紧密连接蛋白claudins应用于肿瘤治疗的进展
2019-07-10陈思远刘雪罗文新
陈思远,刘雪,罗文新
紧密连接蛋白claudins应用于肿瘤治疗的进展
陈思远,刘雪,罗文新
厦门大学 生命科学学院 公共卫生学院 国家传染病诊断试剂与疫苗工程技术研究中心 分子疫苗学和分子诊断学国家重点实验室,福建 厦门 361102
Claudins蛋白家族是组成紧密连接(Tight junctions,TJs) 必不可少的骨架蛋白,在维持上皮和内皮细胞中的细胞极性、细胞间的粘附固定、细胞旁路的离子运输等发挥重要作用。近年来大量的研究结果证明,claudins在许多人类恶性肿瘤中异常表达。因此,claudins也被作为癌症治疗的潜在靶标。文中就claudin蛋白家族在肿瘤中的表达情况及其相关药物的研究进展进行阐述。
claudin,肿瘤,梭菌肠毒素,抗体,靶向治疗
紧密连接 (Tight junctions,TJs)又叫occluding junctions或zonulae occludentes,是细胞黏附结构的重要连接形式之一。紧密连接包括跨膜蛋白occludins和claudins (CLDNs) 以及膜外周蛋白如ZO-1[1-3],其缝合了相邻极化的上皮细胞间或内皮细胞间的缝隙,使之具有屏障功能,同时调控着离子、水、大分子甚至癌细胞通过旁细胞途径的转运[4]。
Claudins的异常表达使得TJs功能受损,屏障功能降低,从而导致组织通透性提高,最终导致包括遗传性、过敏性疾病,各个系统感染性疾病乃至肿瘤等多种疾病的发生[5]。Claudins表达水平的上调或下调对肿瘤的发生发展具有促进或抑制作用,在肿瘤的增殖、侵袭、迁移与转移过程中扮演重要角色,其在肿瘤诊断和治疗中具有潜在价值,可作为诊断标志,又可作为免疫治疗的靶点[6]。本文主要就claudin蛋白家族的结构功能、在恶性肿瘤中的异常表达情况及其作为靶标的肿瘤抗体的研究进展进行了综述,为研究者进一步研究claudins提供参考。
1 Claudin蛋白家族及其结构与功能
Claudin蛋白家族最早在1998年由Furuse Mikio从鸡肝中克隆得到并为之命名[7]。从线虫到人类,claudins的结构十分相似,高度保守。迄今为止,claudins已经发现有27个成员的蛋白家族,其中有24种在哺乳动物中表达,分子量从20–35 kDa不等,此外,人类和黑猩猩缺少CLDN13[4]。根据不同claudins的序列同源性的高低可以将其分为两组,分别是经典型claudins和非经典型claudins (图1)[8]。
Claudins成员之间具有相似的结构(图2),包括胞内一个短的N端(Amino-terminal),四次穿膜形成一大一小的两个胞外loop区,以及一个胞内的C端(Carboxy-terminal)[8]。在较大的胞外loop区上含有带电的氨基酸,可以调节细胞旁路对阴阳离子的选择透过性。在这个loop上,claudins有两个高度保守的半胱氨酸残基(Cysteine)。较小的loop则和其他的细胞膜上的claudins通过芳香族残基的疏水作用形成二聚体。除此之外,CLDN3、4、6、9的小loop是梭菌肠毒素enterotoxin (CPE) 的受体。C端是claudins中差异最大的部分。除了CLDN12之外,其他的claudins成员在C端都有PDZ结构域(Post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1) and Zonula occludens-1 protein (ZO-1)),这一区域使得claudins可以和包括TJs相关的ZO family的适配体在内的细胞质支架蛋白直接互相作用[8]。这一特点使其在细胞信号转导中发挥重要作用,同时也使claudins能与肌动蛋白间接作用,对TJs间的稳定性和选择透过性起重要作用[9]。Claudins在TJs中的正确定位需要PDZ结合元件的上游的C端序列。这一区域含有的氨基酸残基有利于翻译后的各种修饰(图2),影响claudins的定位和功能[3, 10-14]。

图1 Claudin蛋白家族成员
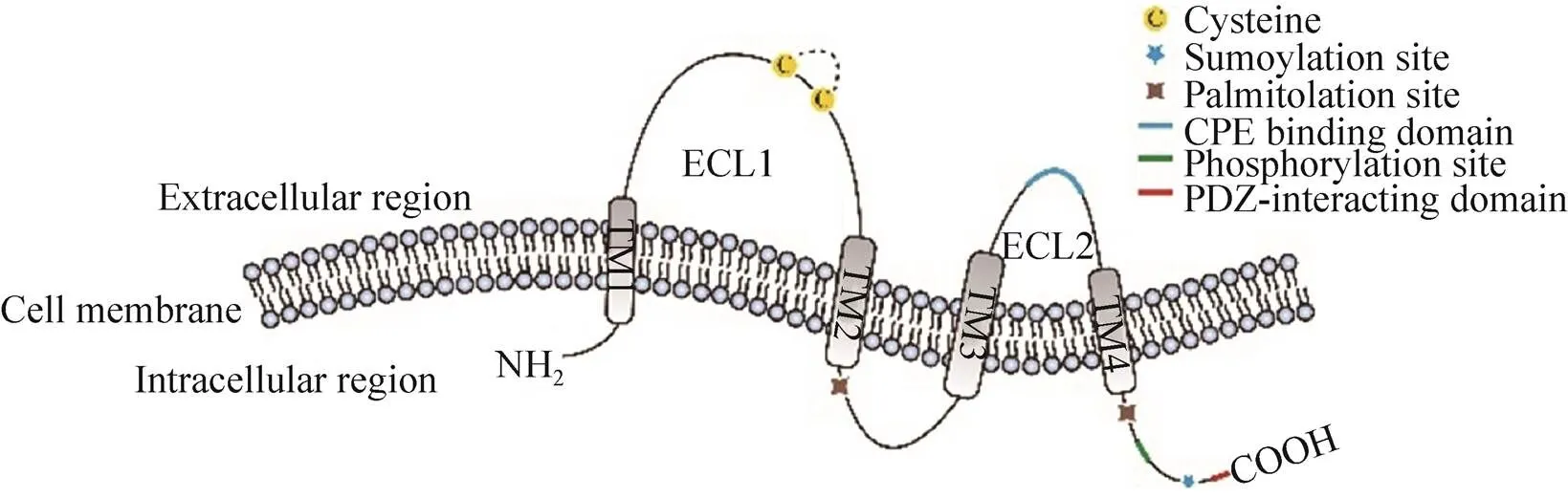
图2 Claudins蛋白结构及关键区段
2 Claudins在肿瘤中的表达及可能的机理
Claudins的异常表达可导致上皮细胞、内皮细胞的结构破坏及功能受损,其在多种上皮来源的肿瘤中异常表达,表明claudins可能在肿瘤的侵袭和转移中发挥着重要的作用。细胞间的吸附消失被认为和肿瘤的侵略性有关,因此有假说认为,在肿瘤代谢中,claudins表达水平的下调加强了肿瘤的转移和入侵。但另一方面,也有研究发现有些claudins的过量表达可能导致在某些肿瘤中蛋白的定位和功能异常,从而促进了肿瘤侵袭和转移能力。因此,在上皮/内皮相关肿瘤发生过程中,很可能看到claudins的异常表达[4]。
如表1所示,一些claudins在癌症中表达下调,这被认为是由于这些claudins在TJs形成和细胞黏附的屏障作用,从而抑制了肿瘤发生和发展。例如,在胸腺癌中,CLDN7在原位和浸润性导管癌的表达降低,并且与肿瘤的组织化程度呈负相关,在高度病变肿瘤中CLDN7的表达明显降低。在siRNA介导敲除CLDN7的TE食管鳞状细胞系中,细胞的生长和转移增加,伴随钙粘蛋白(E-cadherin) 的表达降低。而CLDN7的高表达逆转了这些表型,使得细胞间黏附更强而入侵能力更弱,同时钙粘蛋白表达增加[45]。类似的现象也有在CLDN1有关的一些癌症中发现,在人胃癌细胞中敲除CLDN1促进了肿瘤的发生[46],而在肺癌中CLDN1的过表达抑制肿瘤的分裂以及肿瘤细胞的迁移、入侵和代谢[15]。相关研究认为,一种细胞膜蛋白激酶RON可能在胸腺癌中CLDN1的下调中有重要作用[47]。肿瘤发生过程中上/内皮细胞向间充质细胞转化 (Epithelial/endothelialto mesenchymal transition,EMT) 途径的激活被提出用于解释这些现象。原发性肿瘤细胞的相关的claudins表达受到抑制从而降低,细胞极性被破坏同时细胞黏附性降低,肿瘤侵入性增强,透过基底膜入侵进入血流。接着这些循环的肿瘤细胞退出血液进行间充质细胞向上/内皮细胞转化 (Mesenchymal to epithelial/endothelial transition,MET) 形成微转移[48](图3)。

表1 部分claudins在一些癌症组织中的表达变化
另一方面,也可以在人卵巢癌表面上皮细胞中过量表达CLDN3与CLDN4,使其在转移、入侵和生存能力上都有提升[49]。CLDN4的过表达促进肿瘤细胞的入侵能力,这在肠道癌细胞系Caco-2中也有被发现,而这被认为可能与基质金属蛋白酶 (Matrix metalloproteinase,MMP) 中的MMP-2和MMP-9的激活有关[50],这二者有能力降解基底膜的三重螺旋的Ⅳ和Ⅴ型胶原蛋白,从而导致恶性肿瘤的侵袭和转移[51]。在人肾上皮细胞293T细胞上的一些实验表明包括CLDN1、3、4、5在内的许多种claudins可以介导pro-MMP2的激活,从而促进肿瘤的侵袭和转移。同时另有研究表明胃癌中CLDN4在MMP-9的表达中或许十分重要,并且可能决定了弥漫性胃癌中肠型的发展[52-53]。在肝癌细胞中,CLDN10的表达促进了细胞的存活、运动和入侵,同时MMP-2表达上调[54]。除此之外,也有研究发现,肝转移中的CLDN2表达水平升高可以促进肿瘤细胞/细胞外基质粘附或促进肿瘤细胞与驻留肝细胞之间的相互作用,从而增强乳腺癌细胞的存活[55]。

图3 Claudins有关的肿瘤细胞转移
3 以claudins为肿瘤治疗靶点的相关研究
在许多癌症中claudins的异常表达和其在质膜上的定位,使他们成为癌症治疗的潜在靶标候选。其中,CLDN3、4、6、18.2在许多癌症中表达量都有显著增高,是肿瘤靶向治疗的热门靶标(表2)。人们越来越关注在癌症恶化过程中针对claudins制定策略,有很多种针对肿瘤细胞表达claudins的策略值得期待,包括:1) CPE结合介导肿瘤细胞溶解;2) C-CPE偶联药物递送细胞毒性药物或小分子抑制剂,或作为示踪试剂;3) 以claudins为靶点的抗体或抗体偶联药物 (Antibody-drug conjugates,ADC)作为递送细胞毒性药物或小分子抑制剂的载体,或连接荧光基团作为示踪试剂;4) 以claudins为靶点的嵌合抗原受体T细胞免疫疗法 (Chimeric antigen receptor T-cell immunotherapy,CAR-T) (图4)。
3.1 以claudins为肿瘤治疗靶点的CPE相关研究
CPE是一个35 kDa的蛋白,其C端在与受体claudins第二个loop结合的过程中诱导穿孔的形成,可导致细胞膜的通透和上皮细胞的溶解,继而引导caspase-3途径的细胞凋亡或细胞胀裂[56]。CPE蛋白可以被分为两部分,N端为细胞毒性域,和寡聚化及穿孔形成有关,C端为受体结合域,即C-CPE[56-57](图5)。CPE特异地与游离的CLDN结合而极少与整合入TJs的claudins结合。许多研究发现,CLDN3、4、6、7、9是具有高亲和力CPE/C-CPE受体,CLDN1、2、8、19是低亲和力CPE/C-CPE受体[58-66]。

表2 Claudins相关临床治疗试验进展
fAll the experimental information above was obtained from the ClinicalTrials.gov website and updated until October 10, 2018.
研究显示,CLDN3和CLDN4在几个卵巢癌病例中的表达量是正常上皮细胞的83–109倍[66]。此外,DNA微阵列分析显示,CLDN3和CLDN4是卵巢癌中表达差异最大的5个基因中的2个[67]。CLDN6和CLDN7在卵巢癌中过表达[39, 68],意味着CPE敏感的几个claudins在卵巢癌中的表达往往是增多的[69]。此外,有研究报道化疗耐药或复发性卵巢癌的CLDN3和CLDN4的表达水平高于化疗敏感性卵巢癌[69-70],这为CPE用于卵巢癌的靶向治疗提供了希望。

图4 靶向claudins的治疗策略
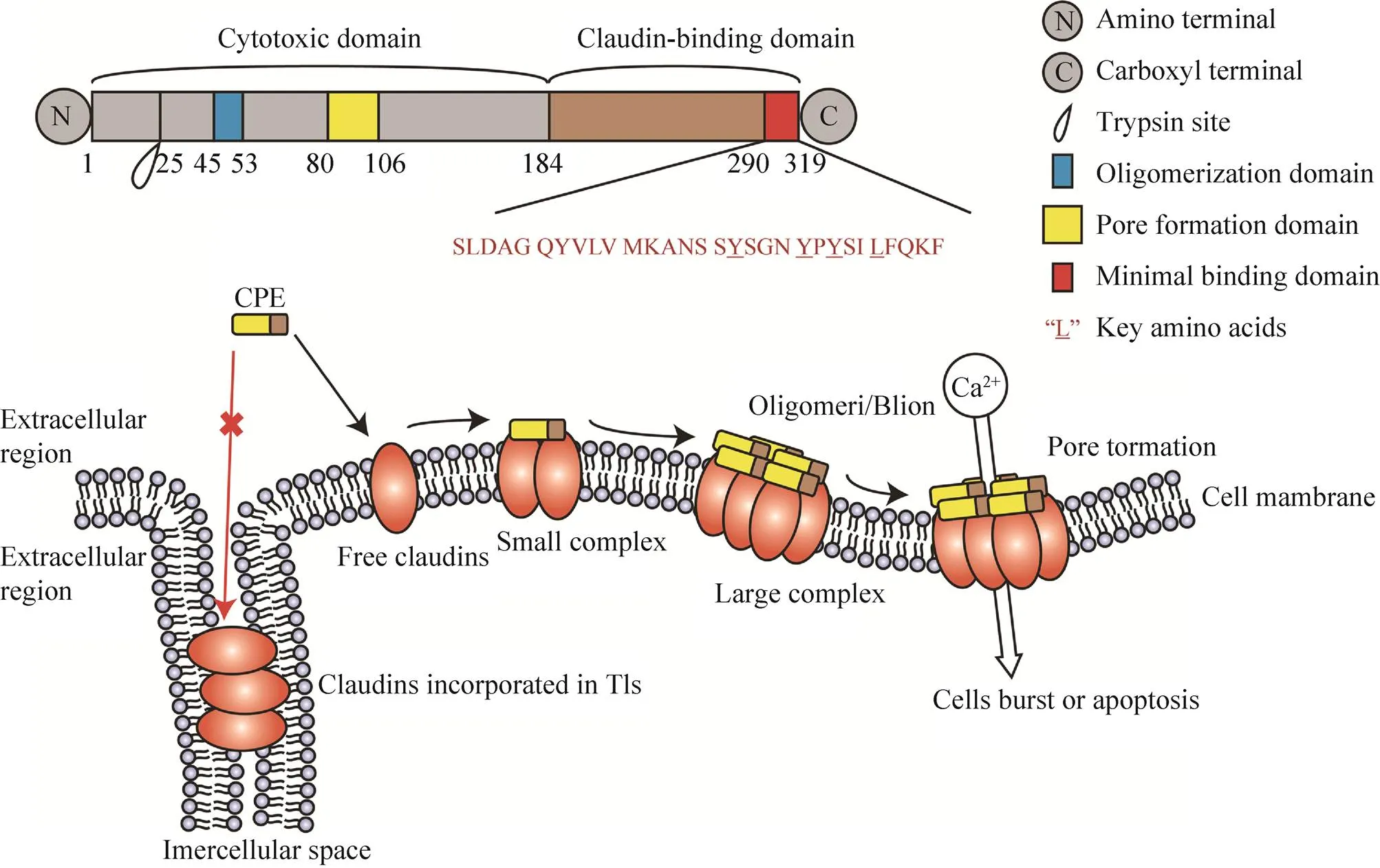
图5 CPE的结构功能区域和细胞裂解作用(改自Hashimoto等[56])
有学者尝试直接使用CPE治疗肿瘤。腹腔注射CPE的抗化疗原发性卵巢癌细胞的小鼠移植模型肿瘤生长得到抑制,且呈剂量依赖,并且并未引发明显的副作用[69, 71]。在胰腺癌细胞Panc-1移植的小鼠体内注射CPE则完全抑制肿瘤生长,导致肿瘤生长明显受抑和坏死,同时未造成任何不良影响[27]。
由于CLDN3/4在肺、胃等正常组织中也有表达,因此也有研究者为了减少CPE潜在的毒副风险,同时也为了使其具有更小的抗原特性,尝试使用C-CPE作为抗肿瘤药物穿透的增强剂并应用于癌症诊断。反复腹腔注射紫杉醇联合C-CPE,在植入皮下肿瘤的小鼠产生了显著的协同抗肿瘤作用,同时发现C-CPE诱导了卵巢癌细胞的形态学改变[72]。对腹腔移植了腹腔转移卵巢癌细胞OSPC-ARK-1的小鼠静脉注射C-CPE联合荧光染料,能够检测到以往视觉观察难以发现的小至1 mm2区域的肿瘤组织[73]。用111共轭谷胱甘肽S-转移酶和C-CPE结合的断层显像研究显示,CLDN4阳性的乳腺癌细胞MDA-MB-468移植小鼠的肿瘤组织中,放射性示踪物的累积与CLDN4阴性移植小鼠对比明显增加[74]。C-CPE联合荧光素在CLDN4阳性的胰腺癌细胞Capan-1小鼠中的累积与CLDN4阴性移植小鼠相比更高[75]。
3.2 以claudins为肿瘤治疗靶点的抗体及CAR-T研究
目前已有许多以claudins为抗原筛选得到的抗体,抗原包含了claudins的短肽、细胞、DNA或是病毒颗粒类似物。另一种靶向肿瘤claudins的治疗策略是特异性抗体的应用,CLDN3、4、6、18.2是该研究中的热门靶标,有许多研究团队已经筛选了特异性针对肿瘤细胞高表达的claudins胞外区的抗体。
如前文所述,CLDN3和CLDN4在许多卵巢癌病例中的表达量异常增高,因此靶向CLDN3/4抑或二者的抗体有作为治疗手段的潜力,一些抗体也被证明在临床前动物模型上有抗肿瘤作用,更是有抗体已经进入了临床阶段(表2)。筛选得到能够识别并结合CLDN4胞外第2个loop的鼠抗KM3900并人源化后得到的抗体KM3934,在体外实验中表现出抗体依赖性的细胞毒性 (Antibody dependent cytotoxicity,ADCC) 和补充依赖性细胞毒性 (Complement dependent cytotoxicity,CDC),并在移植人卵巢癌细胞系MCAS或人胰腺癌细胞系CFPAC-1的免疫缺陷小鼠体内能够显著抑制肿瘤的生长[76]。CLDN6在一定比例的晚期卵巢癌中高水平表达,在正常成人组织中没有发现。IMAB027是一株特异性结合CLDN6的单克隆抗体。临床前实验证明这种抗体有抑制肿瘤生长和杀死癌细胞的ADCC和CDC效应,目前正在进行复发性晚期卵巢癌患者的临床Ⅰ期试验(NCT02054351)。早期的数据表明,IMAB027具有良好的耐受性[77]。CLDN18.2参与肿瘤的发展和进展,暴露在细胞外的loop可用于单克隆抗体结合。这些生物学特性表明它是靶向治疗的理想分子,是目前claudins相关肿瘤临床治疗研究中最热门的靶标。除了胃内层的正常组织中,CLDN18.2在70%–90%的胃、胰、胆管癌中高表达。IMAB362就是一株抗CLDN18.2的单克隆抗体[78],能够诱导ADCC和CDC效应,及介导肿瘤被破坏。来自二期临床研究的发现表明,单纯化疗相比,将实验性抗体IMAB362 (Ganymed药物) 添加到标准化疗中,可以提高以前未经治疗的胃癌患者的整体生存3–5个月,而在CLDN18.2表达超过70%的肿瘤上,甚至能由9个月提高至16.7个月[79]。
如表2所示,目前也有针对CLDN18.2的CAR-T细胞疗法的研究在一期临床招募阶段。CAR-T细胞设计的基本原理涉及结合抗原结合和T细胞激活功能的重组受体,从人身上取出T细胞,通过基因工程修饰,并将它们重新输回患者体内,以便攻击癌细胞。一旦T细胞被设计成CAR-T细胞,它就会成为患者体内的“活药物”[80]。目前CAR-T疗法在急性白血病和非霍奇金淋巴瘤的治疗上显示出良好的潜力并且即将上市,科济(CARsgen) 公司开发的CAR-T疗法主要针对CLDN18.2过表达的晚期胃腺癌、胰腺癌,在上海长海医院(现为海军军区大学第一附属医院)进行临床招募,为针对claudins表达异常的肿瘤治疗提供了新的思路。
4 总结与展望
Claudins在肿瘤代谢过程中的一些具体机理机制和关键步骤仍不清楚,但其作为肿瘤治疗靶标的潜力毋庸置疑。临床前实验和临床研究已充分证实了claudins作为肿瘤治疗靶标及相关应用的可能并且取得了一定进展:CPE及其衍生物用于肿瘤治疗和诊断的临床前研究效果显著;靶向CLDN18.2的抗体IMAB362和靶向CLDN4/6的抗体IMAB027已经进入临床研究阶段,IMAB027更是已获得FDA和欧盟授予的治疗胃癌和胰腺癌的孤儿药资格;科济生物医药(上海) 有限公司所开发的针对CLDN18.2的CAR-T疗法也已进入临床研究阶段,用于癌症的免疫治疗将会有很光明的前景。
即便如此,研究过程中仍存在一些需要解决的问题和可以改进的方面,比如:是否能通过C端的蛋白修饰来调控claudins的功能;如何进一步避免CPE及其衍生物带来的毒副作用;如何确保治疗过程中claudins靶向的特异性。因此,还需更深入研究肿瘤细胞异常表达的claudins在肿瘤代谢中的作用机制,一些假说尚需更多实验去探索,临床取得的成果也需要进一步的验证。综合来看,将claudins作为靶标用于肿瘤治疗的前景光明,给目前的肿瘤治疗带来新的选择。
[1] Markov AG. Claudins as tight junction proteins: the molecular element of paracellular transport. Ross Fiziol Zh Im I M Sechenova, 2013, 99(2): 175–195.
[2] Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol, 2004, 286(6): C1213–C1228.
[3] Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mole Cell Biol, 2001, 2(4): 285–293.
[4] Krause G, Winkler L, Mueller SL, et al. Structure and function of claudins. Biochim Biophys Acta, 2008, 1778(3): 631–645.
[5] Sawada N, Murata M, Kikuchi K, et al. Tight junctions and human diseases. Med Electron Microsc, 2003, 36(3): 147–156.
[6] Tabariès S, Siegel PM. The role of claudins in cancer metastasis. Oncogene, 2017, 36(9): 1176–1190.
[7] Furuse M, Fujita K, Hiiragi T, et al. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol, 1998, 141(7): 1539–1550.
[8] Lal-Nag M, Morin PJ. The claudins. Genome Biol, 2009, 10(8): 235.
[9] Gonzalez JE, Digeronimo RJ, Arthur DE, et al. Remodeling of the tight junction during recovery from exposure to hydrogen peroxide in kidney epithelial cells. Free Radic Biol Med, 2009, 47(11): 1561–1569.
[10] Van Itallie CM, Tietgens AJ, Logrande K, et al. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. J Cell Sci, 2012, 125: 4902–4912.
[11] van Itallie CM, Mitic LL, Anderson JM. SUMOylation of claudin-2. Ann N Y Acad Sci, 2012, 1258: 60–64.
[12] González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta, 2008, 1778(3): 729–756.
[13] van Itallie CM, Gambling TM, Carson JL, et al. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci, 2005, 118: 1427–1436.
[14] Rüffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur J Cell Biol, 2004, 83(4): 135–144.
[15] Chao YC, Pan SH, Yang SC, et al. Claudin-1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med, 2009, 179(2): 123–133.
[16] Nakagawa S, Miyoshi N, Ishii H, et al. Expression of CLDN1 in colorectal cancer: a novel marker for prognosis. Int J Oncol, 2011, 39(4): 791–796.
[17] Szász AM, Nyirády P, Majoros A, et al. β-catenin expression and claudin expression pattern as prognostic factors of prostatic cancer progression. BJU Int, 2010, 105(5): 716–722.
[18] Sheehan GM, Kallakury BVS, Sheehan CE, et al. Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas. Human Pathol, 2007, 38(4): 564–569.
[19] Huang J, Li JF, Qu Y, et al. The expression of claudin 1 correlates with β-catenin and is a prognostic factor of poor outcome in gastric cancer. Int J Oncol, 2014, 44(4): 1293–1301.
[20] Kleinberg L, Holth A, Trope CG, et al. Claudin upregulation in ovarian carcinoma effusions is associated with poor survival. Human Pathol, 2008, 39(5): 747–757.
[21] Choi YL, Kim J, Kwon MJ, et al. Expression profile of tight junction protein claudin 3 and claudin 4 in ovarian serous adenocarcinoma with prognostic correlation. Histol Histopathol, 2007, 22(11): 1185–1195.
[22] Rangel LB, Agarwal R, D’souza T, et al. Tight junction proteins claudin-3 and claudin-4 are frequently overexpressed in ovarian cancer but not in ovarian cystadenomas. Clin Cancer Res, 2003, 9(7): 2567–2575.
[23] Szasz AM, Tokes AM, Micsinai M, et al. Prognostic significance of claudin expression changes in breast cancer with regional lymph node metastasis. Clin Exp Metastas, 2011, 28(1): 55–63.
[24] Missiaglia E, Blaveri E, Terris B, et al. Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int J Cancer, 2004, 112(1): 100–112.
[25] Liu JX, Wei ZY, Chen JS, et al. Prognostic and clinical significance of claudin-4 in gastric cancer: a meta-analysis. World J Surg Oncol, 2015, 13: 207.
[26] Ohtani S, Terashima M, Satoh J, et al. Expression of tight-junction-associated proteins in human gastric cancer: downregulation of claudin-4 correlates with tumor aggressiveness and survival. Gastr Cancer, 2009, 12(1): 43–51.
[27] Michl P, Buchholz M, Rolke M, et al. Claudin-4: a new target for pancreatic cancer treatment usingenterotoxin. Gastroenterology, 2001, 121(3): 678–684.
[28] Jung JH, Jung CK, Choi HJ, et al. Diagnostic utility of expression of claudins in non-small cell lung cancer: different expression profiles in squamous cell carcinomas and adenocarcinomas. Pathol Res Pract, 2009, 205(6): 409–416.
[29] Zhu J, Wang R, Cao H, et al. Expression of claudin-5, -7, -8 and -9 in cervical carcinoma tissues and adjacent non-neoplastic tissues. Int J Clin Exp Pathol, 2015, 8(8): 9479–9486.
[30] Lal-Nag M, Battis M, Santin AD, et al. Claudin-6: a novel receptor for CPE-mediated cytotoxicity in ovarian cancer. Oncogenesis, 2012, 1(11): e33.
[31] Wang Q, Zhang Y, Zhang T, et al. Low claudin-6 expression correlates with poor prognosis in patients with non-small cell lung cancer. OncoTargets Ther, 2015, 8: 1971–1977.
[32] Yamamoto T, Oshima T, Yoshihara K, et al. Reduced expression of claudin-7 is associated with poor outcome in non-small cell lung cancer. Oncol Lett, 2010, 1(3): 501–505.
[33] Kominsky SL, Argani P, Korz D, et al. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinomaand invasive ductal carcinoma of the breast. Oncogene, 2003, 22(13): 2021–2033.
[34] Németh Z, Szász AM, Tátrai P, et al. Claudin-1, -2, -3, -4, -7, -8, and -10 protein expression in biliary tract cancers. J Histochem Cytochem, 2009, 57(2): 113–121.
[35] Grone J, Weber B, Staub E, et al. Differential expression of genes encoding tight junction proteins in colorectal cancer: frequent dysregulation of claudin-1, -8 and -12. Int J Colorect Dis, 2007, 22(6): 651–659.
[36] Huang GW, Ding X, Chen SL, et al. Expression of claudin 10 protein in hepatocellular carcinoma: impact on survival. J Cancer Res Clin Oncol, 2011, 137(8): 1213–1218.
[37] Agarwal R, Mori Y, Cheng Y, et al. Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS ONE, 2009, 4(11): e8002.
[38] Morita K, Morita NI, Nemoto K, et al. Expression of claudin in melanoma cells. J Dermatol, 2008, 35(1): 36–38.
[39] Davidson B, Zhang Z, Kleinberg L, et al. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from diffuse malignant peritoneal mesothelioma. Clin Cancer Res, 2006, 12: 5944–5950.
[40] Martin TA, Harrison GM, Watkins G, et al. Claudin-16 reduces the aggressive behavior of human breast cancer cells. J Cell Biochem, 2008, 105(1): 41–52.
[41] Singh P, Toom S, Huang YW. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol, 2017, 10: 105.
[42] Wöll S, Schlitter AM, Dhaene K, et al. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer, 2014, 134(3): 731–739.
[43] Martin TA, Lane J, Ozupek H, et al. Claudin-20 promotes an aggressive phenotype in human breast cancer cells. Tissue Barr, 2013, 1(3): e26518.
[44] Pitule P, Vycital O, Bruha J, et al. Differential expression and prognostic role of selected genes in colorectal cancer patients. Anticancer Rese, 2013, 33(11): 4855–4865.
[45] Lioni M, Brafford P, Andl C, et al. Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am J Pathol, 2007, 170(2): 709–721.
[46] Chang TL, Ito K, Ko TK, et al. Claudin-1 has tumor suppressive activity and is a direct target of RUNX3 in gastric epithelial cells. Gastroenterology, 2010, 138(1): 255–265.e3.
[47] Wu CM, Lee YS, Wang TH, et al. Identification of differential gene expression between intestinal and diffuse gastric cancer using cDNA microarray. Oncol Rep, 2006, 15(1): 57–64.
[48] Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science, 2011, 331(6024): 1559–1564.
[49] Agarwal R, D’souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res, 2005, 65(16): 7378–7385.
[50] Takehara M, Nishimura T, Mima S, et al. Effect of claudin expression on paracellular permeability, migration and invasion of colonic cancer cells. Biol Pharm Bull, 2009, 32(5): 825–831.
[51] Vihinen P, Kähäri VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer, 2002, 99(2): 157–166.
[52] Lee LY, Wu CM, Wang CC, et al. Expression of matrix metalloproteinases MMP-2 and MMP-9 in gastric cancer and their relation to claudin-4 expression. Histol Histopathol, 2008, 23(5): 515–521.
[53] Miyamori H, Takino T, Kobayashi Y, et al. Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J Biol Chem, 2001, 276(30): 28204–28211.
[54] Ip YC, Cheung ST, Lee YT, et al. Inhibition of hepatocellular carcinoma invasion by suppression of claudin-10 in HLE cells. Mol Cancer Ther, 2007, 6(11): 2858–2867.
[55] Kimbung S, Kovács A, Bendahl PO, et al. Claudin-2 is an independent negative prognostic factor in breast cancer and specifically predicts early liver recurrences. Mol Oncol, 2014, 8(1): 119–128.
[56] Hashimoto Y, Yagi K, Kondoh M. Roles of the first-generation claudin binder,enterotoxin, in the diagnosis and claudin-targeted treatment of epithelium-derived cancers. Pflugers Arch, 2017, 469(1): 45–53.
[57] Hardy SP, Denmead M, Parekh N, et al. Cationic currents induced bytype A enterotoxin in human intestinal CaCO-2 cells. J Med Microbiol, 1999, 48(3): 235–243.
[58] Saitoh Y, Suzuki H, Tani K, et al. Tight junctions. Structural insight into tight junction disassembly byenterotoxin. Science, 2015, 347(6223): 775–778.
[59] Protze J, Eichner M, Piontek A, et al. Directed structural modification ofenterotoxin to enhance binding to claudin-5. Cell Mol Life Sci, 2015, 72(7): 1417–1432.
[60] Shrestha A, Mcclane BA. Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects ofenterotoxin. mBio, 2013, 4(1): e00594-12.
[61] Robertson SL, Smedley III JG, Mcclane BA. Identification of a claudin-4 residue important for mediating the host cell binding and action ofenterotoxin. Infect Imm, 2010, 78(1): 505–517.
[62] Kimura J, Abe H, Kamitani S, et al.enterotoxin interacts with claudins via electrostatic attraction. J Biol Chem, 2010, 285(1): 401–408.
[63] Winkler L, Gehring C, Wenzel A, et al. Molecular determinants of the interaction betweenenterotoxin fragments and claudin-3. J Biol Chem, 2009, 284(28): 18863–18872.
[64] Fujita K, Katahira J, Horiguchi Y, et al.enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett, 2000, 476(3): 258–261.
[65] Sonoda N, Furuse M, Sasaki H, et al.enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol, 1999, 147(1): 195–204.
[66] Hough CD, Sherman-Baust CA, Pizer ES, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res, 2000, 60(22): 6281–6287.
[67] Santin AD, Zhan FH, Bellone S, et al. Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer, 2004, 112(1): 14–25.
[68] Tassi RA, Bignotti E, Falchetti M, et al. Claudin-7 expression in human epithelial ovarian cancer. Int J Gynecol Cancer, 2008, 18(6): 1262–1271.
[69] Santin AD, Cané S, Bellone S, et al. Treatment of chemotherapy-resistant human ovarian cancer xenografts in C. B-17/SCID mice by intraperitoneal administration ofenterotoxin. Cancer Res, 2005, 65(10): 4334–4342.
[70] Yoshida H, Sumi T, Zhi X, et al. Claudin-4: a potential therapeutic target in chemotherapy-resistant ovarian cancer. Anticancer Res, 2011, 31(4): 1271–1277.
[71] Casagrande F, Cocco E, Bellone S, et al. Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration ofenterotoxin. Cancer, 2011, 117(24): 5519–5528.
[72] Gao ZJ, Xu XY, Mcclane B, et al. C terminus ofenterotoxin downregulates CLDN4 and sensitizes ovarian cancer cells to taxol and carboplatin. Clin Cancer Res, 2011, 17(5): 1065–1074.
[73] Cocco E, Shapiro EM, Gasparrini S, et al.enterotoxin C-terminal domain labeled to fluorescent dyes forvisualization of micrometastatic chemotherapy-resistant ovarian cancer. Int J Cancer, 2015, 137(11): 2618–2629.
[74] Mosley M, Knight J, Neesse A, et al. Claudin-4 SPECT imaging allows detection of aplastic lesions in a mouse model of breast cancer. J Nucl Med, 2015, 56(5): 745–751.
[75] Neesse A, Hahnenkamp A, Griesmann H, et al. Claudin-4-targeted optical imaging detects pancreatic cancer and its precursor lesions. Gut, 2013, 62(7): 1034–1043.
[76] Suzuki M, Kato-Nakano M, Kawamoto S, et al. Therapeutic antitumor efficacy of monoclonal antibody against claudin-4 for pancreatic and ovarian cancers. Cancer Sci, 2009, 100(9): 1623–1630.
[77] Sahin U, Jaeger D, Marme F, et al. First-in-human phase I/II dose-escalation study of IMAB027 in patients with recurrent advanced ovarian cancer (OVAR): Preliminary data of phase I part. Evolution, 2015, 67(12): 3442–3454.
[78] Singh P, Toom S, Huang YW. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol, 2017, 10: 105.
[79] Antibody improves survival in gastric cancer. Cancer Discov, 2016, 6(8): OF8.
[80] Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov, 2013, 3(4): 388–398.
Advances in the application of claudins to tumor therapy
Siyuan Chen, Xue Liu, and Wenxin Luo
State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, School of Life Sciences, Xiamen University, Xiamen 361102, Fujian, China
Claudin proteins are the most crucial components of tight junctions, and play an essential role in maintaining cell polarity, regulating cell permeability and the intercellular ion. In recent years, many studies have shown that abnormality of claudins expression is implicated in the tumor progression. The expression correlates with tumor prognosis and can serve as a biomarker of prognosis and potential therapeutic targets. This review summarizes the current knowledge regarding claudin dysregulation in cancer and highlights the progress in claudin-based treatments.
claudin, tumor,enterotoxin, antibody, targeted therapy
October 25, 2018;
December 28, 2018
National Natural Science Foundation of China (No. 31870925), Major Projects of Infectious Diseases (No. 2017ZX10202203-001).
Wenxin Luo. Tel: +86-592-2188657; Fax: +86-592-2181258; E-mail: wxluo@xmu.edu.cn
国家自然科学基金 (No. 31870925),传染病重大专项 (No. 2017ZX10202203-001) 资助。
10.13345/j.cjb.180435
陈思远, 刘雪, 罗文新. 紧密连接蛋白claudins应用于肿瘤治疗的进展. 生物工程学报, 2019, 35(6): 931–941.
Chen SY, Liu X, Luo WX. Advances in the application of claudins to tumor therapy. Chin J Biotech, 2019, 35(6): 931–941.
(本文责编 陈宏宇)
