Novel risk scoring system for prediction of pancreatic fistula after pancreaticoduodenectomy
2019-06-13YeLiFangZhouDongMingZhuZiXiangZhangJianYangJunYaoYiJunWeiYaLingXuDeiChunLiJianZhou
Ye Li, Fang Zhou, Dong-Ming Zhu, Zi-Xiang Zhang, Jian Yang, Jun Yao, Yi-Jun Wei, Ya-Ling Xu, Dei-Chun Li,Jian Zhou
AbstractBACKGROUND The available prediction models for clinically relevant postoperative pancreatic fistula (CR-POPF) do not incorporate both preoperative and intraoperative variables.AIM To construct a new risk scoring system for CR-POPF that includes both preoperative and intraoperative factors.METHODS This was a retrospective study of patients who underwent pancreaticoduodenectomy (PD) or pylorus-preserving PD (PPPD) between January 2011 and December 2016 at the First Affiliated Hospital of Soochow University. Patients were divided into a study (01/2011 to 12/2014) or validation(01/2015 to 12/2016) group according to the time of admission. POPF severity was classified into three grades: Biochemical leak (grade A) and CR-POPF(grades B and C). Logistic regression was used to create a predictive scoring system.RESULTS Preoperative serum albumin ≥ 35 g/L [P = 0.032, odds ratio (OR) = 0.92, 95%confidence interval (CI): 0.85-0.99], hard pancreatic texture (P = 0.004, OR = 0.25,95%CI: 0.10-0.64), pancreatic duct diameter ≥ 3 mm (P = 0.029, OR = 0.50, 95%CI:0.27-0.93), and intraoperative blood loss ≥ 500 mL (P = 0.006, OR = 1.002, 95%CI:1.001-1.003) were independently associated with CR-POPF. We established a 10-point risk scoring system to predict CR-POPF. The area under the curve was 0.821 (95%CI: 0.736-0.905) and the cut-off value was 3.5. Including drain amylase levels improved the predictive power of the model.CONCLUSIONThis study established a 10-point scoring system to predict CR-POPF after PD/PPPD using preoperative and intraoperative parameters. Ultimately, this system could be used to distinguish between high- and low-risk populations in order to facilitate timely interventions after PD.
Key words: Postoperative; Pancreatic fistula; Pancreaticoduodenectomy; Risk factor;Predictive model; Complications; Scoring system
INTRODUCTION
Despite recent advances in surgical technique and perioperative management, the morbidity rate after pancreaticoduodenectomy (PD) remains high at 30%-65%[1,2].Postoperative pancreatic fistula (POPF) is one of the most common complications after PD and is the most prevalent cause of intra-abdominal abscesses or hemorrhages in these patients[3-5]. According to the 2016 edition of the International Study Group of Pancreatic Surgery (ISGPS), POPF occurs in 15%-45% of patients who undergo PD,and is subsequently associated with a mortality rate of up to 9%[3]. POPF can be classified by severity according to the 2016 ISGPS guidelines. The original grade A, or biochemical leak (BL), has no clinical impact. On the other hand, clinically relevant POPF (CR-POPF) has serious consequences and the clinical management strategy has changed from a passive “wait and see” approach to a proactive strategy that requires early prediction and timely intervention[6-9].
Many risk factors have been independently associated with POPF, including age,body mass index (BMI), preoperative serum total bilirubin (STB), operative time,operative blood loss, pancreatic duct diameter, and pancreatic texture[10-12].Nevertheless, accurate predictions of CR-POPF require the successful integration of preoperative, intraoperative, and postoperative variables[13]. Currently, POPF prediction tools incorporate only preoperative or intraoperative variables[7,11,13-16].
Therefore, the aim of the present study was to construct a new risk scoring system for CR-POPF that included both preoperative and intraoperative variables. We hypothesized that this risk scoring system would effectively predict CR-POPF. A complementary measure was also determined using the level of postoperative drain amylase to enhance the predictive power of this scoring system.
MATERIALS AND METHODS
Patients
This was a retrospective study of patients who underwent PD or pylorus-preserving PD (PPPD) between January 2011 and December 2016 at the First Affiliated Hospital of Soochow University. The study was approved by the ethics committee of the First Affiliated Hospital of Soochow University. The need for individual consent was waived by the committee because of the retrospective nature of the study.
The inclusion criteria were: (1) Patients who met the indications for PD or PPPD; (2)No history of radiotherapy or chemotherapy; and (3) No distant metastasis at the time of diagnosis. The exclusion criteria were: (1) Total pancreatectomy; (2) Emergency operation for trauma; or (3) Incomplete medical records.
The patients were divided into a study or a validation group according to the time of admission. Patients admitted from January 2011 to December 2014 were included in the study group and used to evaluate the risk factors for POPF and to develop the risk scoring system. Patients admitted from January 2015 to December 2016 were included in the validation group and used to validate this system.
Data collection
Variables that might be associated with POPF were analyzed. Preoperative variables included patient characteristics and the results of laboratory tests (STB, pre-albumin,serum albumin, serum globulin, hemoglobin, white blood cells, and platelets).Intraoperative variables included pancreas texture, main pancreatic duct diameter,pancreatojejunostomy anastomosis, pancreatic duct stent, vascular invasion, operative time, and estimated blood loss. Postoperative variables included serum albumin,serum globulin, hemoglobin, and drain amylase level.
Surgical procedures and perioperative management
The operation was performed by four pancreatic surgeons. The main surgeon and the first assistant were two senior surgeons with > 20 years of surgical experience.Resection and reconstruction of the mesenteric or portal vein were performed when the tumor was located close to the portal vein and locally invaded the portal vein or superior mesenteric vein. Pancreatic anastomosis was performed using the duct-tomucosa method or by the dunking method when the pancreatic duct could not be found or had a diameter < 3 mm.
Pancreaticojejunostomy was performed using the duct-to-mucosa method and by the dunking method. For the duct-to-mucosa method, the antimesenteric seromuscular layer at the site on the jejunum loop was selected for the anastomosis and incised to expose an area identical to the section of the pancreas. A small hole was made at the middle of the jejunum mucosa for the anastomosis with the pancreatic duct. Then, a 5-0 absorbable thread was used for the interrupted suturing of the pancreatic parenchyma at the section to the posterior wall of the seromuscular layer of the jejunum. Interrupted suturing of the pancreatic duct and the posterior wall of the jejunal mucosa was conducted using 2-4 stitches. The pancreatic duct supporter was implanted (or not in some cases), and interrupted suturing of the pancreatic duct and the anterior wall of the jejunal mucosa was conducted using 2-4 stitches. Interrupted suturing of the pancreatic parenchyma at the section and seromuscular layer of jejunum was conducted. Finally, strengthening suturing using one stitch each at the upper and lower ends of the pancreaticojejunostomy was conducted.
For the dunking method, end-to-side anastomosis was performed. The antimesenteric seromuscular layer at the site on the jejunum loop was selected for the anastomosis and incised to expose an area identical to the section of the pancreas. An atraumatic thread (4-0) was used for the continuous suturing of the pancreatic parenchyma at the section and the posterior wall of the full layer of the jejunum.Afterwards, a 4-0 atraumatic thread was used for the continuous suturing of the pancreatic parenchyma at the section and the anterior wall of the full layer of the jejunum. Then strengthening suturing for one stitch each at the upper and lower end of the pancreaticojejunostomy was conducted.
Two soft drainage tubes were placed near the pancreatic anastomosis. After operation, an antibiotic (third generation cephalosporin, 3 g bid for 5 d) and a H2blocker (Losec, 40 mg qd for 5 d) were used routinely through postoperative day(POD) 5.
Laboratory tests and the levels of drain amylase were routinely monitored at POD 1, 3, and 5. Drainage tubes were removed at POD 5 in patients without POPF. An abdominal computerized tomography scan was performed at POD 7 and the replacement of tubes was performed as necessary when encapsulated effusion was found.
Regarding prophylactic jaundice reduction in the patients with jaundice, the practice at our center is that preoperative jaundice reduction should only be conducted for the patients with total bilirubin > 400 μmol/L. Percutaneous transhepaticcholangial drainage (PTCD) and plastic stents were the preferred choice for the prophylactic jaundice reduction, which could reduce the inflammation in the surgical area. Implantation of a metal stent under PTCD or ERCP was conducted for jaundice reduction only for the jaundice patients with local progression and in whom the lesions could not be resected, or those who refuse radical operations.
Definition of POPF
POPF was defined according to the 2016 ISGPS guidelines as a drain output of any measurable volume of fluid with amylase levels greater than three times the upper institutional limit of normal for serum amylase, and associated with clinically relevant development. POPF was classified into three grades: A-C. BL implied that there was no deviation in the normal postoperative procedure and no effect on the postoperative duration of stay. Grade B POPF required a change in the management of the expected postoperative pathway, including persistent drainage > 3 wk,percutaneous or endoscopic drainage, and angiographic procedure for bleeding.Grade C POPF led to organ failure, a secondary operation, or subsequent POPFrelated mortality. Grades B and C were defined as CR-POPF.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 for Windows (IBM, Armonk, NY,United States). Categorical variables are described using frequencies and percentages,and were analyzed using the Fisher exact test. Continuous variables are reported as the mean ± standard deviation, and were analyzed using the independent samples ttest. P values < 0.05 were considered statistically significant. Variables with a significant difference in univariate analyses were entered in a multivariate logistic regression model to determine the independent risk factors for POPF. A predictive scoring system was developed using each independent risk factor, based on the regression coefficient of the logistic regression model. The performance of the predictive scoring system was evaluated using the receiver operating characteristic(ROC) curve and the corresponding area under the curve (AUC). The Youden index(sensitivity + specificity - 1) was used to determine the optimal cut-off value to divide the risk strata.
RESULTS
Characteristics of the patients
A total of 298 consecutive patients were included in this study. Their mean age was 62.1 ± 10.1 years (range, 19-86 years) and the male-to-female ratio was 176:122. Of these patients, 158 (53.0%) underwent classic PD and 140 (47%) underwent PPPD. The baseline characteristics and intraoperative status of the patients are shown in Tables 1 and 2. According to the 2016 ISGPF classification, the study group included 34 (18.0%)BL cases, 32 (17.0%) grade B POPF, six (3.2%) grade C POPF, and 117 (61.9%) without POPF. In the validation group, 18 (16.5%) patients were BL cases, 20 (18.3%) were grade B POPF, three (2.8%) were grade CPOPF, and 68 (62.4%) had no POPF. Six patients died in hospital, four in the study group and two in the validation group.Hemorrhage after POPF was the primary cause of death. There were no significant differences in baseline and clinical data between the study and validation groups (P >0.05 for all) (Table 1).
The baseline and clinical data of the CR-POPF and the non-CR-POPF groups are shown in Table 2. There were no significant differences in baseline and clinical data between the two groups (P > 0.05 for all), except that the non-CR-POPF group had higher preoperative albumin levels (P = 0.002), higher frequency of hard pancreas (P <0.001), larger main pancreatic duct (P = 0.001), and less blood loss (P = 0.001).
Preoperative and intraoperative risk factors for CR-POPF
The univariate and multivariate analyses identified risk factors that could predict CRPOPF (Table 3). The univariate analyses revealed that preoperative serum albumin (P< 0.001), pancreatic texture (P < 0.001), pancreatic duct diameter (P < 0.001), and estimated blood loss (P = 0.001) successfully predicted CR-POPF. The forest plot of the odds ratio (OR) of predictive factors for CR-POPF is shown in Figure 1. Preoperative serum albumin ≥ 35 g/L, hard pancreatic texture, and pancreatic duct diameter ≥ 3 mm were associated with lower CR-POPF occurrence. Estimated blood loss ≥ 500 mL was the primary risk factor for CR-POPF, based on ROC analysis. The multivariate analysis revealed that preoperative serum albumin ≥ 35 g/L [P = 0.032,OR = 0.92, 95% confidence interval (CI): 0.85-0.99], hard pancreatic texture (P = 0.004,OR = 0.25, 95%CI: 0.10-0.64), pancreatic duct diameter ≥ 3 mm (P = 0.029, OR = 0.50,95%CI: 0.27-0.93), and estimated blood loss ≥ 500 mL (P = 0.006, OR = 1.002, 95%CI:1.001-1.003) were independently associated with CR-POPF.
Risk scoring system for CR-POPF
Based on the regression coefficient of the logistic regression model, a predictive modelwas established to assess the risk of CR-POPF. The risk rate was calculate using the formula Y = ex/(1+ex), where X = 4.881-0.08*ALB (g/L)-1.375*(Texture: soft, 1; hard,2)-0.7*Diameter (mm)+2*EBL/1000 (mL). The ROC curve of this predictive model showed that the AUC was 0.808 (95%CI: 0.724-0.893) and the cut-off value was 0.252,with a sensitivity and specificity of 76.3% and 80.8%, respectively (Figure 2A).
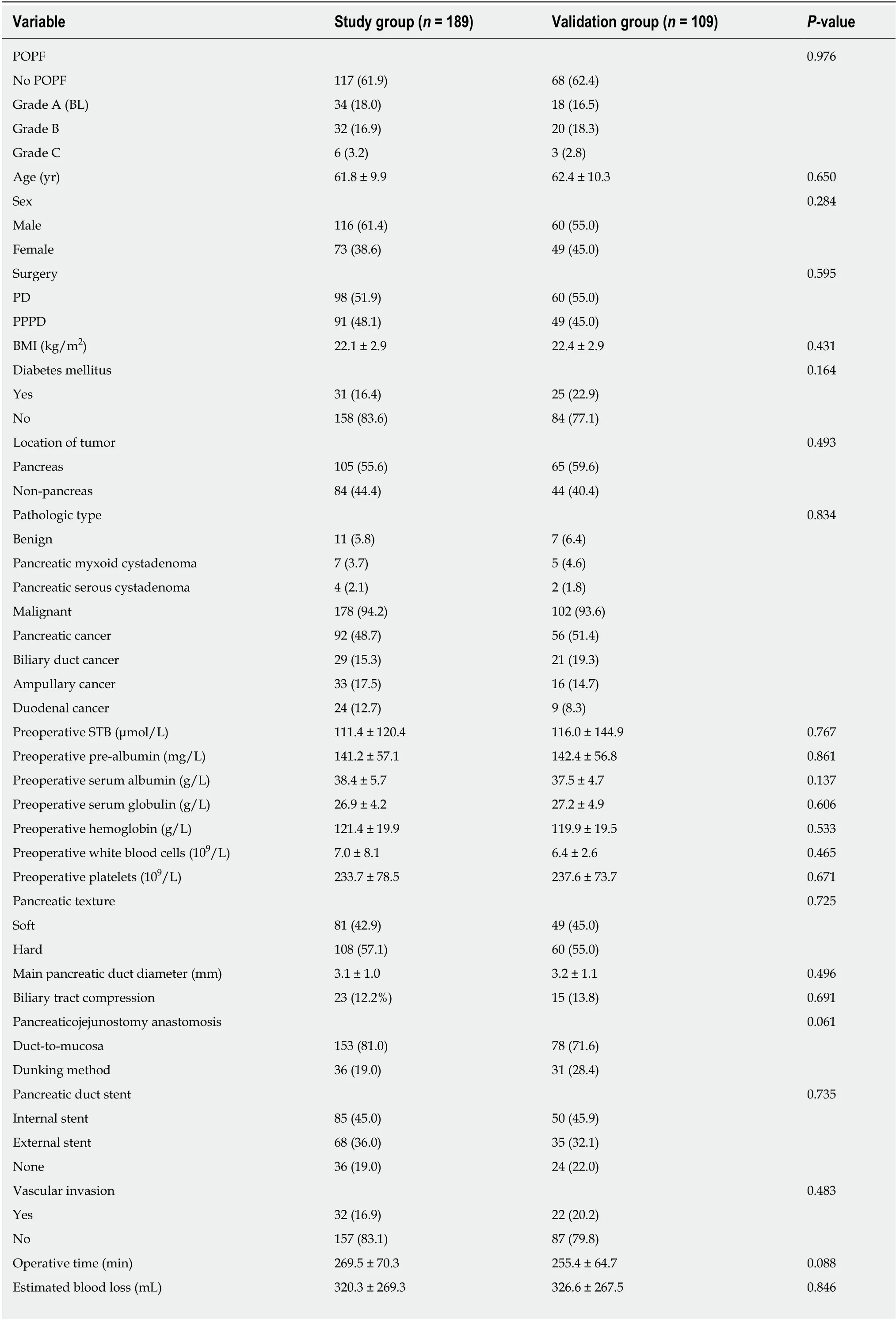
Table 1 Demographic and clinical characteristics of the study and validation groups n (%)

POPF: Postoperative pancreatic fistula; BL: Biochemical leak; BMI: Body mass index; PD: Pan-creaticoduodenectomy; PPPD: Pylorus-preserving pancreaticoduodenectomy; POD: Postoperative day; STB: Serum total bilirubin.
For simplicity, we developed the risk scoring system by adding up the scores for each of the four identified risk factors (Table 4). The possible scores ranged from 0 to 10. The AUC of this risk scoring system was 0.821 (95%CI: 0.736-0.905) (Figure 2B).The optimal cut-off value was a score of 4 points, with a sensitivity of 81.5% and specificity of 76.1%. Then, we tested this risk scoring system in the validation group of 109 patients. The AUC was 0.835 (95%CI: 0.736-0.936) (Figure 2C), and using a score of 4 points as the cut-off value, the sensitivity of this risk scoring system was 78.3% and the specificity was 82.6%. The AUC of this risk scoring system was 0.824 (95%CI:0.759-0.889) in all 298 patients (Figure 2D). As shown in Table 5, in the study group,the risk scoring system successfully distinguished between the low-risk patient group represented by a 5.7% (7/122) CR-POPF rate and the high-risk group with a corresponding 46.3% (31/67) CR-POPF rate. The risk scoring system also correctly distinguished between the low- and high-risk patient populations for the validation and overall patient groups.
Elevated serum total bilirubin, pancreatic duct stent, and drain amylase levels
The univariate and multivariate analyses revealed that STB was not a risk factor for CR-POPF in all groups. Using our risk scoring system, 33 of the 48 patients with elevated STB were classified as high-risk CR-POPF (P < 0.05)(Supplementary Table 1). Similarly, in patients with normal STB, 14 of 20 patients with CR-POPF were predicted as high-risk (P < 0.05) (Supplementary Table 1). The analyses showed that having a pancreatic duct stent did not protect against CR-POPF(OR = 1.71). Nevertheless, subgroup analysis revealed that there were 36 patients with grade B/C POPF in the internal stent group and 15 patients in the external stent group (Supplementary Table 2). Specifically, significantly fewer patients (P = 0.024) in the external stent group had CR-POPF relative to the internal stent group.
Twelve patients classified as low-risk developed CR-POPF (seven in the study group and five in the validation group). Therefore, to refine the model, drain amylase levels were investigated (Table 6). On POD 1, there was no significant difference in the drain amylase levels between grade B/C and none/BL POPF. On POD 3 and 5,the drain amylase levels were significantly higher for patients with grade B/C POPF relative to those with none/BL POPF. The ROC curves of drain amylase levels on POD 3 and 5 are shown in Figure 3A. The AUC were 0.859 and 0.748 for POD 3 and 5,respectively. Based on these results, the value of drain amylase levels on POD 3 was the best indicator for the low-risk group to identify patients with potential CR-POPF.The optimal cut-off value for drain amylase level on POD 3 was 4021.5 U/L, which corresponded to a sensitivity and specificity 77.8% and 95.4%, respectively.
In the high-risk group, there was no significant difference in the drain amylase levels between grade B/C POPF and none/BL POPF on POD 3 and 5. On POD 1, the mean drain amylase levels were significantly higher for patients with grade B/C POPF relative to none/BL POPF (P < 0.001). These results indicate that for the highrisk CR-POPF group, the drain amylase levels on POD 1 can further distinguish patients with a lower risk of B/C grade POPF. The ROC curve of drain amylase level on POD 1 is shown in Figure 3B. The AUC was 0.850 and the optimal cut-off value for drain amylase level was 921.7 U/L, which corresponded to a sensitivity and specificity of 78.9% and 82.8%, respectively.
DISCUSSION
CR-POPF is the most serious complication of PD and can induce intra-abdominal infection, hemorrhage, and even death without early and proper treatment[8,17]. The early identification of patients with a high risk of CR-POPF or potential development is critical to improving perioperative management. In the present study, we established a scoring system based on preoperative and intraoperative parameters to predict the risk of CR-POPF after PD. Additionally, postoperative drain amylase was verified as an efficient postoperative supplement to the scoring system. The risk scoring system incorporated multiple independent risk factors and satisfactorily predicted CR-POPF.
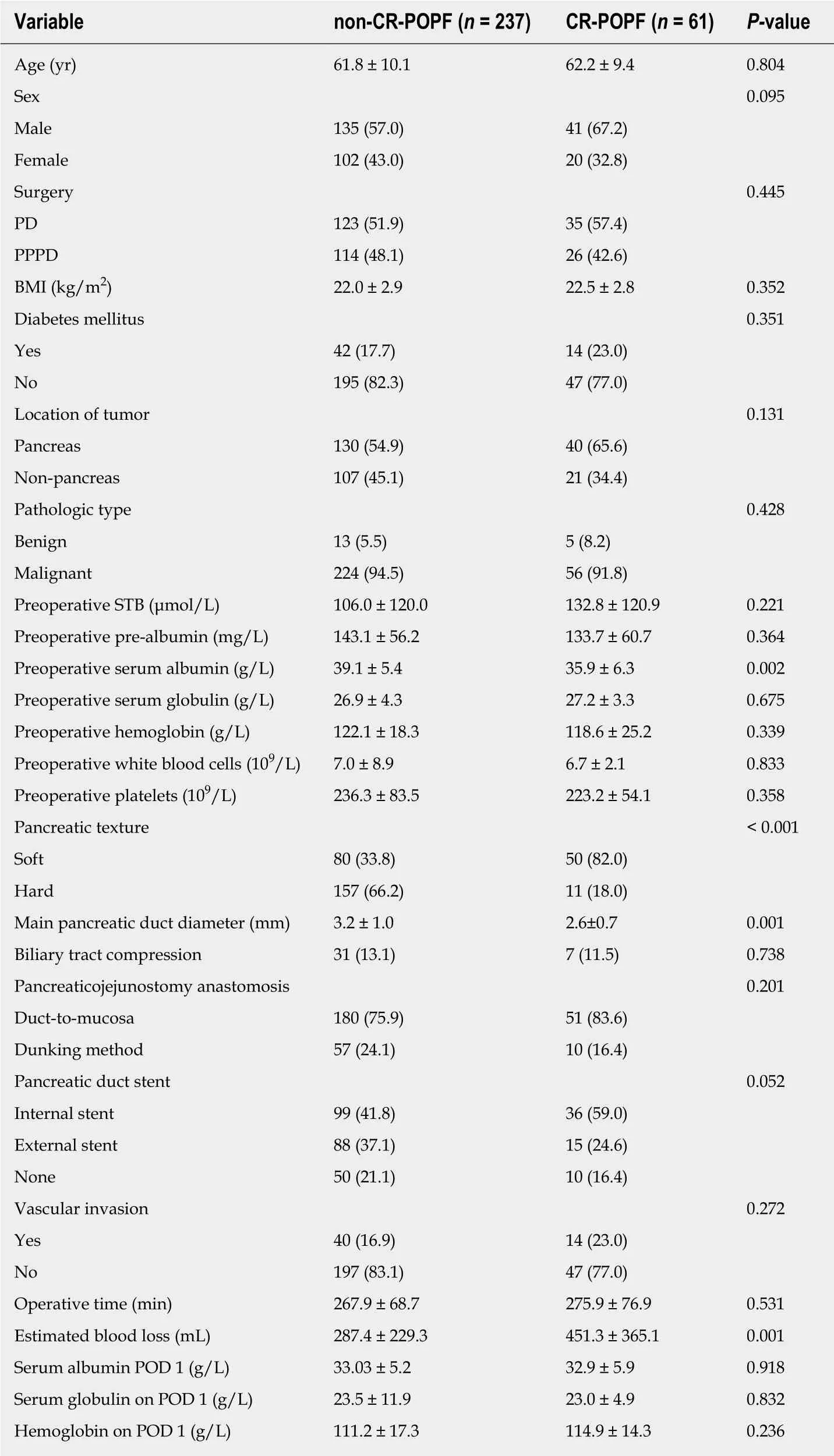
Table 2 Demographic and clinical characteristics of the clinically relevant postoperative pancreatic fistula and non-clinically relevant postoperative pancreatic fistula groups n (%)
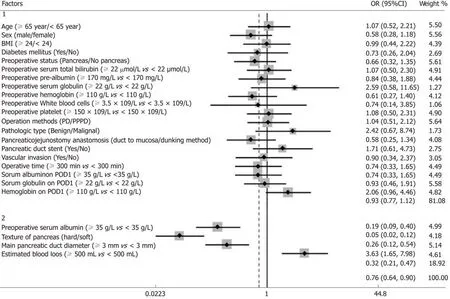
Figure 1 Forest plot of the predictive factors for clinically relevant pancreatic fistula in the study group by univariate analysis. Group 1: Factors with no statistical significance; Group 2: Factors with statistical significance. BMI: Body mass index; PD: Pancreaticoduodenectomy; PPPD: Pylorus-preserving pancreaticoduodenectomy; POD: Postoperative day; CI: Confidence interval; OR: Odds ratio.
Previous studies have reported associations between POPF and many independent variables including sex[14], age[11,18], BMI[14,15,18,19], main pancreatic duct diameter[7,14,15,20],texture of the pancreatic parenchyma[7,19,20], estimated blood loss[7,21], pathology of the pancreatic tumor[7,14], drain amylase[18,22], and postoperative C-reactive protein[23,24]. In this study, the univariate and multivariate analyses revealed that preoperative serum albumin ≤ 35 mg/L, soft pancreatic texture, pancreatic duct diameter ≤ 3 mm, and intraoperative estimated blood loss ≥ 500 mL were independent risk factors for CRPOPF. As a result, our risk predictive scoring system included both preoperative and intraoperative factors. Of these factors, pancreatic duct diameter and pancreatic texture were inherent characteristics of the patients and were associated with difficulty of pancreatojejunostomy anastomosis. Several studies have confirmed that soft pancreatic texture and narrowed pancreatic duct diameter increase the incidence of POPF[9,12]. Moreover, soft pancreatic texture and narrowed pancreatic duct diameter indicate that the exocrine function of pancreas is nearly normal. These patient characteristics indicate increased secretion of pancreatic juices after pancreatectomy[7,24].
In our risk predictive scoring system, preoperative serum albumin ≤ 35 mg/L was considered an independent risk factor for CR-POPF. Serum albumin maintains a stable plasma colloid osmotic pressure and transports endogenous and exogenous compounds[25]. Although transfusing human blood albumin cannot enhance the immune response and resistance to CR-POPF, low serum albumin is usually associated with poor nutritional status and is considered an independent risk factor for increased mortality and severity complications in patients[26]. In contrast, some studies have shown that a low level of preoperative serum albumin does not increase the incidence of POPF[27,28]. These discrepancies might be related to the relatively long time required for hypo-albuminemia to affect the occurrence of POPF. Furthermore,our risk predictive scoring system identified estimated blood loss as an intraoperative risk factor for CR-POPF. The amount of blood loss is associated with the experience level of the operating surgeons and the extent of vascular invasion. Blood loss also cause ischemia and tissue edema and may directly affect healing of the pancreatic anastomosis[7,29].
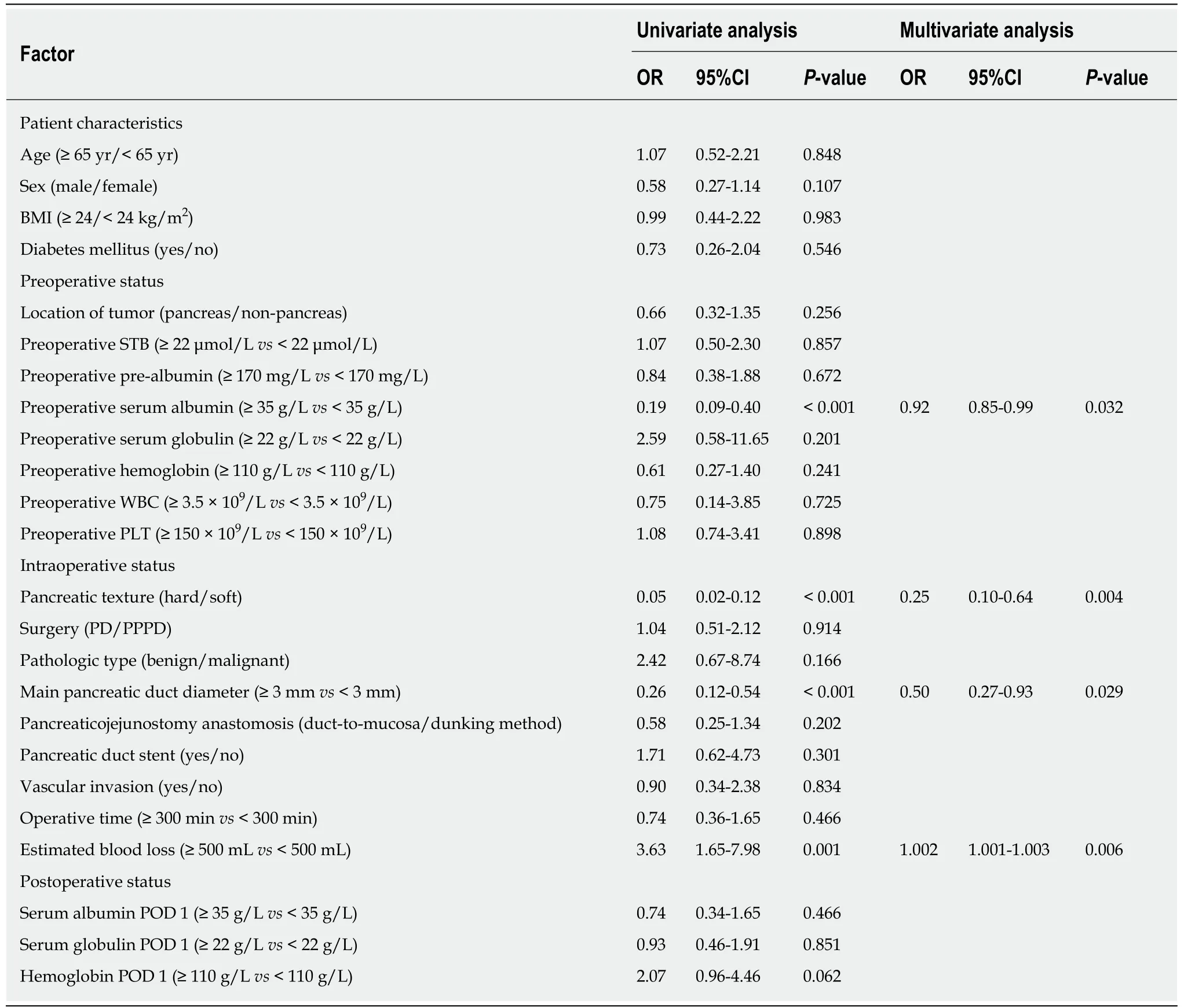
Table 3 Univariate and multivariate analyses of predictive factors for clinically relevant pancreatic fistula in the study group (n = 189)
The relationship between the use of pancreatic duct stents and CR-POPF incidence is highly controversial[30,31]. Some studies demonstrated that internal pancreatic duct stenting does not reduce the incidence of pancreatic fistula and related complications[30,32]. Others have shown that the use of an external stent through the pancreatic anastomosis reduces the pancreatic fistula rate[31,33]. In the present study,the use of pancreatic duct stents did not significantly protect against or reduce the occurrence of CR-POPF. Nevertheless, the subgroup analysis revealed that external stenting reduced the incidence of CR-POPF relative to internal stenting. This suggests that pancreatic external stents might be more effective among this subgroup, but future studies are necessary to investigate this hypothesis. We compared the diagnostic value of our risk scoring system to three previously established scoring tools using all 298 patients (Figure 4). The first tool[19], Test1, included two risk factors(BMI and pancreatic duct diameter), while the second[9], Test2, included four risk factors (BMI, pancreatic duct diameter, pancreatic texture, and blood loss). Test3,included four risk factors (pancreatic texture, main pancreatic duct diameter,extended lymphadenectomy and POD 1 serum albumin)[34]. Supplementary Table 3 describes the sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio of the three scoring systems and our risk scoring system. The AUCs of Test1, Test2, and Test3 were 0.668,0.760, and 0.749, respectively (Figure 4). This analysis shows that our risk scoring system is at least not worse in predicting CR-POPF than the previously established systems. Other risk systems are also available. Callery et al[7]created a model that included pancreatic duct size, pancreatic texture, and certain pathologies and that correlated with the occurrence of CR-POPF. Hence, additional factors should be explored.
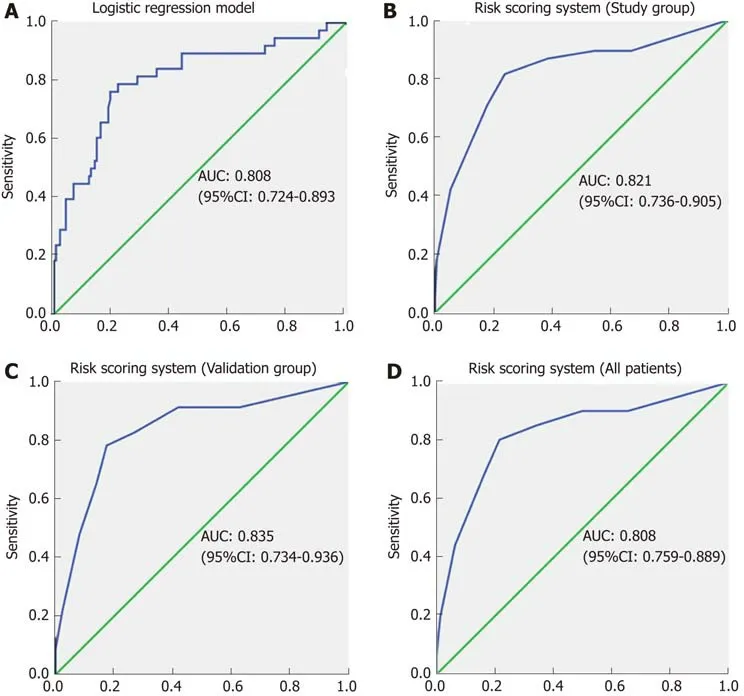
Figure 2 Receiver operating characteristic curves. A: Receiver operating characteristic (ROC) curve for the logistic regression model. The area under the curve(AUC) was 0.808; B: ROC curve for the risk scoring system in the study group. The AUC was 0.821; C: ROC curve for the risk scoring system in the validation group.The AUC was 0.835; D: ROC curve for the risk scoring system in all patients. The AUC was 0.824. ROC: Receiver operating characteristic; AUC: Area under the curve; CI: Confidence interval.
Nevertheless, compared with the three previous predictive systems, our risk scoring system has its superiority. First, our research is based on a single center analysis. Thus, the perioperative management and surgical methods had a unified standard. Second, our predictive scoring system included both preoperative and intraoperative factors, with the postoperative factor, drain amylase level, applied as a beneficial supplement. To the best of our knowledge, our study is the first to report that “double-checks” could predict CR-POPF more accurately. That is, our risk scoring system evaluated the risk of CR-POPF according to the preoperative and intraoperative indicators of the patients, and the patients were divided into high-risk group and low-risk group. Then a secondary evaluation was done according to the post-drainage amylase levels. Finally, our predictive scoring system predicted POPF more accurately than previous systems for high-risk POPF patients. Our novel scoring system renders the following improvements to current perioperative management after PD: (1) The ability to select low-risk patients for early removal or retention of drainage based on the threshold of drain amylase level on POD 3; and (2) The ability to identify high-risk patients for early intervention, such as application of somatostatin and analogues or the performance of continuous low pressure suction.
There were several limitations in this study. First, pancreatic texture was classified as either hard or soft simply based on the surgeon's subjective judgments. Second, the use of pancreatic duct stents depended on the surgeon's clinical experience. Third,this was a retrospective study and the sample size was small. Finally, the levels of drain amylase were not examined every day. Therefore, further prospective studies are necessary to validate these risk factors and evaluate the predictive value of this risk scoring system for CR-POPF after PD.
In conclusion, this study established a scoring system to predict the risk of CRPOPF after PD. This system incorporates both preoperative and intraoperative parameters, with the levels of postoperative drain amylase included as a supplement to this system. This risk scoring system significantly predicted CR-POPF and ultimately, may be used to distinguish between high- and low-risk CR-POPF populations and facilitate timely therapeutic interventions after PD.
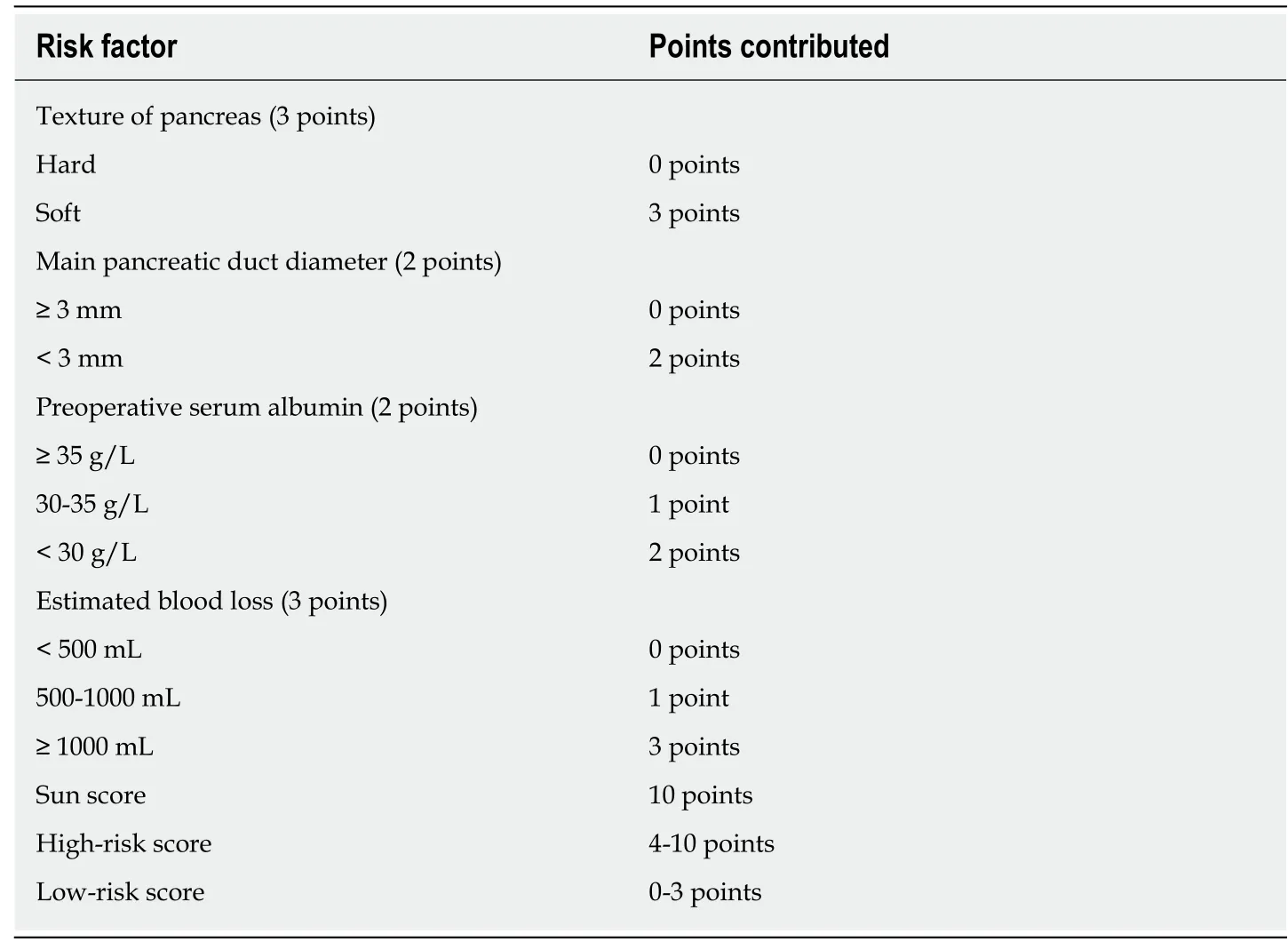
Table 4 Risk scoring system for clinical relevant pancreatic fistula
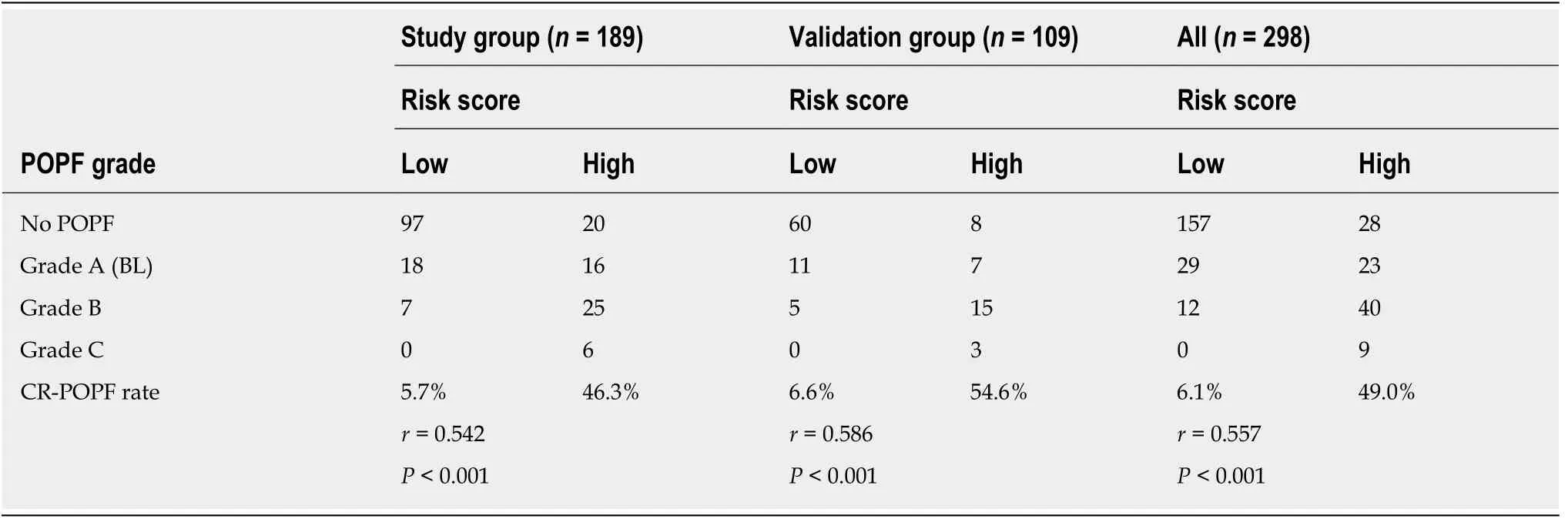
Table 5 Correlation between risk score and postoperative pancreatic fistula
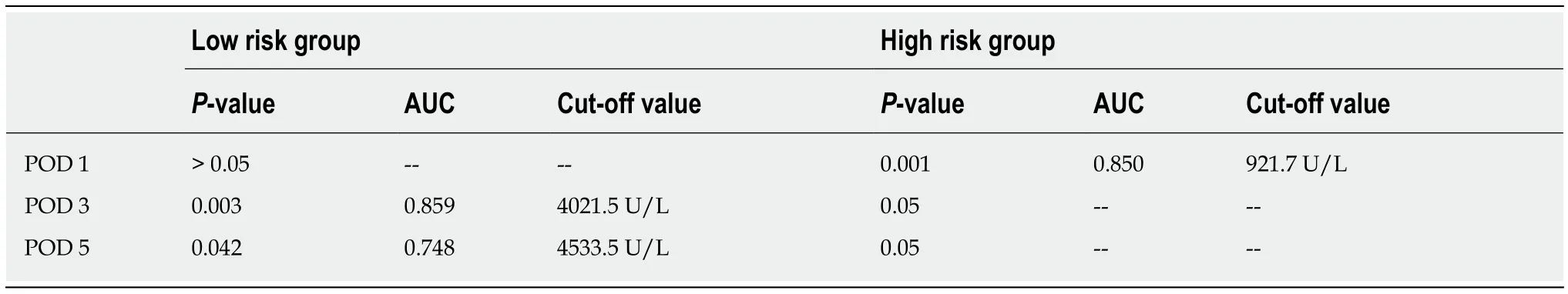
Table 6 Relationship between postoperative drain amylase levels and clinically relevant postoperative pancreatic fistula
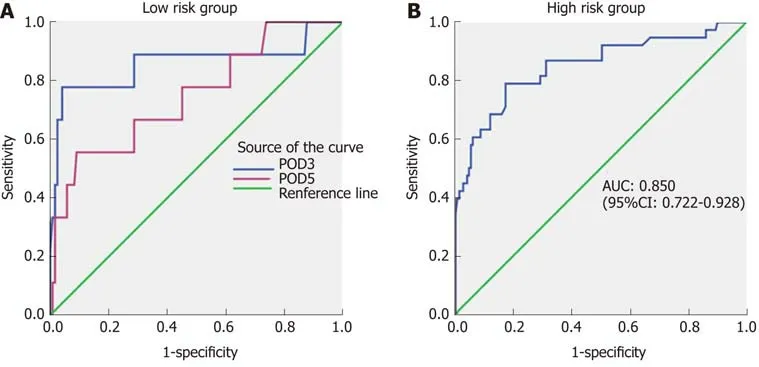
Figure 3 Receiver operating characteristic curves of drain amylase levels for low and high-risk groups. A: Receiver operating characteristic (ROC) curves of drain amylase levels on postoperative day (POD) 3 and POD 5 in the low risk group. The areas under the curve (AUCs) were 0.859 and 0.748, respectively; B: ROC curve of drain amylase level on POD 1 in the high-risk group. The AUC was 0.850. ROC: Receiver operating characteristic; POD: Postoperative day; AUC: Area under the curve; CI: Confidence interval.
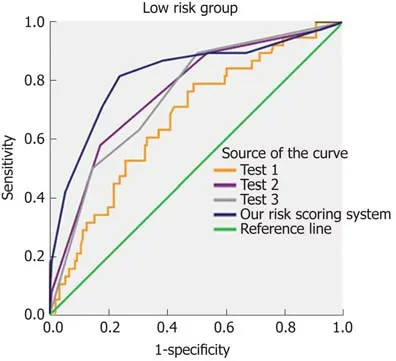
Figure 4 Comparison with three other predictive scoring tools (Test1, Test2, and Test3). The area under the curve (AUC) of Test1 was 0.688 [95% confidence interval (CI): 0.575-0.762]. The AUC of Test2 was 0.760 (95%CI: 0.675-0.846). The AUC of Test3 was 0.749 (95%CI: 0.665-0.833). The AUC of our risk scoring system was 0.824 (95%CI: 0.759-0.889). AUC: Area under the curve; CI: Confidence interval.
ARTICLE HIGHLIGHTS
Research background
Pancreaticoduodenectomy (PD) is associated with significant postoperative morbidity. Clinically relevant postoperative pancreatic fistula (CR-POPF) is among the most common complications after PD and may have serious consequences for the patients. Factors such as age, body mass index, preoperative serum total bilirubin, operative time, operative blood loss, pancreatic duct diameter, and pancreatic texture are known to influence the occurrence of CR-POPF.
Research motivation
Both preoperative and intraoperative variables should be included in the same model for the prediction of CR-POPF, but the available models do not incorporate both preoperative and intraoperative variables.
Research objectives
This study aimed to construct a new risk scoring system for CR-POPF that include both preoperative and intraoperative factors.
Research methods
This was a retrospective study of patients who underwent PD or pylorus-preserving PD (PPPD)between January 2011 and December 2016 at the First Affiliated Hospital of Soochow University.Patients were divided into a study (01/2011 to 12/2014) or validation (01/2015 to 12/2016)group according to the time of admission. POPF severity was classified into three grades:Biochemical leak (grade A) and CR-POPF (grades B and C). Logistic regression was used to create a predictive scoring system.
Research results
Preoperative serum albumin ≥ 35 g/L [P = 0.032, odds ratio (OR) = 0.92, 95% confidence interval(CI): 0.85-0.99], hard pancreatic texture (P = 0.004, OR = 0.25, 95%CI: 0.10-0.64), pancreatic duct diameter ≥ 3 mm (P = 0.029, OR = 0.50, 95%CI: 0.27-0.93), and intraoperative blood loss ≥ 500 mL(P = 0.006, OR = 1.002, 95%CI: 1.001-1.003) were independently associated with CR-POPF. We established a 10-point risk scoring system to predict CR-POPF. The area under the curve was 0.821 (95%CI: 0.736-0.905) and the cut-off value was 3.5. Including drain amylase levels improved the predictive power of the model. Taken together, these results suggest that this 10-point risk scoring system could predict CR-POPF after PD/PPPD.
Research conclusions
The present study established a 10-point scoring system to predict CR-POPF after PD/PPPD using preoperative and intraoperative parameters. Ultimately, this system could be used to distinguish between high- and low-risk populations in order to facilitate timely interventions after PD.
Research perspectives
This system is original and has not been proposed before. Nevertheless, this scoring system will have to be validated prospectively. We hypothesize that this risk scoring system will effectively predict CR-POPF in clinical practice.
杂志排行
World Journal of Gastroenterology的其它文章
- Predicting (side) effects for patients with inflammatory bowel disease: The promise of pharmacogenetics
- Diagnostic and therapeutic challenges of gastrointestinal angiodysplasias: A critical review and view points
- Colorectal cancer screening from 45 years of age: Thesis, antithesis and synthesis
- Gastric per-oral endoscopic myotomy: Current status and future directions
- Liver transplantation for hepatocellular carcinoma: Where do we stand?
- Systems pharmacology approach reveals protective mechanisms of Jian-Pi Qing-Chang decoction on ulcerative colitis
