Gastric per-oral endoscopic myotomy: Current status and future directions
2019-06-13AlexanderPodboyJooHaHwangLindaNguyenPatriciaGarciaThomasZikosAfrinKamalGeorgeTriadafilopoulosJohnClarke
Alexander Podboy, Joo Ha Hwang, Linda A Nguyen, Patricia Garcia, Thomas A Zikos, Afrin Kamal,George Triadafilopoulos, John O Clarke
Abstract Gastroparesis, or symptomatic delayed gastric emptying in the absence of mechanical obstruction, is a challenging and increasingly identified syndrome.Medical options are limited and the only medication approved by the Food and Drug Administration for treatment of gastroparesis is metoclopramide, although other agents are frequently used off label. With this caveat, first-line treatments for gastroparesis include dietary modifications, antiemetics and promotility agents, although these therapies are limited by suboptimal efficacy and significant medication side effects. Treatment of patients that fail first-line treatments represents a significant therapeutic challenge. Recent advances in endoscopic techniques have led to the development of a promising novel endoscopic therapy for gastroparesis via endoscopic pyloromyotomy, also referred to as gastric per-oral endoscopic myotomy or per-oral endoscopic pyloromyotomy. The aim of this article is to review the technical aspects of the per-oral endoscopic myotomy procedure for the treatment of gastroparesis,provide an overview of the currently published literature, and outline potential next directions for the field.
Key words: Gastroparesis; Gastric per-oral endoscopic myotomy; Per-oral endoscopic pyloromyotomy; Myotomy; Endoscopic myotomy; Peroral endoscopic myotomy; Gastric emptying
INTRODUCTION
Gastroparesis, characterized by the symptom constellation of early satiety, nausea,vomiting, bloating, postprandial fullness, and upper abdominal pain in the presence of delayed gastric emptying, is an increasingly recognized cause of significant foregut morbidity. Initially thought to be an uncommon disease entity, gastroparesis has experienced a marked increase in prevalence over the past decade; with a growing number of reports indicating increasing emergency room visitations, hospitalizations and health care utilization[1-4].
Gastroparesis is a heterogeneous condition most commonly idiopathic in nature,but also frequently identified in diabetes, connective tissues disease, neurologic disorders, and as a complication of intrathoracic or intrabdominal procedures[5,6].Numerous underlying pathophysiologic mechanisms have been proposed, but the true pathophysiology of gastroparesis is not well elucidated[5,6]. Because of this limitation, the medical treatment for gastroparesis is largely empiric with the goal of increasing upper gastrointestinal (GI) motility and providing supportive symptomatic relief[5-8]. The only medication that is approved by the Food and Drug Administration for the treatment of gastroparesis at present is metoclopramide; although other agents are frequently used off label. Other medical treatments for gastroparesis includes the use of prokinetic agents such as dopaminergic antagonists like domperidone, utilizing the antibiotic erythromycin's effects on motilin receptors in the gut to stimulate gastric motility, employment of anti-emetics, or use of neuromodulators[5-9].Unfortunately, the efficacy of these agents has been limited by the presence of tachyphylaxis, or significant cardiovascular or neurologic side effect profiles that restrict the ability to continue these medical therapies long term[5-9].
In 1986, Mearin et al[10]published a landmark paper that demonstrated abnormally prolonged, high-amplitude pyloric contractions within a subset of patients with gastroparesis. Since that publication, suspected “pylorospasm”, has been the target of a multitude of surgical and endoscopic therapies. These therapies include pyloric injection of botulinum toxin, endoscopic dilatation, placement of trans-pyloric stents,and surgical pyloroplasty or pyloric myotomy[11-18]. However, widespread application of these modalities has been dampened by either the invasiveness of the procedure or a failure to demonstrate consistent long-term efficacy[5,6,11-18]. However, recent advances in endoscopic submucosal dissection have led to the development of novel endoscopic tunneling techniques that have been associated with significant therapeutic potential. Pasricha et al[19]in 2007 incorporated these submucosal tunneling techniques along with an endoscopic myotomy in pigs for treatment of achalasia and subsequently Inoue et al[20]in 2010 first reported the initial clinical experience of these techniques in humans. Since publication of these experiences,these techniques have been increasingly applied to good effect for the treatment of functional esophageal disease[21,22]. Utilizing this successful model as a template, the technical feasibility of per-oral endoscopic pyloromyotomy (G-POEM or POP) was initially described in 2012 by Kawai et al[23]in a porcine model. Following this publication, the first successful G-POEM in humans was described by Khashab et al[24]in 2013. This report details a patient with refractory debilitating gastroparesis who underwent a successful endoscopic pyloromyotomy with marked improvement in symptoms 12 weeks following the procedure[24]. After this initial report, over a dozen published reports of successful G-POEM procedures have been identified in the English literature indicating that we have now entered an era of increased utilization of per-oral endoscopic myotomy for the treatment of gastroparesis[25-34]. The aim of this article is to review the technical aspects of the G-POEM procedure for the treatment of gastroparesis, provide an overview of the currently published literature, and outline potential next directions for the field.
TECHNICAL ASPECTS OF THE PER-ORAL ENDOSCOPIC PYLOROMYOTOMY PROCEDURE
In our experience, G-POEM for the treatment of gastroparesis is divided into 5 stages:(1) The initial endoscopic inspection; (2) Initial mucosotomy; (3) Submucosal tunnel formation; (4) Pyloric myotomy; and (5) Mucosal Closure (Figure 1).
Step 1: Initial endoscopic inspection
In stage 1, the initial endoscopic inspection is performed to clear the surgical field of food or residual debris and to identify any contraindications such as mass or ulceration which would preclude continuation of the procedure. At our institution,we commonly assess pyloric distensibility utilizing a 325/8 cm sized endoscopic Functional Lumen Imaging Probe (FLIP, EndoFlip, Medtronic, Sunnyvale, California)during this step (Figure 1A).
Step 2: Mucosotomy
The submucosa is injected with 4-6 mL of lifting solution [0.25% indigo carmine into 500 mL HESPAN (6% Hetastarch in 0.9% Sodium Chloride solution)] 4-5 cm proximal to the pyloric channel along the greater curvature of the stomach. We utilize a triangle tip (TT) endoscopic knife (KD640L, Olympus, Tokyo, Japan) using EndoCut Q setting at 50 W, effect 2 (VIO300D, ERBE, Tϋbingen, Germany) to create a 1-1.5 cm transverse mucosal incision above the submucosal injection (Figure 1B and C).
Step 3: Submucosal tunnel formation
Upon creation of the mucosotomy, the endoscope is advanced into the submucosal space. Submucosal dissection is performed using either the TT or IT-nano (KD 604L or KD 612L, Olympus, Tokyo, Japan) endoscopic knifes, based on anatomic orientation.The submucosal fibers are dissected using spray coagulation mode (50-80 W, effect 2)or forced coagulation mode (50-80 W, effect 2). The submucosal tunnel is extended until the pyloric ring is visualized (Figure 1D). Frequent orientation by exiting the tunnel and visualizing the pylorus is performed throughout creation of the submucosal tunnel to ensure appropriate directionality. Lifting solution is intermittently applied via a spray catheter to aid visualization of the submucosal plane and better demarcate the mucosa and submucosal layers. Any obstructing submucosal vessels are cauterized using coagulation graspers (FD 410 LR, Olympus,Tokyo, Japan) in soft coagulation mode (80 W, effect 3).
Step 4: Myotomy
Upon visualization of the pyloric ring, a full thickness pyloromyotomy down to the serosal layer is performed using either the TT or IT-nano endoscopic knifes with an EndoCut Q current (50 W, effect 2). The myotomy is extended approximately 1-2 cm proximal to the pyloric ring to ensure that the pyloric sphincter has been completely cut (Figure 1D and E).
Step 5: Closure
The mucosal defect is then closed with endoscopic sutures using the OverStitch™Endoscopic Suturing System (Apollo Endosurgery Inc, Austin, Texas) (Figure 1E). In our practice all patients with medically refractory gastroparesis or with symptom severity sufficient to justify endoscopic intervention are considered for the procedure.Nausea and vomiting are subjectively the symptoms we feel most respond to intervention and we focus on people with these symptoms specifically, rather than those that present primarily with pain. Our practice is to perform a baseline gastric emptying, symptom assessment and assessment of pyloric distensibility (FLIP) prior to (or during) the G-POEM procedure.
Patients are kept nil per os the night before the procedure. If there is a history of gastric food remnant in the stomach, a longer period of a liquid diet is employed to ensure that the stomach is empty for the procedure. Anesthesia is employed to ensure that the patient is kept motionless during the procedure.
Following the procedure, the patient is admitted to the hospital for post procedural observation, initiated on post procedural IV antibiotics and keep on gut rest until a follow up upper GI gastrografin study is obtained the following morning. If no extraluminal contrast is identified the patient's diet is advanced to a full liquid diet,and the patient is discharged on 5 d of oral antibiotic therapy and acid suppression via twice daily proton pump for 4-8 wk. Our average length of hospitalization is 1-2 d.Repeat gastric emptying, pyloric distensibility and symptom assessment is typically obtained 12 wk post procedure.
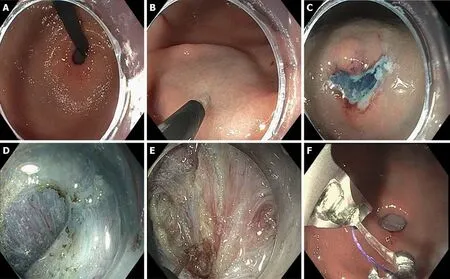
Figure 1 Per-oral endoscopic pyloromyotomy procedure in 5 steps. A: Initial inspection; B and C: Mucosotomy; D: Submucusal tunnel dissection with identification of pyloric ring; E: Pyloromyotomy; F: Mucosal closure.
CURRENT DATA
Table 1[25-34]depicts the preclinical, procedural and post-procedural outcomes in the largest reported G-POEM or POP reports published to date. When the data are aggregated, a total of 325 patients underwent the G-POEM procedure with 100%noting technical success. Major complications were noted in 8.3% of all patients noted,the most common of which were capnomediastinum or capnoperitoneum followed by antral or prepyloric ulceration and GI bleeding. Only 3 perforations were reported,and all healed with supportive care. The mean length of stay ranged from 1-5 d with a mean procedural length ranging from 37-120 min. Rodriguez et al[29]noted decreased procedural times with increased familiarity with the procedure. The majority of patients were female in their 5thto 6thdecade of life and the etiology of gastroparesis was noted to be idiopathic in 45%, diabetic in 28%, and post-surgical in 22%. Other than medical therapy, a wide range of previous treatments for gastroparesis were reported with botulinum toxin and gastric stimulator placement being the two most commonly reported.
Clinical success was noted in 68%-90% of patients. 3 studies defined clinical success as any symptomatic improvement[25,27,34], with 4 studies identifying any decrease in Gastroparesis Cardinal Symptom Index (GCSI) or Clinical Patient Grading Assessment Scale score as clinical success[26,30,32,33]. Only one study utilized an absolute GCSI decrease (0.75 points) as a marker for success[31]. Two studies did not define parameters for clinical success[28,29]. An improvement in gastric emptying scores at 4 hours was reported in 67%-90% of all patients with follow up Gastric Emptying Study(GES) studies. Normalization of the GES at 4 h was noted in 0-66% of all gastric emptying studies, although significant drop out rates were reported. Eight studies reported follow up intervals of less than 7 mo.
No clinical parameters were identified that reliably predicted G-POEM success.Gonzalez et al[26]noted that female gender and diabetes were associated with a higher rate of failure in a univariant analysis; however, these results were not corroborated in a multivariate analysis. Malik et al[30]and Jacques et al[31]were the only authors to study the pre- and post-pyloric distensibility with FLIP. While Malik et al[30]identified a larger cross sectional area at 40 mL balloon in those that improved with G-POEM,Jacques et al[31]identified that at 50 ml a Distensibility Index of < 9.2 mm2/mmHg was 100% specific and 72.2% sensitive with regards to clinical efficacy of G-POEM. No other parameters were identified that predicted success or failure of the G-POEM procedure. Table 2 depicts the procedural variability across the studies. Note the large variation between studies in regard to the site of mucosotomy, endoscopic equipment used, and post-operative testing and care including antibiotics and acid suppression.
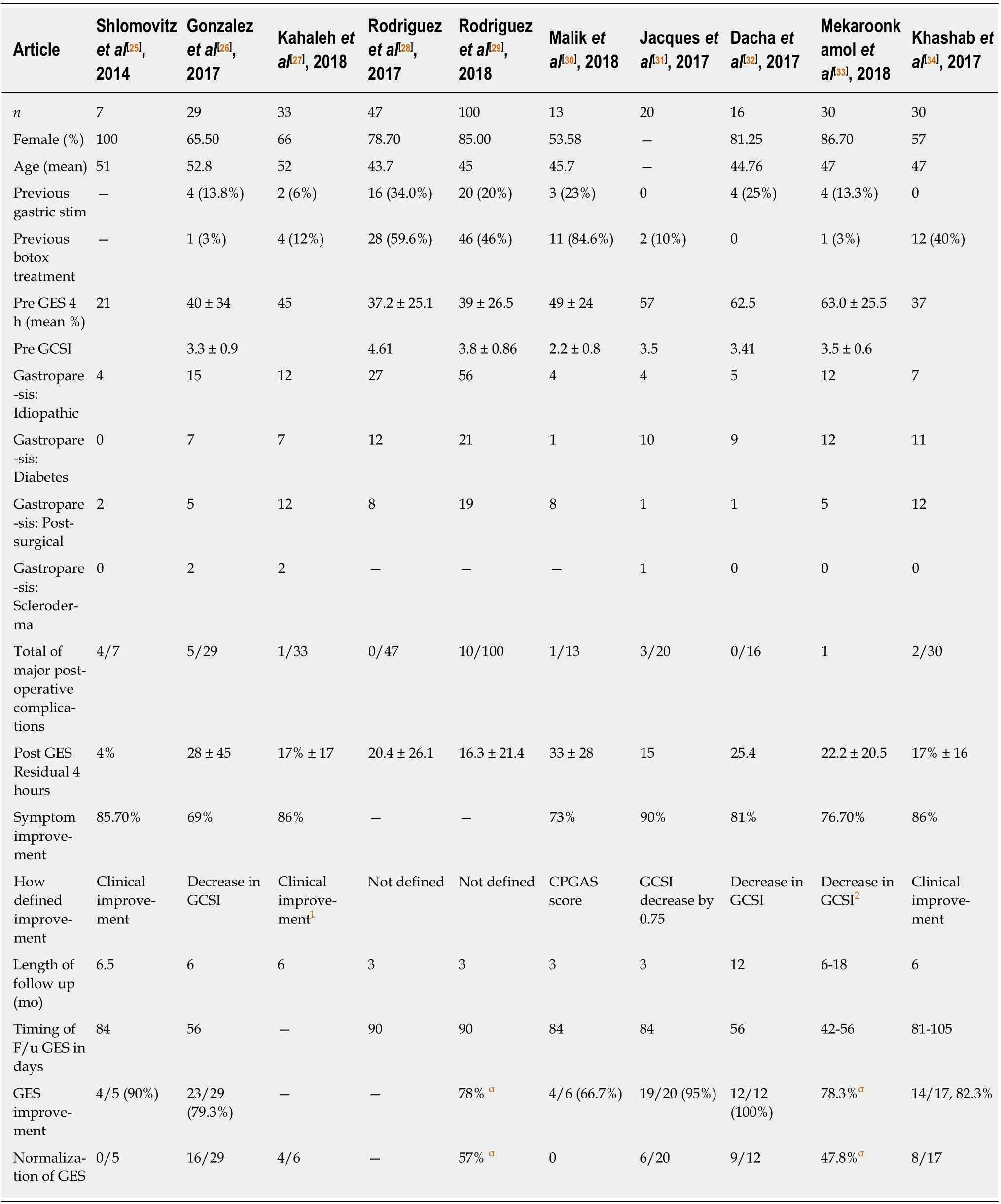
Table 1 Depicts pre-clinical demographic and clinical data and post-operative changes to symptoms and gastric emptying
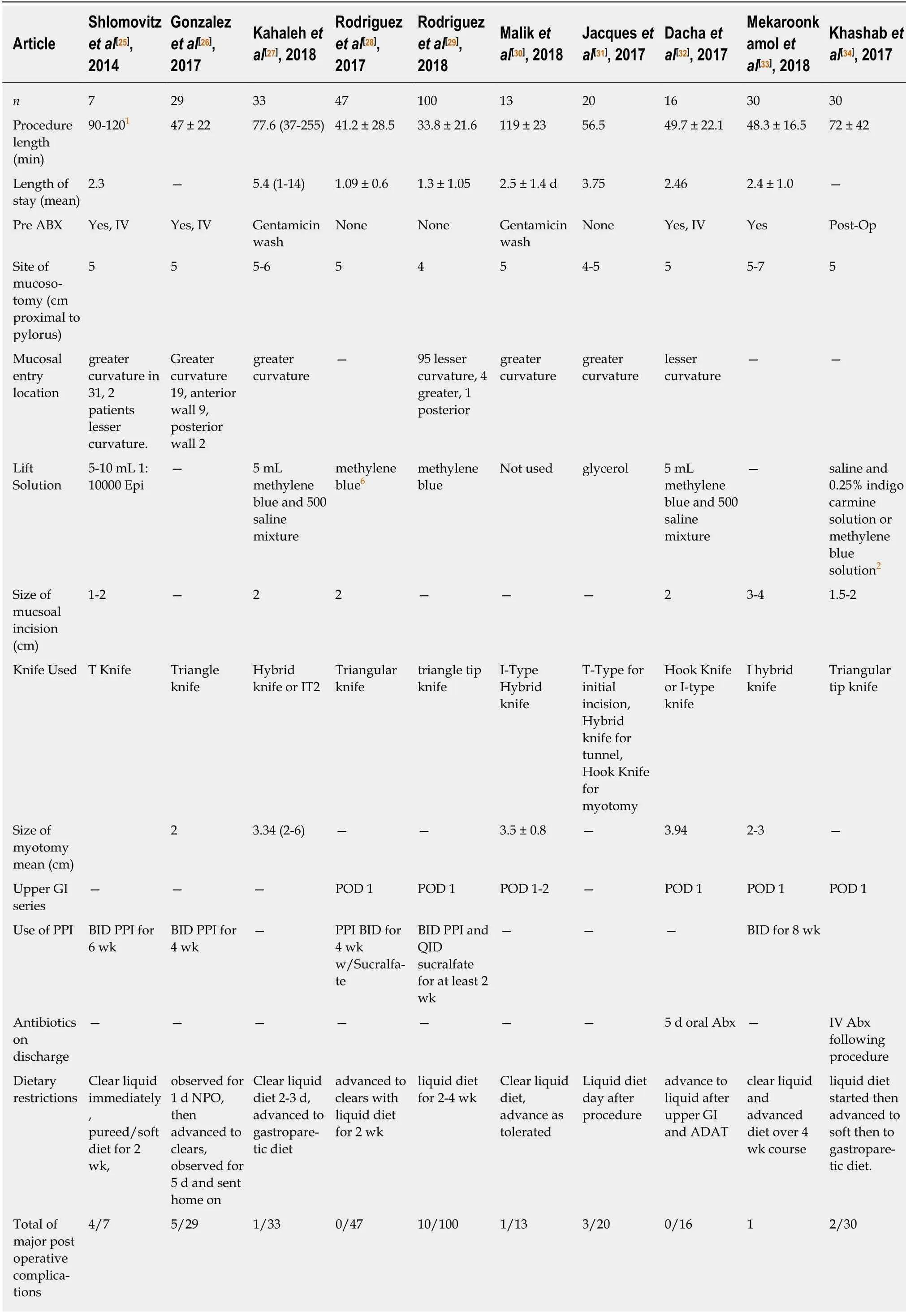
Table 2 Demonstrates procedural data including equipment used and post-operative practice reported across the studies
LIMITATIONS
There are several limitations to these published studies. Mainly there is significant heterogeneity between the studies with regards to demographic data, patient selection, pre- and post-operative testing, and in determination and assessment of clinical response. Additionally, the published reports are largely retrospective in nature with no true control groups; moreover, they experienced significant drop out rates in regard to follow up assessment and testing without inclusion of any intention to treat analysis. There was no standard approach towards type or duration of premyotomy medications or interventions and no standard approach in determining preprocedural symptom severity. Furthermore, as highlighted in Table 2, there was no standardized technique with regards to the G-POEM procedure itself, with significant differences in location of myotomy position, myotomy size, equipment used and postprocedural medications and testing.
WHERE DO WE GO FROM HERE?
The above heterogeneity highlights a need for validated formalized pre- and postoperative testing, and careful stratification of symptoms. This would allow objective measurement of gastroparesis severity, which could allow subsequent accurate determination of optimal procedural candidates. Additionally, concurrent or perioperative use of novel assessments of pyloric pressure and distension via FLIP[35-37],classification of gastric contractile patterns via electrogastrography[38], or response to previous pyloric-directed therapies including pyloric botulinum toxin injection or pyloric stent placement[11-18]could prove useful in identifying a subgroup of patients that would be best served by G-POEM. Moreover, identifying and classifying patients based on emerging histopathologic features of gastroparesis such as loss of intestinal cells of Cajal, degree of collagen fibrosis, smooth muscle abnormalities, or alterations in the gastric muscle macrophage population[39-42]all may prove useful in further stratifying patients into subgroups that may provide further clarity into which patients would best benefit from endoscopic pyloromyotomy.
While the current published literature demonstrates significant optimism regarding the efficacy of G-POEM for the treatment of gastroparesis, gastroparesis itself is a markedly heterogeneous disease entity and caution should be used in interpretation of short term follow-up data in a disease that has demonstrated significant placebo response in the past[43]. Based on this, there is a clear need for a randomized blinded prospective trial, although overall the design of this trial remains unclear.Consideration for G-POEM vs sham procedure is ideal but may have ethical and blinding considerations. Alternative options including randomized crossover studies utilizing G-POEM vs other pyloric-directed therapies, including botulinum toxin injection, pyloric stenting, or surgical pyloromyotomy, is an attractive option that may help mitigate some of the ethical and blinding concerns. Regardless of the approach,these studies are likely best served via multicenter investigation secondary to the total numbers required to power such a study.
Questions including the optimal number of training cases prior to competency also require consideration. Previously published literature in POEM has identified a wide range of conflicting reports for the number of required cases prior to accepted clinical competency, with ranges from 20-100[21-44], and it is not clear if the learning curve for G-POEM follows a similar pattern to POEM. Other potential procedural issues that require future attention include determination/comparison of the various technical aspects of the procedure (i.e., type or location or myotomy) and determination of the optimal timing of G-POEM either sequentially or in combination with other therapeutic modalities for gastroparesis.
Additionally, it is not clear if the ultimate therapeutic efficacy of G-POEM is secondary to pyloric disruption alone or if pyloric disruption results in yet undetermined physiologic alterations. Rao et al[45]previously established that fundic distension via balloon insufflation led to increases in phasic motor activity within the antrum and duodenum. It is unclear whether similar adaptive changes in the fundus or duodenum are seen following endoscopic pyloromyotomy.
CONCLUSION
Gastroparesis is a chronic disease with significant associated morbidity. G-POEM demonstrates an exciting potential therapeutic addition to a limited armamentarium and may ultimately be a safer option than many of the medical options currently available. However, more investigation is needed to identify optimal candidates,including a standardized approach to pre- and post-operative testing, developing a standard endoscopic approach and further studies into the physiologic effects of GPOEM. Ultimately, we suspect that GPOEM may be an excellent candidate for a subgroup of gastroparesis patients with pyloric dysfunction; however, standardization and data are needed to ensure that this procedure is best positioned to help those patients who may most benefit from pyloric intervention.
杂志排行
World Journal of Gastroenterology的其它文章
- Predicting (side) effects for patients with inflammatory bowel disease: The promise of pharmacogenetics
- Diagnostic and therapeutic challenges of gastrointestinal angiodysplasias: A critical review and view points
- Colorectal cancer screening from 45 years of age: Thesis, antithesis and synthesis
- Liver transplantation for hepatocellular carcinoma: Where do we stand?
- Systems pharmacology approach reveals protective mechanisms of Jian-Pi Qing-Chang decoction on ulcerative colitis
- Endoscopic resection of the pancreatic tail and subsequent wound healing mechanisms in a porcine model
