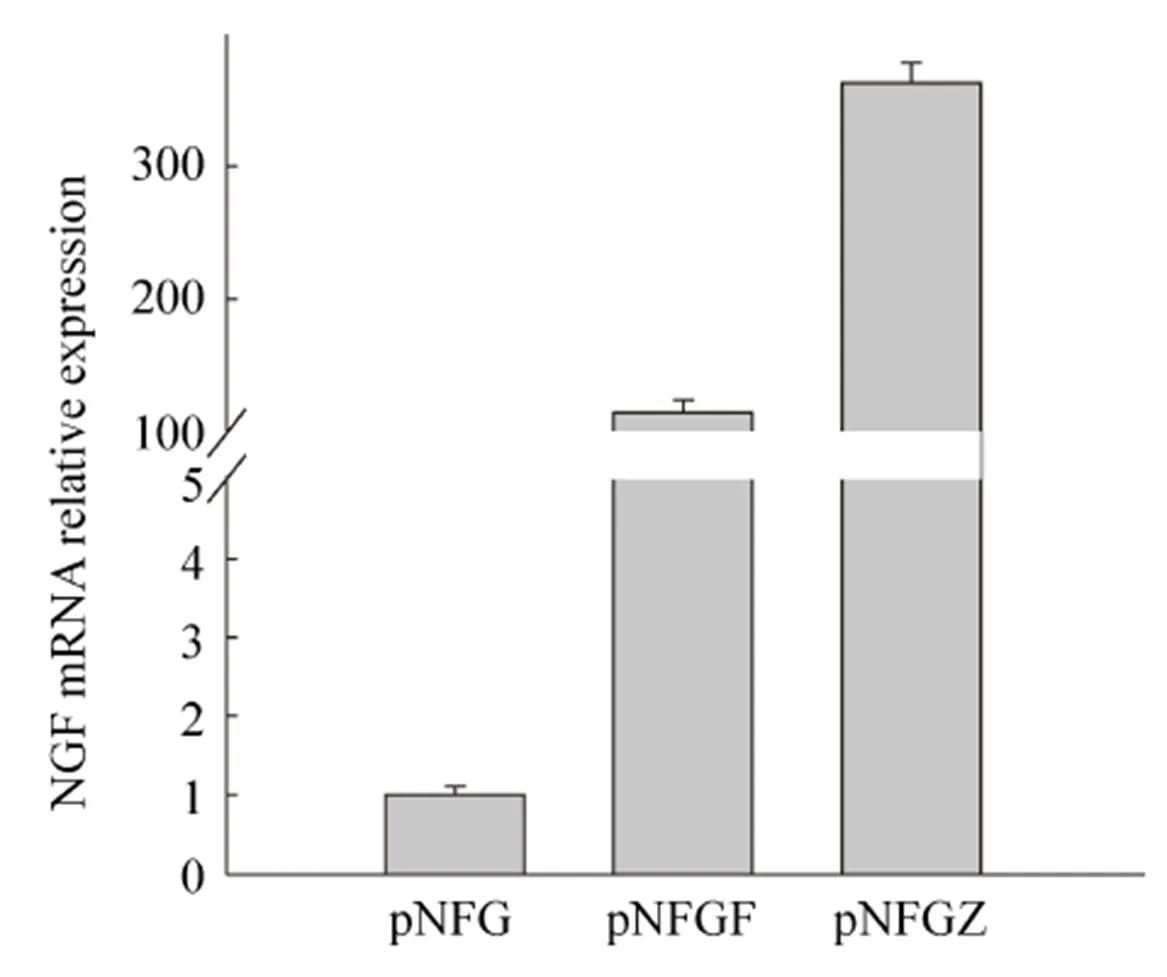
图3 荧光定量PCR检测NGF mRNA的相对表达量
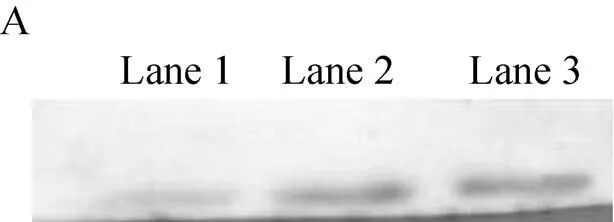
Western blotting results also showed that the activity of NGF protein in pNFGZ and pNFGF vectors containing introns was higher than that of pNFG, while pNFGZ had a higher protein activity than pNFGF (Fig. 5).
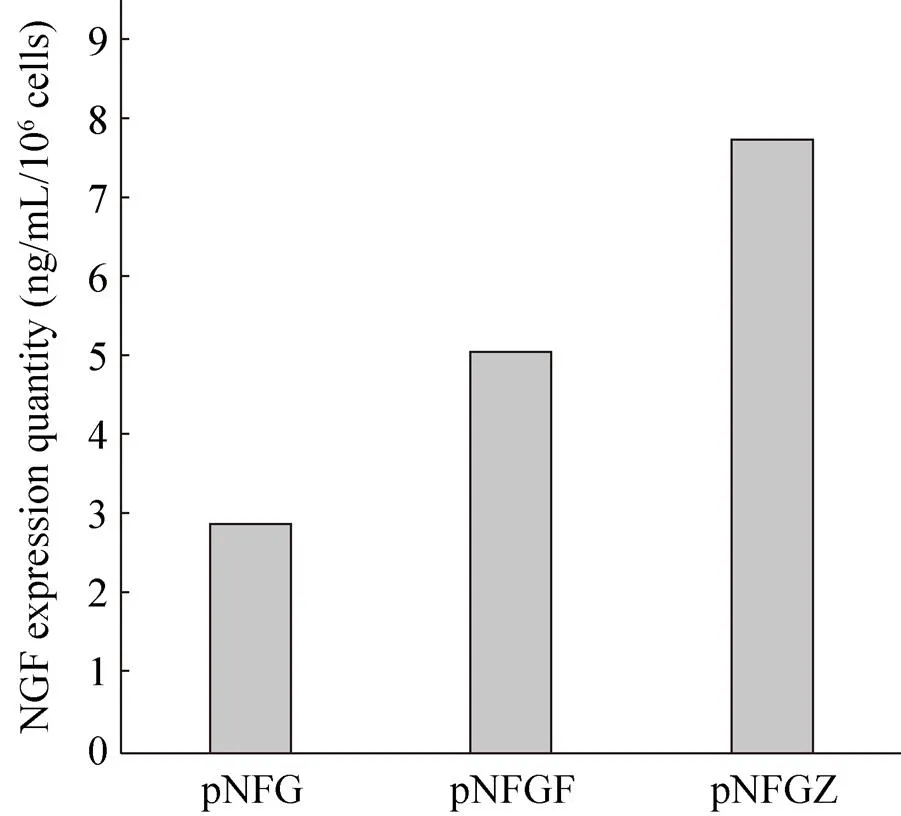
图4 ELISA检测NGF蛋白的表达量
2.4 Analysis of transcription-factor-binding sites
Transcription-factor-binding sites of the forward and reverse intron sequences were analyzed using the MatInspector software. The results showed that there were 27 transcription factors that could combine with the forward intron sequence. These included the Zinc finger BED domain-containing protein, heat shock factors, and GATA binding factors. In addition, 22 transcription factors could combine with the reverse intron sequence, including the C2H2zinc finger transcription factors, two-handed zinc finger homeodomain transcription factors, and Hepatic Nuclear Factor 1. The forward intron sequence could combine with a greater number of transcription factors, thereby, being more efficient in improving the gene transcription. The reverse intron sequence presented a lower number of transcription-factor-binding sites, consistent with its lower ability to enhance gene expression.
3 Discussion
Designing efficient expression vectors is an important way of improving gene expression systems. cHS4 (chicken β-globin 5ʹ hypersensitive site) and ubiquitous chromatin opening element (UCOE) are examples of cis-elements that are typically used to improve transgenic expression[18,22]. In addition, introns are effective elements to enhance transgene expression. Cooper[9]reported that lack of introns can lead to a decline in gene expression. Hermening et al., Hasannia et al. and Kim et al.[10-12]reported that introns can improve the expression of foreign genes. Different types of introns enhance the expression of recombinant proteins to different degrees, and the maximum change reported to date has been 20-fold.Possible ways in which introns enhance gene expression are: 1) introns may contain enhancers or other acting elements, which could bind to proteins to affect the initiation and extension of transcription; 2) splicing of introns may increase the stability of mRNA in nucleus, leading to greater accumulation of mature mRNA in the cytoplasm; and 3) introns may contain sequences that can improve gene expression by changing the nuclear components, location, etc.
In this study, recombinant nerve growth factor (NGF) gene expression vectors were constructed, and chimeric introns comprising the 5ʹ-end splice sequence of the first intron of human β-globin and splice sequences of the 3ʹ-end of the human immunoglobulin heavy chain variable region intron were inserted into the 5ʹ-upstream region of thegene. Chimeric introns in different directions could potentially enhance the expression of thegene differently. The forward intron not only enhanced the NGF mRNA expression level, but was also able to increase the amount of NGF protein produced. However, the effect on the protein expression level was not as significant as that on the mRNA expression level. This difference may be explained by the fact that there are more transcription factor binding sites in the forward intron sequence, which could greatly improve the level ofgene transcription, and the translation process could be affected by mRNA degradation. The reverse intron could also promote the transcription and translation process of thegene, thereby increasing the quantity of both NGF mRNAs and proteins. Our results are similar to those reported by Hermening et al. and Kim et al.[10-12], i.e. introns can improve the expression of transgenes, but different introns exhibit different degrees of improvement. This may be related to the choice of intron types, the source of introns, and the position of the insertion. We determined that the direction of the chimeric introns could affect gene expression. A possible reason is that introns inserted in different positions have different sequences, which may be combined with different transcription factors, thereby presenting different abilities of gene expression regulation.
4 Conclusion
Chimeric introns can enhance the expression of exogenous genes. Different direction introns have different capacities of gene expression regulation. In our study, the forward introns could greatly promote the transcription of a foreign gene. They could also significantly improve the process of translation of a foreign gene. Reverse introns could also improve the expression of foreign genes, but not as effectively as the forward intron.
[1] Mock U, Thiele R, Uhde A, et al. Efficient lentiviral transduction and transgene expression in primary human B cells. Hum Gene Ther Methods, 2012, 23(6): 408–415.
[2] Fath S, Bauer AP, Liss M, et al. Multiparameter RNA and codon optimization: a standardized tool to assess and enhance autologous mammalian gene expression. PLoS ONE, 2011, 6(3): e17596.
[3] Williams S, Mustoe T, Mulcahy T, et al. CpG-island fragments from thegenomic locus reduce silencing and enhance transgene expression from the hCMV promoter/enhancer in mammalian cells. BMC Biotechnol, 2005, 5: 17.
[4] Mariati, Ng YK, Chao SH, et al. Evaluating regulatory elements of human cytomegalovirus major immediate early gene for enhancing transgene expression levels in CHO K1 and HEK293 cells. J Biotechnol, 2010, 147(3/4): 160–163.
[5] Iwai R, Kumagai Y, Fujiwara M, et al. Combination of cytomegalovirus enhancer with human cellular promoters for gene-induced chondrogenesis of human bone marrow mesenchymal stem cells. J Biosci Bioeng, 2010, 110(5): 593–596.
[6] Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther, 2011, 19(1): 16–27.
[7] Wang TY, Zhang JH, Jing CQ, et al. Positional effects of the matrix attachment region on transgene expression in stably transfected CHO cells. Cell Biol Int, 2010, 34(2): 141–145.
[8] Wang F, Wang TY, Tang YY, et al. Different matrix attachment regions flanking a transgene effectively enhance gene expression in stably transfected Chinese hamster ovary cells. Gene, 2012, 500(1): 59–62.
[9] Luan W. Introns and their role in gene expression. Yunnan Agr Sci & Technol, 2008, (S2): 182–186 (in Chinese).栾薇. 内含子及其在基因表达中的作用.云南农业科技, 2008, (S2): 182–186.
[10] Cooper AR, Lill GR, Gschweng EH, et al. Rescue of splicing-mediated intron loss maximizes expression in lentiviral vectors containing the human ubiquitin C promoter. Nucleic Acids Res, 2015, 43(1): 682–690.
[11] Hermening S, Kügler S, Bähr M, et al. Increased protein expression from adenoviral shuttle plasmids and vectors by insertion of a small chimeric intron sequence. J Virol Methods, 2004, 122(1): 73–77.
[12] Hasannia S, Lotfi AS, Mahboudi F, et al. Elevated expression of human alpha-1 antitrypsin mediated by yeast intron in. Biotechnol Lett, 2006, 28(19): 1545–1550.
[13] Kim SY, Lee JH, Shin HS, et al. The human elongation factor 1 alpha (EF-1α) first intron highly enhances expression of foreign genes from the murine cytomegalovirus promoter. J Biotechnol, 2002, 93(2): 183–187.
[14] Choi T, Huang M, Gorman C, et al. A generic intron increases gene expression in transgenic mice. Mol Cell Biol, 1991, 11(6): 3070–3074.
[15] Gong YP, Tao J, Wang H, et al. Effect of chimeric intron on the Expression of Anti-bFGF antibody genes in 293T cells. China Biotechnol, 2010, 30(3): 9–14 (in Chinese).龚义平,陶俊,王宏,等.嵌合内含子对抗bFGF抗体基因在293T细胞中表达的影响. 中国生物工程杂志, 2010, 30(3): 9–14.
[16] Liu XL, Zhang W, Tang SJ. Intracranial transplantation of human adipose-derived stem cells promotes the expression of neurotrophic factors and nerve repair in rats of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol, 2014, 7(1): 174–183.
[17] Li CJ, Ma YH, Yi KL, et al. The interactions between nerve growth factor and gonadotrophins in bovine oviduct. Animal Reprod Sci, 2014, 149(3/4): 117–123.
[18] Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, et al. Position-effect protection and enhancer blocking by the chicken β-globin insulator are separable activities. Proc Natl Acad Sci USA, 2002, 99(10): 6883–6888.
[19] Fath S, Bauer AP, Liss M, et al. Multiparameter RNA and codon optimization: a standardized tool to assess and enhance autologous mammalian gene expression. PLoS ONE, 2011, 6(3): e17596.
[20] Lin Y, Li ZX, Wang TY, et al. MAR characteristic motifs mediate episomal vector in CHO cells. Gene, 2015, 559(2): 137–143.
[21] Wang F, Zhang JH, Wang TY, et al. Regulating effects of insertion direction of matrix attachment regions on transgenic expression in stably transformed Chinese hamster ovary cells. Genet Mol Res, 2015, 14(2): 7031–7038.
[22] Xu L, Li YH, Shi XC, et al. Expression, purification, and characterization of recombinant mouse nerve growth factor in Chinese hamster ovary cells. Protein Expr Purif, 2014, 104: 41–49.
[23] Wang XY, Zhang JH, Sun QL, et al. Characteristic element of matrix attachment region mediates vector attachment and enhances nerve growth factor expression in Chinese hamster ovary cells. Genet Mol Res, 2015, 14(3): 9191–9199.
Increasing transgenic expression in recombinant Chinese hamster ovary cells using introns in different directions
Weihua Dong1,2, Cuiping Li1, Yun Yang1, Tianyun Wang1,2, and Fang Wang1
1 Department of Biochemistry and Molecular Biology, Basic Medical School, Xinxiang Medical University, Xinxiang 453003, Henan, China 2 Molecular Diagnostic and Medical Test Technology Collaborative Innovation Center of Henan Province, Xinxiang 453003, Henan, China
The aim of this study is to investigate the effect of the chimeric intron in different directions on the expression of the nerve growth factor (NGF) in recombinant Chinese hamster ovary (CHO) cells. The chimeric intron that contained the splice sequence of the first intron of the human β-globin and the human immunoglobulin heavy chain variable region intron was used. NGF gene was cloned into the expression vectors containing the chimeric intron in the forward or reverse direction, followed by transfecting into CHO cells, and screened under G418 to produce the stable transfected CHO cells. Fluorescence quantitative PCR, ELISA, and Western blotting were performed to detect the recombinant NGF gene expression in CHO cells. The results showed that the chimeric introns could significantly enhance the expression of NGF in recombinant CHO cells. Moreover, the enhancing effect on NGF expression level by the intron in the forward direction showed stronger than that of the reverse direction both at mRNA and protein level. In conclusion, the chimeric intron could increase NGF expression in stably transfected CHO cells and the effect is associated with the direction of the intron insertion.
Chinese hamster ovary cells, gene expression, intron, nerve growth factor
December 11, 2018;
February 11, 2019
Science and Technology Research Projects of Henan Province (No. 182102311184), National Natural Science Foundation of China (No. 81673337).
Tianyun Wang. Tel: +86-373-3029488; E-mail: wtianyuncn@126.com
河南省科技攻关项目 (No. 182102311184),国家自然科学基金 (No. 81673337) 资助。
10.13345/j.cjb.180516
董卫华, 李翠萍, 杨赟, 等. 不同方向内含子对重组CHO细胞中神经生长因子表达的影响. 生物工程学报, 2019, 35(6): 1071–1078.
Dong WH, Li CP, Yang Y, et al. Increasing transgenic expression in recombinant Chinese hamster ovary cells using introns in different directions. Chin J Biotech, 2019, 35(6): 1071–1078.
(本文责编 郝丽芳)
