Intestinal microflora in patients with liver disease
2019-05-17ManManWuHaiNingYuShengRongShen
Man-Man Wu , Hai-Ning Yu, Sheng-Rong Shen
Key words: Intestinal microflora, Liver disease, Endotoxin, Metabolism.
Abbreviations
Lipopolysaccharide (LPS); Toll-like receptors4 (TLR4); Interleukin-6 (IL-6); Tumor necrosis factor alpha (TNFα);non-alcoholic steatohepatitis (NASH); Methionine-Choline-Deficient (MCD); Non-alcoholic fatty liver disease (NAFLD);
hepatic stellate cell (HSC); Transforming growth factor-b (TGF-b); Transforming growth factor-β (TGF-β); Short
chain fatty acids (SCFAs); Monocarboxylate transporters-1 (MCT-1); Sodium-coupled monocarboxylate transporter-1
(SMCT-1); Tricarboxylic Acid (TCA); G protein-coupled receptors41 (Gpr41); G protein-coupled receptors43 (Gpr43);
Adenosine 5'-monophosphate-activated protein kinase (AMPK); Triglyceride(TG); Total-Cholesterol(TC); Cholesterol
7 alpha-hydroxylase (CYP7A1); Farnesoid X receptor (FXR); The G protein-coupled bile acid receptor (TGR5); Ursodeoxycholic Acid (UDCA); Trimethylamine (TMA); Trimethylamine N-oxide (TMAO); Cytochrome P450 2E1 (CYP2E1)
Introduction
T here exists over 1014microflora from dozens of genera in human intestinal tract, mainly in the colon[1]. The normal intestinal microflora is involved in the progresses of digestion, decomposition, absorption and synthesis of various substances (Such as carbohydrates, fats, amino acids,cholesterol, bile, etc.). In the physiological state, intestinal microflora is a natural barrier and play an important role in health. The changes of intestinal microflora in variety and quantity will affect the physiologic functions[2]. Recently there's increasing studies have reported the involvement of intestinal microflora in live diseases.
The liver has two blood supplies-portal venous system and hepatic arterial system and about 75% of the blood is from the hepatic portal vein. The hepatic portal vein carries all blood received from the intestines through the liver, which formed the“intestinal-liver axis”. The nutrients from intestinal blood can activate the liver. In turn, the liver can affect intestinal function by secreting bile to intestinal tract [3].
The liver, as the first organ of the portal vein, is very susceptible to the intestinal microflora and its metabolites. Liver diseases are associated with changes of qualitative (Composition of flora) and quantitative(Growth) of intestinal microflora [4].When intestinal microflora is out of balance, harmful substances in the intestinal tract cannot be fully decomposed. Ammonia, phenols, endotoxins and other substances are produced in large via various ways and absorbed by the liver, thus increases the liver detoxif ication burden and f inally lead to the occurrence of liver diseases. On the other hand, when chronic liver disease occurs, the liver can affect the activity of intestinal microflora via interfering the process of bile metabolism and reducing the blood f low and squirming of intestinal. More over, flora imbalance can in turn worsen the liver damage [5]. In conclusion, the imbalance of intestinal microf lora can cause or exacerbate liver diseases, and liver disease can also aggravate intestinal microf lora imbalance, as a vicious circle.
1 The mechanism of intestinal microflora in liver diseases
1.1 Effect of intestinal microflora on endotoxin (lipopolysaccharide).
Overgrown of intestinal Gram-negative bacteria (Such as Escherichia coli, proteus etc.) [6] produces plenty of metabolites (Lipopolysaccharides) and increases intestinal barrier permeability, which enables lipopolysaccharide(LPS) enter mesenteric lymph nodes or other parenteral organs. Once LPS enter the liver and combined with toll-like receptors 4 (TLR4), it can mediate multiple intracellular signal transduction pathways and increase the release of pro-inf lammatory factors (TNFα, IL-6) [7], which lead to liver inf lammation.
Studies have shown that LPS binding to TLR4 can activate Kupffer cells, which further increase reactive oxygen species (ROS) and TNFα. It is an important pathway in the pathogenesis of alcoholic liver disease [8], which can also lead to the occurrence of non-alcoholic steatohepatitis(NASH) [9]. The effect of LPS on NASH has also been found in genetic obesity rats and mice which are more sensitive to LPS-mediated liver injury [10]. Compared with wild-type mice in non-alcoholic fatty liver disease(NAFLD) models induced by high fructose or Methionine-Choline-Def icient (MCD) diets, TLR4-def icient mice showed a reduction of liver damage, inflammation, and lipid accumulation [11]. NASH may also develop to liver f ibrosis, cirrhosis, and eventually hepatocellular carcinoma[12]. LPS - TLR4 pathway also plays an important role in the occurrence of liver f ibrosis. It can activate hepatic stellate cells (HSC) to produce various chemokines and adhesion molecules, which can induce recruitment of liver macrophages -Kupffer cells. These Kupffer cells enhance the production of transforming growth factor-β (TGF-β)and bind to TGF-β receptors on HSC, thereby activating liver f ibrosis [13]. It has also been suggested that the LPS- TLR4 axis is an important pathway involved in viral hepatitis-cirrhosis-liver cancer [14]. Other studies have suggested that bacterial translocation and endotoxin are important factors for the occurrence and development of liver failure [15]. When liver failure occurs, the number of Bifi dobacteria and Lactobacillus in the intestinal tract will be significantly reduced, affecting the immune function and thus damaging the immune barrier of the intestinal tract [16]. Incomplete intestinal barrier function will cause endotoxemia, and endotoxin circulation, which further aggravates liver injury. Dapito et al. [7] demonstrated that the activation of TLR4 induced by LPS is essential for liver cancer through chronic damage, but is not essential for the occurrence of hepatocellular carcinoma (HCC). TLR4 is the important pathway for liver cell proliferation and evading apoptosis. YAO et al. [17] also showed that primary liver cancer patients prone to small intestine bacterial.Enterogenous endotoxemia, and endotoxin as well as the active TLR4 caused by intestinal microf lora imbalance can activate TLR4 signal pathways, which further deteriorate liver cancer.
Nowadays, LPS has been a potential therapeutic target.Some therapies, such as anti-LPS immunoglobulin and microbiota related metabolite, has been proposed by many studies. For example, oral IMM-124E, an LPS-rich bovine colostrum, could alleviate the related chronic inf lammation and liver damage in both NASH model mouse [18] and NASH patients [19].

Figure 1: Eff ect of lps-tlr4 pathway on liver disease
1.2 Effect of intestinal microflora on short-chain fatty acids.
Intestinal microflora can ferment dietary fiber and produce short-chain fatty acids (SCFAs), including acetate,propionate and butyrate, which play an important role in lipid metabolism. Although most SCFAs act in the intestinal tract, some SCFAs still bound to monocarboxylate transporters-1 (MCT-1) and Sodium-coupled monocarboxylate transporter-1 (SMCT-1) and are transported to the liver via the portal vein to participate in the Tricarboxylic Acid(TCA) cycle. In addition, SCFAs can also bind with the G protein-coupled receptors 41 (Gpr41) and G protein-coupled receptors 43 (Gpr43) in the liver [20] to affect liver and fat accumulation in adipose tissue. Activated Gpr41 receptor stimulates endocrine cells to secrete tyrosin,which reduces intestinal peristalsis and promotes intestinal absorption of more fatty acids [21]. Activation of GPR43 receptor can not only regulate insulin sensitivity, but also promote the decomposition of adipocytes and enhance the intestinal permeability [22]. Furthermore, SCFAs can also can influence the adenosine 5'-monophosphate-activated protein kinase (AMPK) signal pathway to change the oxidation of hepatic fatty acids, thus affecting the hepatic lipid metabolism [23-24].
The type and quantity of intestinal microflora can affect the SCFAs from fermentation sources, and different SCFAs are produced by different intestinal microflora [25].When intestinal microflora is out of balance, long-term changes of the type and number of SCFAs producing bacteria (Such as Lactobacillus, Bacteroides and Bifidobacterium) will lead to liver fat accumulation degeneration [26].Over time it can develop into non-alcoholic steatohepatitis.Exogenous SCFAs can improve NASH by reducing inflammatory signals, which can improve lipid accumulation in animal NAFLD models by regulating intestinal microbiota and intestinal barrier function, liver inflammation [27]and inhibition of inflammatory pathways [27].
1.3 Effect of intestinal microflora on fatty acid metabolism
A large number of studies have confirmed that intestinal microflora can directly or indirectly affect the lipid metabolism, which further affects the physiological function of the liver, and even lead to the occurrence and development of certain liver diseases. Bacteria associated with lipid metabolism include Bacteroidetes, Enterobacteria, Bifidobacterium, and Lactobacilli. Bacteroidetes can promote the expression of fatty acid related synthesis enzymes, but inhibit the fatty acid oxidase, leading to liver lipid deposition[28-29]. The increase of Enterobacterium was positively correlated with TG and TC levels in the blood [30]. Bifidobacterium, lactobacillus and Akkermansia are related to liver fatty acid metabolism. Akkermansia is a Gram-negative bacterium in the subfamily verruca microflora.Recently, a number of evidences have shown that a huge amount of Akkermansia in vivo can decrease expression of fatty acid related synthesis enzymes in the liver, reduce the generation of TG, and maintain the balance of lipid metabolism in the liver [31]. At the same time, Bifidobacterium and lactobacillus also have similar functions of regulating liver fatty acid [32].
The first severe blow to the development of NAFLD is hepatocytes TG deposition. Subsequently, the accumulation of toxic lipid metabolites and excessive production of active oxidative substances lead to hepatocyte apoptosis and abnormal activation of inflammatory reactions, and which promotes the progresses of non-alcoholic steatohepatitis [33-34]. At present, intestinal microflora has been the target for the treatment of NAFLD which improve the disease by supplementing probiotics. For example, the accumulation of TG in the liver of mice with non-alcoholic steatohepatitis caused by eye feeding of high-fat words can be significantly reduced by exogenous oral Bifidobacteria[35].
1.4 Effects of intestinal microflora on bile acid metabolism
The liver is the main site for the synthesis of bile acid.Cholesterol forms primary bile acids in liver cells through a series of enzymatic reactions. It is discharged into the intestinal tract through the bile duct and further metabolized into secondary bile acid under the action of intestinal microflora. Cholesterol 7 alpha-hydroxylase (CYP7A1) is the rate-limiting enzyme of bile acid biosynthesis pathway,and is a crucial enzyme of liver metabolizing cholesterol to primary bile acid [36]. The expression of CYP7A1 is regulated by farnesoid X receptor (FXR), which is highly expressed in the ileum and liver [37].
Changes on intestinal microflora can regulate the size of the bile acid pool and the proportion of each bile acid elements [38]. When intestinal microflora is out of balance,some bacteria (Bacteroides and Proteus, etc.) will stimulate the up-regulation of FXR or the G protein-coupled bile acid receptor (TGR5) expression and inhibit the CYP7A1,thereby inhibiting the synthesis of bile acid. The intestinal firmicutes and actinomyces can stimulate the synthesis of bile acids. Bile acid, as a signal molecule, plays a role not only in adjusting its biosynthesis, also by activating the nuclear receptor such as FXR and TGR5 to regulate cholesterol, triglycerides, glucose metabolism, and the key metabolic pathways involved in energy consumption[39]. FXR plays a critical role in the de novo synthesis of adipose and the process of TG transport and output. The application of TGR5 ligands could lower the levels of TG in the liver, thereby reducing the degree of liver steatosis.Dekaney et al. [40] found that compared with wild-type mice, sterile mice with FXR deletion inhibited the expression of bile acid transporter gene, increased the production of TG and cholesterol, and eventually led to fatty hepatitis.Bechmann et al. [41] followed up 113 NAFLD patients and found that the expression levels of CYP7A1 and bile acid transporters both increased with the increase of fatty acid level. They preliminarily suggested that the synthesis rate and the concentration of bile acid in the blood are correlated with the severity of NAFLD. Jao et al. [42]found that changes in intestinal microf lora led to increased concentration of bile acid. It activated inflammation and oxidative stress in the liver, and then led to steatosis, even liver cells apoptosis and necrosis, and eventually to liver f ibrosis and cirrhosis.Drugs targeting NAFLD bile acid disorders, including FXR agonists, PPARα agonists, Ursodeoxycholic Acid(UDCA) and their derivatives, have entered different stages of clinical trials, and they have shown promising therapeutic effects. Sherriff et al. [43] found that the use of double FXR/ TGR5 agonists in the treatment of obese mice signif icantly improved the histological manifestations of non-alcoholic liver disease in mice.
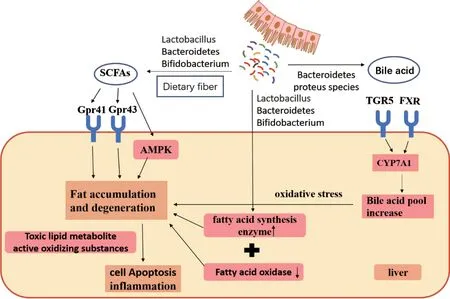
Figure 2: Correlation of SCFAs and bile acid metabolism with liver disease
1.5 Eff ect of intestinal microf lora on choline metabolism
Choline is an important component of cell membrane and a necessary nutrient for human body, which can obtain from food. Choline promotes fat transport in the form of liver cells' phospholipids and prevents liver abnormal fat accumulation. When intestinal microecology is out of balance, the body may be in a state of choline def iciency, and when cells are def icient in choline, liver cell death and liver steatosis usually occur [44]. There are microorganisms in the intestinal tract that can metabolize choline, mainly including firmicutes, proteobacteria and actinomycetes.The number of these bacteria will increase for the intestinal microflora's disorder, and then choline is acted by these bacteria to produce toxic derivatives dimethylamine and Trimethylamine (TMA). These toxic amines can reach the liver through the portal vein and then be oxidized by the monooxygenase in the liver to form Trimethylamine N-oxide (TMAO), which can cause liver inflammation[45].
1.6 Eff ect of intestinal microf lora on endogenous alcohol production.
Elevated Escherichia coli leads to increased endogenous alcohol production in the intestine, which increases intestinal mucosal permeability. Endotoxin, alcohol and other substances directly attack the liver through the portal vein [46]. It will aggravate the oxidative stress reaction and inflammation of the liver. In addition, in the liver,ethanol dehydrogenase metabolizes ethanol into toxic acetaldehyde, which, due to its electrophilic nature, can form adducts with proteins and other molecules in cells, and results in loss of structure and function. Cytochrome P450 2E1 (CYP2E1) in the liver not only metabolizes acetaldehyde to acetic acid, but also increases the production of toxic intermediates and also metabolizes other molecules.It is associated with oxidative stress levels in the liver and is an effective pro-fibrotic signal that ultimately leads to liver f ibrosis [47].

Figure 3: Correlation of choline and endogenous alcohol pathway with liver disease
2 Summary and prospect.
Intestinal microflora is closely related to the occurrence and development of liver disease, and they inf luence each other (Figure 4). At present, plenty of researches have proved that intestinal microflora can be used as a breakthrough for the diagnosis, treatment and prevention of liver diseases. There are many ways to improve the intestinal microflora, such as diet, probiotics, antibiotics, etc.In addition, for different types of patients, it can realize individualized application through the intervention means of targeting the intestinal microflora and its metabolites.High-quality evidence-based and mechanism research can provide reliable evidence for the diagnosis, treatment and prevention of liver diseases.

Figure 4: The pathways of liver damage caused by intestinal f lora
杂志排行
Food and Health的其它文章
- Green tea and its active compounds in cancer prevention andtreatment
- Effects of medicinal diets on patients with non-small cell lung cancer undergoing chemotherapy
- Reporting and methodological quality of systematic reviews or meta-analyses in nasogastric and nasojejunal enteral nutrition for severe acute pancreatitis
- Progress of Bai He Di Huang decoction on intestinal flora of mouse with depression
