Effect of transcranial direct current stimulation on the level of consciousness in patients with traumatic coma:study protocol for a self-controlled trial
2019-06-05NilpadmaSarkarSubhasishChatterjeeAjayGehlot
Nilpadma Sarkar,Subhasish Chatterjee, ,Ajay Gehlot
1 Maharishi Markandeshwar Institute of Physiotherapy,Mullana,Ambala,Haryana,India
2 Department of Neurosurgery,Maharishi Markandeshwar Institute of Medical Science and Research,Mullana,Ambala,Haryana,India
Abstract
Key words: coma; consciousness; coma recovery scale-revised; motor cortex; transcranial direct current stimulation; traumatic brain injury,unconsciousness
INTRODUCTION
Traumatic brain injury (TBI) is a serious health problem that mainly leads to disability,morbidity,and mortality globally(Hyder et al.,2007).The main reason behind the traumatic brain injury is road traffic accidents,fall from height,violence,and combination of workplace and sports-related injuries among active and healthy teenagers and young adults(Kraus et al.,1984; Gururaj,2002).TBI alters the the level of consciousness which includes coma and vegetative state(Sfdpwfsz and Dpotdjpvtoftt,2006).One in ten patients with severe head injury remains in prolonged state of coma (Pierce et al.,1990).In Indian scenario,2 million persons are injured and 200000 persons die annually due to TBI.The younger people are more prone to TBI.The male and female ratio of TBI is 4:1 (Gururaj,2002; Bruns and Hauser,2003).
Unconsciousness is mainly consisting of coma,vegetative state,and minimally conscious state (Laureys et al.,2006; Sarà et al.,2009).Coma is a complete loss of arousal system where patient's eyes are closed and application of vigorous sensory stimulation is unable to alert the person (Giacino et al.,2002).Vegetative state is a complete loss of behavioral evidence without awareness (Laureys et al.,2010).In minimally conscious state,consciousness is severely altered but behavioral evidence of awareness is minimal (Jennett and Plum,1972).According to the level of non-reflexive responsiveness of the patients,minimally conscious state is to be subclassified into Minimally Conscious State Minus and Minimally Conscious State Plus and it can be validated with fluorodeoxyglucose positron emission tomography (PDG-PET) (Sarà et al.,2009).
Transcranial direct current stimulation (tDCS) is a weak constant electrical current flow which administrates into the cerebral cortexviascalp electrodes.This technique has been investigated in 1960 to produce localized changes of cerebral excitability (Nitsche and Paulus,2000).Transcranial direct current stimulation effects on resting potential of the neural membrane but does not induce any nerve action potentials(Angelakis et al.,2014).There are two electrodes in tDCS-one is anodal (positive) which has excitatory effects,and the other is cathodal (negative) which has inhibitory effects on the underlying cortex (Bindman et al.,1964).There is no known side effect of tDCS but some studies have reported skin lesions at the area under the electrode whereas it is minimized by using saline water (Kang et al.,2012).
There is no available study for the treatment of unconsciousness.There are some stimulation techniques of motor cortex that improve the level of consciousness but they are time consuming and costly techniques (Demirtas-Tatlidede et al.,2012).The study aimed to assess the effect of tDCS on unconsciousness of patients with vegetative state and minimally conscious state after traumatic brain injury.
METHODS/DESIGN
Study design
The single-subject ABA design single-center study will be conducted in the Neuro-Surgery ICU,Maharishi Markandeshwar Hospital,India (Figure1).ABA is meaning with assessment(pre),intervention with assessment,assessment (post).It is a single group quasi experimental study.Patient's conscious level will be assessed by JFK Coma recovery scale- Revised(CRS-R) (Angelakis et al.,2014).A score 6-10 suggest persistent vegetative state,unresponsive wakefulness syndrome,minimally conscious state (Angelakis et al.,2014).So a Legal Authorized Guardians of all the patients will receive a written explanation of the trial and the written informed consent forms(Additional file 1) will be signed prior to the patients being involved in the trial.The study was registered with ctri.nic.in(CTRI/2019/01/017186) on January 22,2019.
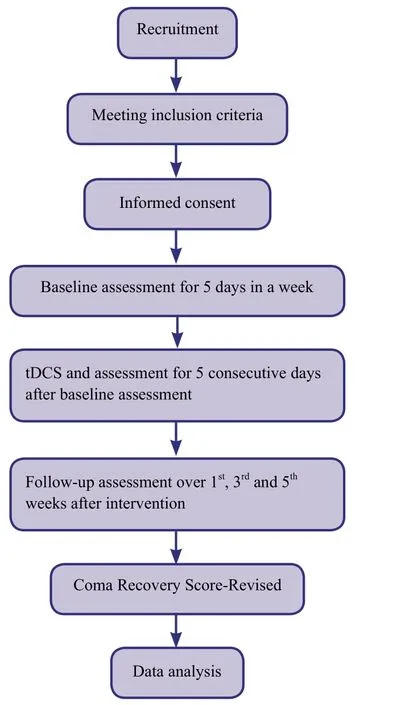
Figure1: Study flow chart.
Participant recruitment
Patients with traumatic coma will be recruited from inpatients at Neuro-Surgery ICU of Maharishi Markandeshwar Hospital.Individuals will be included based are patients with age between 15-60 years,both male and female,patients with traumatic brain injury,GCS score ≤ 9.Exclusion criteria will be open wound in the skull and metallic implants,previous diagnose with epilepsy,post traumatic CNS infection,and brain herniation.
Outcome measurement
Pre intervention,during intervention,post intervention and follow up outcome measurement will be documented by JFK Coma Recovery Scale-Revised (CRS-R) (Løvstad et al.,2010).The scale consists of six sub-functional scale- auditory function scale (0-4),visual function scale (0-5),motor function scale (0-6),verbal function scale (0-3),communication scale(0-2) and arousal scale (0-3).The total highest score of the
Coma Recovery Scale -Revised is 23 (Schnakers et al.,2008).Baseline measurement will be taken for 5 consecutive days with JKF CRS-R,in this period there will be no intervention,after that intervention will be given with tDCS for 20 minutes twice a day with 2.0 mA and the outcome measurement will be continued (Fregni et al.,2005; Lang et al.,2007; Thibaut et al.,2014).After intervention,follow-up assessment will be taken on every first day of the 1st,3rdand 5thweek (Table1).
· Baseline assessment for 5 days in a week
· Intervention twice a day and assessment for 5 consecutive days
· Follow-up assessment for every 1stday over 1st,3rd,and 5thweeks post intervention.

Table1: Meseaures and time frame
Intervention
The electrodes of tDCS (Walnut MedicalTM) will be placed over F3 and C3 region based on 10/20 international electroencephalography system,to get behavioural,cognitive and emotional affects with anodal tDCS (Boggio et al.,2007).All participants will also receive conventional medical treatment,includes management of intracranial hypertension and secondary brain injury,maintenance of cerebral perfusion pressure,and ensuring adequate oxygen delivery to injured brain tissue that includes airway control and ventilation,fluid management,sedation and analgesia,Intracranial pressure management,osmotherapy,multimodal neuromonitoring,anticonvulsant therapy,temperature management,glycemic control,decompressive craniotomy,nutrition,and antibiotic therapy (Bates,1993; Dash and Chavali,2018).
Data management
All the baseline,treatment and pose treatment assessment will be taken by blind observer with postgraduate degree in physiotherapy with 3 years of experience in Neuro-Surgery ICU.
Statistical analysis
Statistical analyses will be conducted using SPSS version 20.0(IBM Corp.,Armonk,NY,USA),and results will be considered significant at thep< 0.05 level.Measurements will be taken at three time points: baseline (pre-intervention),during treatment session and after treatment session (post).Prior to statistical comparisons,all data will be examined for normal distribution using the Shapiro-Wilk test.
The auditory function,visual function,motor function,verbal function,communication,and arousal of Patients with acute traumatic coma at baseline will be compared using the pairedt-test or Wilcoxon signed rank test based on the normality distribution of the data.
Ethics and dissemination
Ethical approval has been obtained from the Institutional Ethical Committee (IEC) of Maharishi Markandeshwar (Deemed to be University) (IEC/MMDU/2018/1183) on June 6,2018(Additional file 2) and adheres to the Standard Protocol Items:Recommendations for Interventional Trials (SPIRIT) guidance (Additional file 3) (Chan et al.,2013).The study will be performed in accordance with the ethical guidelines laid out by theDeclaration of Helsinki(Revised 2013).
DISCUSSION
Present study aims to check the effect of tDCS on traumatic coma patients.Coma is a frequent complication in traumatic head injury patients which needs to be addressed during management.Management options in coma are very limited.tDCS is a form of noninvasive brain stimulation which can alter the excitability of brain neurons based on the polarity (Angelakis et al.,2014).This study will provide a preliminary evidence,so that tDCS can be used for effective management of Coma .
TRIALS sTATUS
Patient recruitment is ongoing.
Additional files
Additional file 2: Ethical approval documentation.
Additional file 3: SPIRIT Checklist.
Author contributions
The 1stauthor: Nilpadma Sarkar,contributes to write the manuscript and conduct the study work.Correspondence author: Subhasish Chatterjee,contributes to topic selection and guides the study work.The 3rdauthor: Dr.Ajay Gehlot,contributes to as a co-supervisor and guides to take the assessment.
Conflicts of interest
None declared.
Financial support
The authors received no specific funding for this work.
晚清关税制度变迁过程是一个被动变迁的过程,是鸦片战争失败造成的后果。除此之外,国际、国内政治经济环境的改变,迫使清朝政府重建经济秩序以维护自身的统治,这是关税变迁的间接原因。
Institutional review board statement
This study protocol has been approved by the Institutional Ethical Committee (IEC) of Maharishi Markandeshwar (Deemed to be University) (IEC/MMDU/2018/1183) on June 6,2018.The study will be performed in accordance with the ethical guidelines laid out by theDeclaration of Helsinki(Revised 2013).
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms.In the forms,the patients' guardians will give their consent for patients' images and other clinical information to be reported in the journal.The patients' guardians understand that patients' names and initials will not be published and due efforts will be made to conceal patients' identity.
Reporting statement
This study followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidance for protocol reporting.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Maharishi Markandeshwar (Deemed to be University),Mullana,Ambala,Haryana,India.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
For data sharing,individual participant data will not be available.However,the study protocol and informed consent form will be made available beginning 3 months and ending 5 years following article publication to investigators whose proposed use of the data has been approved by an independent review committee identified to achieve aims in the approved proposal.In order to gain access,data requestors will need to sign a data access agreement.Proposals should be directed to subhasish.physio@gmail.com.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
猜你喜欢
杂志排行
Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Efficacy and safety of traditional Chinese medicine combined with western medicine for early-phase treatment of acute ischemic stroke based on the primary syndrome elements: protocol for a randomized controlled trial
- Efficacy of entacapone and pramipexole in treating non-motor symptoms of Parkinson's disease: a prospective randomized controlled trial
- Influence of bladder management on long-term quality of life in patients with neurogenic lower urinary tract dysfunction
