Development of Helicobacter pylori treatment: How do we manage antimicrobial resistance?
2019-05-08ShoSuzukiMitsuruEsakiChikaKusanoHisatomoIkeharaTakujiGotoda
Sho Suzuki,Mitsuru Esaki,Chika Kusano,Hisatomo Ikehara,Takuji Gotoda
Abstract
Key words:Helicobacter pylori;Antibiotic resistance;Antimicrobial resistance;Dual therapy;Vonoprazan
INTRODUCTION
Helicobacter pylori(H.pylori) infection,one of the most common bacterial infections,affects approximately 50% of the world’s population[1].H.pyloriinfection is a major cause of gastritis,gastric and duodenal ulcers,mucosal associated lymphoid tissue,and gastric cancer[2].H.pylorieradication treatment has been proven to improve gastric inflammation,promote ulcer healing,and reduce the incidence of gastric cancer[3,4].Furthermore,a “test-and-treat” approach is advocated for detecting and eradicatingH.pyloriin patients with dyspeptic symptoms but low gastric cancer risk[5].
H.pylorieradication treatment is becoming more challenging due to increasing antimicrobial resistance.Previously,a 7-d standard triple therapy consisting of a proton pump inhibitor (PPI),amoxicillin (AMPC),and clarithromycin (CAM) was recommended for eradicatingH.pylori[6].However,there has been a significant reduction in the eradication rate achieved with this regimen due to the increase in antimicrobial resistance ofH.pylori.Resistance ofH.pylorihas reached alarming levels worldwide,which greatly affects the efficacy of treatment.The World Health Organization (WHO) recently published its first ever list of antimicrobial resistant“priority pathogens,” which is a catalogue of 12 families of bacteria posing the greatest threat to human health.They indicated three priority statuses-critical,high,and medium-with CAM-resistantH.pyloribeing categorized as a high priority bacterium in the same tier as vancomycin-resistantEnterococcus faeciumand methicillin-resistantStaphylococcus aureus.Furthermore,resistance to metronidazole(MNZ) and fluoroquinolones,which are mainly used as rescue therapies[7],has also increased more recently to over 15% in many regions of the world[8,9].Thus,the mere avoidance of CAM inH.pylorieradication treatment is not enough to prevent and decrease antimicrobial resistance ofH.pylori.Actually,a recent study of the influence of a government-introduced,restrictive antibiotic policy on the rates of resistance ofH.pyloriin Taiwan indicated an increase in levofloxacin resistance since the restriction of macrolides[10].
RECENT STANDARD H.PYLORI THERAPIES AND THE CONCERN FOR ANTIMICROBIAL RESISTANCE
Treatment regimens are expected to overcome the increasing prevalence of resistant strains of H.pylori and achieve a > 90% eradication rate.The eradication rates for first-lineH.pyloritreatment regimens published in meta-analysis and in a study of eradication rates of vonoprazan-based dual therapy are shown in Table 1.Recently,bismuth-containing quadruple therapy (BQT) or non-bismuth concomitant quadruple therapy (CQT) has been recommended by international guidelines as a first-line treatment forH.pyloriin areas of high CAM and/or MNZ resistance[5,11,12].Both BQT and CQT contain PPI and two to three kinds of antibiotic agents including AMPC,CAM,MNZ,nitroimidazole,and tetracycline with longer treatment durations of 10-14 d.It is reported that acceptable eradication rates of > 90% have been obtained by both regimens.Although BQT and CQT provide acceptableH.pylorieradication rates,they have many limitations,such as a complicated protocol,high cost,adverse side effects,and poor patient compliance due to multiple drug combinations[13].Furthermore,these regimens must not contribute to antimicrobial resistance ofH.pylori;moreover,they may promote future resistance because of the use of multiple antibiotics for a long duration.The alarming global rates ofH.pyloriresistance in treatment-naïve patients can be correlated with the increasing and uncontrolled use of antibiotics that are commonly used inH.pyloriempirical therapy and in therapy for other common infections in the general population[14].Increased antibiotic usage worldwide has led to antimicrobial resistance among many bacteria,includingH.pylori,resulting in falling success rates ofH.pylorieradication treatment.These regimens could also be improved to optimize antibiotic usage to prevent antimicrobial resistance.
WHO launched the Global Action Plan on Antimicrobial Resistance to ensure,for as long as possible,the continuity of the ability to treat and prevent infectious diseases with effective and safe medicines that are quality-assured,used in a responsible way,and accessible to all who need them.Five objectives are listed in this document and the fourth objective is “to optimize the use of antimicrobial medicines in human and animal health.” They state that “extent of reduction in global human consumption of antibiotics,the consumption of antibiotics used in food production,and the use of medical and veterinary antimicrobial agents for applications other than human and animal health” are a potential measure of effectiveness for optimizing the use of antimicrobial medicines in human and animal health.Thus,the increase in resistance to CAM and the existence of multi-resistance to various families of antibiotics must be addressed by the appropriate use of antibiotics inH.pyloritreatment.Antimicrobial susceptibility testing is the best way to optimize and reduce antibiotics forH.pylorieradication treatment as well as treating other common infections.Antimicrobial susceptibility testing is recommended to enable tailoring of the eradication therapy presented in the international guidelines[5],to ensure successful eradication[15,16].However,antimicrobial susceptibility testing is not a routine clinical practice due to the invasiveness of the endoscopy procedure,time consuming nature,the availability of laboratory culture facilities,and cost considerations[17];non-invasive methods are being developed[18].
PROSPECTS OF NEW STRATEGIES FOR ENSURING ERADICATION OF H.PYLORI AND PREVENTION OF ANTIMICROBIAL RESISTANCE
A new strategy that could provide sufficient eradication rates as well as decrease the amount of antibiotics is essential for the prevention of future antimicrobial resistance ofH.pylori.Dual therapy with AMPC and PPI could be a possible solution because this regimen is a single-antibiotic therapy,and it is well known thatH.pyloriis hardly resistant to AMPC.Currently,the resistance rates ofH.pylorito AMPC remain low(0%-5%)[19,20].A dual therapy comprising a PPI and AMPC was first introduced in the 1990s as a first-line regimen forH.pyloriinfection[21].As dual therapy of PPI and AMPC administered at standard doses did not achieve satisfactory treatment outcomes[22,23],it was subsequently used as a salvage treatment.Recently,Yanget al[24]reported that a high-dose dual therapy consisting of AMPC and rabeprazole achieved an eradication rate of 95.3% in first-line therapy,and 89.3% in rescue therapy.However,this method needed a high frequency and a high dose of AMPC and PPI for a longer duration (e.g.,rabeprazole 20 mg and amoxicillin 750 mg 4 times/d for 14 d)to attain an acceptable eradication rate of > 90%,which led to high cost,adverse side effects,and poor patient compliance.
One interesting possibility is to substitute conventional PPIs with vonoprazan for use in dual therapies.Vonoprazan-based dual therapy could be an alternative treatment regimen forH.pylorieradication,which could provide sufficient eradication rates ofH.pyloriand minimize antimicrobial resistance.The key to a successful dual therapy regimen is a PPI-generated neutral environment suitable for bacterial growth;this causes dormantH.pylorito enter a replicative state and makesH.pylorisensitive to AMPC.Vonoprazan is a novel potassium competitive acid blocker that has a strong and long-lasting effect on inhibition of acid secretion[25,26].In addition,the pharmacokinetic features of vonoprazan are not affected by CYP2C19 polymorphisms[27,28].It is reported that seven days of standard triple therapy containing vonoprazan provided approximately 90% eradication rate attributable to effective gastric acid inhibition and the maintenance of a high gastric pH,and had a high safety profile irrespective of age[29-31].To the best of our knowledge,there is only one study on vonoprazan and AMPC dual therapy;this study showed that a regimen consisting of vonoprazan 20 mg twice per day and AMPC 500 mg three times/d for seven days provided sufficient eradication rates of 93.8% ofH.pyloriinfection[32].This seven-day,vonoprazan-based dual therapy may have additional advantages in terms of treatment compliance and medical costs as fewer agents are used and the durationof the therapy is shorter than that of other recent standard treatment regimens (such as BQT,CQT,and sequential therapies).Vonoprazan-based dual therapy may be a recent breakthrough that could ensure a satisfactory eradication rate with the use of minimum antibiotic agents and a short treatment duration.Furthermore,reducing antibiotics may prevent changes and dysbiosis in gut microbiota composition,which are caused by antibiotics used inH.pylorieradication therapy[33].Although vonoprazan-based dual therapy potentially has these advantages,it also has several limitations for implementation in clinical setting.First,vonoprazan is available in a few countries.Vonoprazan was developed and launched in Japan in 2015.However,it is now available in several Asian countries including Philippine,Singapore,and Thailand,and has been approved in other regions,including South America(countries such as Argentina and Peru).Thus,vonoprazan may become available and can be used forH.pylorieradication therapy worldwide in the near future.Second,this regimen cannot be used in patients with penicillin allergy and thus antimicrobial susceptibility testing should be performed in these patients to optimizeH.pylorieradication therapy.Although the conventional antimicrobial susceptibility testing is invasive due to the need of endoscopy and biopsy as mentioned above,a noninvasive molecular test using fecal sample has also been recently developed[34].This method involves the isolation ofH.pyloriDNA from stool and detection of point mutations conferring antimicrobial resistance by polymerase chain reaction.This method should be considered for testing antimicrobial susceptibility in patients with penicillin allergy beforeH.pylorieradication therapy.Finally,there are few data and studies regarding this regimen.Further studies should be conducted to prove its efficacy and safety profile.
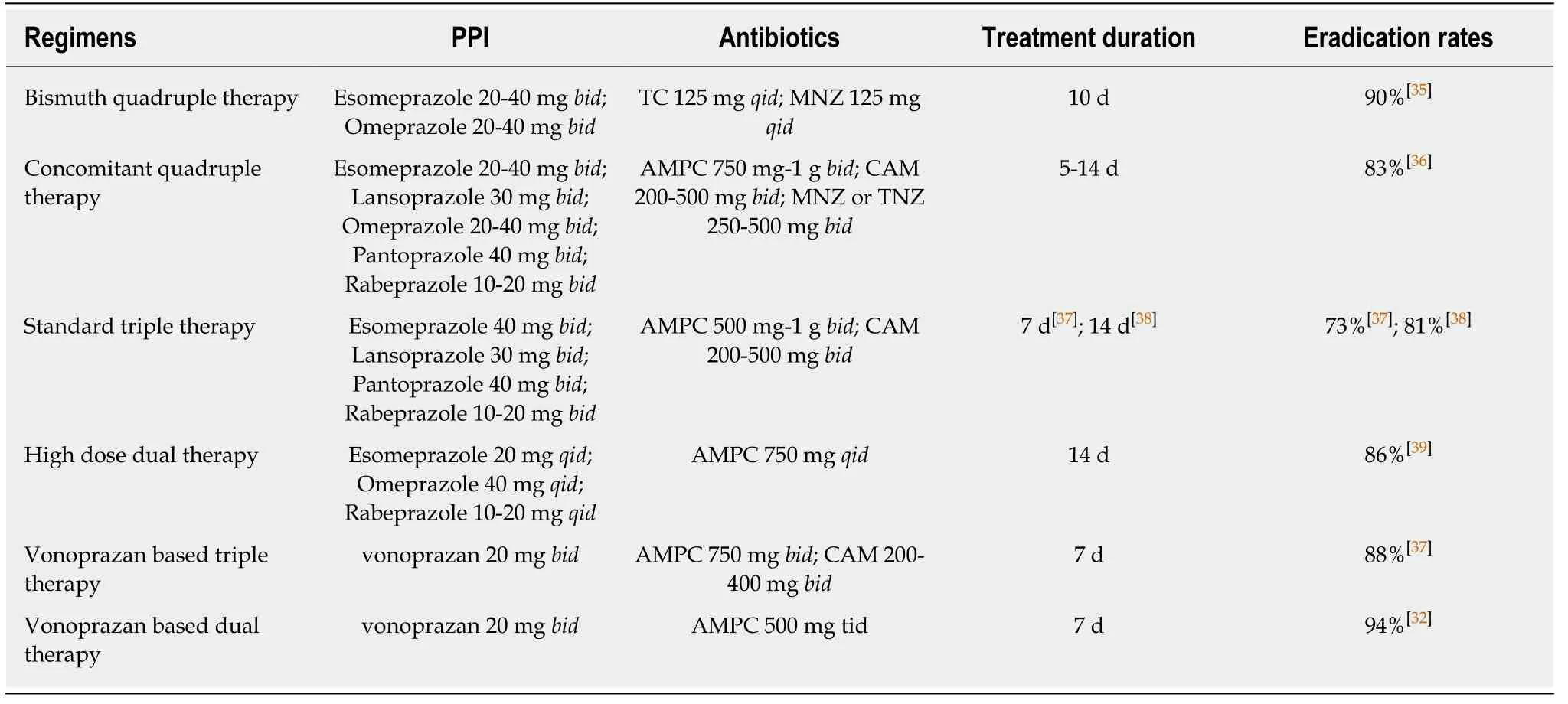
Table1 Treatment regimens for first-line Helicobacter pylori therapies and its successful eradication rates
CONCLUSION
In this review,we outline the urgent,global issue ofH.pyloriantimicrobial resistance and propose our prospects of approach for the issue.H.pyloritreatment is becoming more challenging because of the increasing antimicrobial resistance to not only CAM but also to MNZ and fluoroquinolones.Thus,there is a need to develop newH.pylorieradication therapies that provide an acceptable eradication rate,better safety and tolerability profile,and good patient compliance,while preventing the increase inH.pyloriantimicrobial resistance.One interesting possibility is the use of vonoprazan in dual therapy with AMPC,which has been shown to have over a 90% eradication rate.Large scale,randomized control trials should be conducted to verify and establish this finding in the future.
杂志排行
World Journal of Gastroenterology的其它文章
- Organoids of liver diseases: From bench to bedside
- Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: A pathological review
- Signal transducer and activator of transcription 3 promotes the Warburg effect possibly by inducing pyruvate kinase M2 phosphorylation in liver precancerous lesions
- Immune response pattern varies with the natural history of chronic hepatitis B
- Role and mechanism of circ-PRKCI in hepatocellular carcinoma
- Comparison of decompression tubes with metallic stents for the management of right-sided malignant colonic obstruction
