Solubility and partial molar volume of diacetone-D-glucoseand its derivatives in supercritical carbon dioxide
2019-04-04YANGHaijianBANBinruWANGShengXULingxiaoWANGLihua
YANG Haijian,BAN Binru,WANG Sheng,XU Lingxiao,WANG Lihua
(College of Chemistry and Materials Science,South-Central University for Nationalities,Wuhan 430074,China)
Abstract A series of diacetone-D-glucose(compound 1)derivatives,(diacetone-D-glucose methyl oxalate(compound 2),diacetone-D-glucose ethyl oxalate(compound 3),diacetone-D-glucose methyl malonate(compound 4),were designed and synthesized as carbon dioxide(CO2)-philic compounds for the measurement and correlation of their solubilities in supercritical carbon dioxide(scCO2). The cloud point was measured at different temperatures of 313,323,333 K over the pressure range of 8.0 to 13.6 MPa,then the solubility data were calculated and correlated by five different theoretical semi-empirical models(Chrastil,KJ,SS,MST,JCF). The results showed satisfactory agreements with the experimental data,with the JCF model providing the best fitness,which gave out the lowest average absolute relative deviation(AARD)from 3.13% to 6.22%. Furthermore,the partial molar volumes of these four compounds in the supercritical phase were also calculated according to the Kumar and Johnston theory,with the value between -6479.51 and -630.65 cm3·mol-1.
Keywords supercritical carbon dioxide; diacetone-D-glucose derivatives; solubility; correlation; partial molar volume
Over the past decades,supercritical fluids(SCFs)have been widely utilized in various applications,such as dyeing,food,catalytic and enzymatic reactions,and so forth[1-4]. Among all kinds of SCFs,scCO2is the most investigated and employed SCF because of its non-toxic,non-flammable chemical inertness,abundant and variable density,higher diffusion coefficient,lower viscosity and reasonable accessible critical constants[5-8]. The properties of scCO2are between liquid and gas and have much higher diffusivity and lower viscosity than that of liquid and much stronger solvent power than that of gas[9].Due to its inherent physical properties,carbon dioxide is a non-polar molecule of low polarizability and low dielectric constant,which limits the solubility of many polar compounds. To overcome the drawbacks of lower solubility,one solution suggested is to introduce some CO2-philic compounds into the scCO2system. Up to now,most efficient CO2-philic compounds are fluorides and silicones. Unfortunately,fluorides are very expensive and toxic; silicone- functioned amphiphiles require relatively high pressure to generate a single-phase solution in scCO2. So the design and synthesis of new nonfluorous CO2-philic compounds have become a challeng[10,11].
According to the literatures and based on our research results,substituted hydrocarbons with ether and alkyl group carbonyl groups,especially carbonyl group with suitable length,are easily available,comparably economical,and well dispersive in scCO2,and thus used as desirable alternatives to fluorinated compounds. In present work,we have designed and synthesized three new CO2-philic compounds which contained the carbonyl group and alky group as CO2-philic moieties via the simple modification of diacetone-D-glucose(compound1). Then the solubilities of diacetone-D-glucose and its three derivatives,i.e.,diacetone-D-glucose methyl oxalate(compound2),diacetone-D-glucose ethyl oxalate(compound3),diacetone-D-glucose methyl malonate(compound4) were investigated in scCO2at different temperatures(313,323,333 K)and pressures(8.0-13.6 MPa).
The mathematical modeling of the solubility data is essential to get a better understanding of the dissolution phenomenon in scCO2,as well as to predict the solubility at variously interested pressures and temperatures,which continuously update the development of scCO2technology[12,13]. Therefore,it is necessary to propose a semi-empirical model based on theoretical deduction to predict the solubility of compounds in scCO2accurately. Hence,in this work,the experimental solubility data were correlated by five different theoretical semi-empirical models(Chrastil,KJ,SS,MST,and JCF models). Furthermore,the partial molar volumes of diacetone-D-glucose and three diacetone-D-glucose derivatives in scCO2were also calculated according to the Kumar and Johnston theory.
1 Experimental
1.1 Chemicals and experimental apparatus
Methylchloroglyoxylate,ethyl chlorocarbony formate and methyl malonyl chloride(Alfa Aesar Chem. Co.); Diacetone-D-glucose(98%,Shanghai Darui Finechemical Co.);Triethylamine(99.5%,J&K Chemica Co.);Tetrahydrofuran [THF,Sinopharm Chemical Reagent Co. Ltd(China)]; Carbon dioxide(99.99%,mass fraction,Wuhan Steel Co.,used as a fluid).
A CO2delivery pump(JASCO PU-CO2)was used to cool and deliver CO2fluid and a back-pressure regulator(JASCO BP-1580-81)was used to keep the pressure in the range of 0 to 30.0 MPa. The temperature was controlled using a temperature controller jacket with an accuracy of0.01 K. NMR experiments were performed on a Bruker Al-400 MHz instrument using TMS as an internal standard. IR spectra were recorded on a Perkin-Elmer 2000 FT-IR spectrometer. Elemental analysis was conducted on a PE 2400 series II CHNS/O elemental analyzer.
1.2 Procedure for solubility test in scCO2
The experimental procedure described here is the common one and can be found elsewhere in literature[14-17]. A known amount of compounds being measured was introduced to a high-pressure view cell,which has a volume of 7.11 mL and withstands maximum pressure of 20 MPa,that was consisted of a stainless-steel block with two sapphire windows. The compound in the cell was stirred by a magneton,and the temperature was controlled to obtain near-equilibrium conditions using a constant-temperature water bath. At this point,the vessel was purged three times with low pressure CO2to remove traces of air. The CO2was delivered into the cell using the pump until the vessel was full of CO2at the desired initial pressure and temperature. Stirring was stopped for observation. The pressure was gradually increased(0.2 mL·min-1)until the compound/scCO2mixture began to form a single phase. Then the pressure was further increased until a completely transparent phase was present. The solution was stirred and let to equilibrate at these conditions for 2-3 h. Once this state was achieved the sample pressure was gradually(0.5 MPa every 10 min)lowered until the clear transparent one-phase solution turned hazy due to the Tyndall effect of the precipitated particles. This opalescence indicated the equilibrium condition,and the pressure was recorded as the cloud-point pressure. Then the pressure was increased gradually until the cloudy mixture became transparent again. At each condition,the experiment was repeated at least three times. The uncertainty of the cloud-point pressure was ±0.1 MPa. The cloud-point pressure and temperature were recorded to obtain the density of CO2from the website[15].
1.3 Synthesis of diacetone-D-glucose derivatives(compounds 2-4)
The diacetone-D-glucose derivatives were synthesized according to the method shown in
Fig 1. Their structures have been determined by1H NMR,13C NMR,FT-IR,and elemental analysis.

Fig.1 Synthetic route of compounds 2-4图1 化合物2~4的合成路线
Synthesis ofdiacetone-D-glucose methyl oxalate(compound2): Diacetone-D-glucose(1.3 g,5 mmol)was disolved in 25 mL fresh THF(40)in a 100 mL Schlenk flask under the atmosphere of nitrogen,and then oxalyl dichloride(3 mL,0.035 mol)in fresh THF(40 mL)was dropwise added into the solvent at 0 ℃ within 10 min. Then triethylamine(NEt3)(0.9 mL,10 mmol)was dropwise added to reaction system,the resulting mixture was stirred for 24 h at 70 ℃ and was then cooled to room temperature. After filtration and evaporation,the viscous liquid was dissolved with dichloromethane. Then the reaction mixture was sequentially washed with aqueous HCl(w=10%,mass fraction),saturated aqueous NaHCO3solution and distilled water,the organic phase then was dried over anhydrous sodium carbonate for 10 h. After evaporation under vacuum,the residue was purified by column chromatography on silica with petroleum ether: ethyl acetate(1∶3)as elute to afford compound 2(75% yield,GC purity > 99%)as pale yellow oil.1H NMR(400 MHz; CDCl3):δ1.32(t,J=14.1 Hz,8H),1.36-1.46(m,4H),3.36-3.52(m,3H),3.79(s,1H),4.07(m,J=13.1,8.7,3.7 Hz,2H),4.21-4.24(m,1H),4.59(s,1H),5.32(s,1H),5.89(s,1H).13C NMR(400 MHz; CDCl3):δ13.91(1C),26.24(1C),26.66(1C),41.10(1C),64.39(1C),68.47(1C),76.67(1C),76.99(1C),77.31(1C). 77.78(1C),79.27(1C),83.02(1C),99.99(1C),105.07(1C),112.46(1C). FT-IR [KBr,νmax(film)/cm-1]: 1755.4CO),1167.2(C—O—C). Elemental Anal. calcd for C15H22O9:C 52.02,H 6.40,O 41.58; found C 52.01,H 6.38,O 41.61%.
Compounds3and4were obtained as pale yellow oil following the same procedure.
Synthesis ofdiacetone-D-glucose ethyl oxalate(compound 3): Yield(78 %,GC purity > 99%).1H NMR(400 MHz; CDCl3):δ1.30-1.46(m,12H),1.53(d,J=16.0 Hz,3H),4.00-4.09(s,1H),4.13(s,1H),4.29(t,J=3.2 Hz,2H),4.37(m,J=6.9,5.3 Hz,2H),4.59(t,J=6.4 Hz,1H),5.42(s,1H),5.99(s,1H).13C NMR(400 MHz; CDCl3):δ13.85(1C),26.18(1C),26.77(1C),29.67(1C),30.89(1C),63.68(1C),68.05(1C),68.47(1C). 75.46(1C),76.70(1C),77.22(1C),77.33(1C),79.28(1C),85.15(1C). 104.93(1C),111.95(1C). FT-IR [KBr,νmax(film)/cm-1]: 1760.8(CO),1171.4(C—O—C). Elemental Anal. calcd for C16H24O9:C 53.33,H 6.71,O 39.96; found C 53.32,H 6.74,O 39.98.
Synthesis ofdiacetone-D-glucose methyl malonate(compound4): Yield(70%,GC purity > 99%).1H NMR(400 MHz; CDCl3):δ1.30-1.50(m,12H),3.45(d,J=4.1 Hz,3H),3.78(q,J=13.4,11.0 Hz,2H),4.05-4.14(s,1H),4.07(d,J=19.8 Hz,2H),4.58(q,J=10.1,3.7 Hz,2H),5.32(s,1H),5.89(d,J=3.6 Hz,1H).13C NMR(400 MHz; CDCl3):δ25.87(1C),26.18(1C),40.45(1C),41.00(1C),48.65(1C),52.47(1C),63.86(1C),76.21(1C),76.24(1C),76.72(1C),77.03(1C),77.35(1C),79.28(1C),82.81(1C). 105.12(1C),112.71(1C). FT-IR [KBr,νmax(film)/cm-1]: 1750.6(CO),1161.4(C—O—C). Elemental Anal. calcd for:C16H24O9C 53.33,H 6.71,O 39.96,found: C 53.35,H 6.69,O 39.9.
2 Results and Discussion
2.1 Solubility results of compounds 1-4 in scCO2
The reliability of the experimental apparatus and procedures[16]has been confirmed in our previous work[20]. After confirming that our procedures were able to measure solubilities accurately,the solubilities of compounds1-4were proceeded with measuring in scCO2. The solubilities of these compounds in scCO2were determined at temperatures of 313,323,333 K and pressure of 8.0 to 13.6 MPa. The experimental results average value were shown inTab.1.
Fig.2 showed the comparison of solubility′s experimental and the calculated values for compounds1-4in scCO2at 313,323 and 333 K,which indicated that at the same temperature,the solubilities of these compounds increased with increasing pressure. Nevertheless,at the same pressure,the solubilities of these compounds declined with increasing temperature. It was mainly because the density of CO2decreased with the increasing temperature,and the solvent power of scCO2was stronger at lower temperatures. The results also showed that these compounds were all soluble at readily accessible temperatures and pressures. At the same temperature,the solubility sequence was observed as compound2>3>4>1(Fig.3). The modified compounds2-4showed better solubility than compound1in scCO2. It could be attributed to the CO2-philic carbonyl and ether groups,which are helpful to enhance the interaction of solute-solvent. The solubility of compound2is greater than compound3,which might be attributed to the molar mass difference,i.e.,for similar molecule structures,lower molar mass exhibits better solubility in scCO2[6]. For the structurally similar compounds3and4,compound3is more soluble than compound4in scCO2. This may be because the inserted methylene between two carbonyls moderated the CO2-philic ability of compound4.
Tab.1 Solubility at temperature(T),density(ρ),molar fraction(x)for compounds 1-4a 表1 化合物1~4在不同温度、密度、摩尔分数下的溶解度
aStandard uncertaintiesuareu(T)= 0.1 K,u(P)= 0.1 MPa,ur(ρ)= 0.02,andur(x)=0.03

Fig.2 Comparison of solubility experimental and calculated values for compounds 1-4 in supercritical CO2 at different temperatures图2 化合物1~4在不同温度下在超临界CO2中的溶解度实验值和计算值的比较
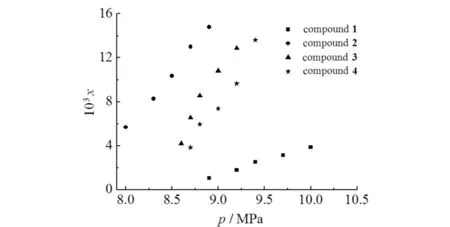
Fig.3 Solubility of compounds1-4 at 313 K 图3 化合物1~4在313 K时的溶解度
2.2 The chrastil model
As one of the most frequently-used and traditional density-based models,theChrastil[12,14]model relates the solute solubility(S,g·L-1)in scCO2,the density of scCO2(ρ,g·L-1),and temperature(T,K)as equation(1):
(1)
whereA1-A3are the adjustable parameters which could be estimated from experimental solubility data in scCO2.
In this article,to justify the selection of the correlation models on a more easily comparable basis,S(g·L-1solute/scCO2)in the Chrastil model is transformed tox(mole fraction solubility of solute),and the parameters of the model are redefined.Scould be calculated by equation(2):
(2)
wherexis the molar fraction of the solute,M1andM2are the molecular weights of CO2and the solute(g·mol-1),respectively.
Combining equations(1)and(2),equation(3)was obtained as following:
(3)
The term 1-xapproximately equals 1 because the mole fraction solubility of the solutexis much smaller than 1. In addition,the termA0-ln(M2/M1)can be redefined as a new association parameterA1. Therefore,equation(3)becomes:
lnx=A1lnρ+A2/T+A3.
(4)
The average absolute relative deviation(AARD)between experimental data and calculated value obtained from the Chrastil model could be calculated using the following formula equation(5):
(5)
wherenis the number of experimental data points,andxi,calandxi,expare the calculated and experimental value of the mole fraction solubility of the solute,respectively.
The results of the solubility data correlation using the Chrastil model were shown in
Tab.2.
Fig.4 exhibited the plots of lnxversus lnρas the result of correlation. The values of AARD were in the range 5.52%-7.18%.

Tab.2 Correlated results of five different theoretical semi-empirical models for the solubility data 表2 5种不同半经验模型理论溶解度相关数据
aCorrelations:Chrastil(lnS=A1lnρ+A2/T+A3),KJ(lnx=B1ρ+B2/T+B3),SS[lnx=(C1+C2/T)lnρ+C3/T+C4],
MST[Tln(xP)=D1ρ+D2T+D3],JCF(lnx=E1P+E2P2+E3PT+E4T/P+E5lnρ+E6)

Fig.4 Plots of lnx versus lnρ for compounds 1-4 using the Chrastil,SS and JCF models at 313,323,333 K图4 313,323,333 K下用Chrastil,SS,JCF模型拟合化合物1~4的lnx与lnρ 值关联曲线
2.3 The KJ model
According to Kumar and Johnston′s theory[11],there′s a linear correlation between lnxand lnρ,and in some cases between lnxandρ,which are system-dependent,and neither can be validly generalized. So similar to equation(1),the linear expression between lnxandρcould be given as equation(6):
(6)
whereB1-B3are adjustable parameters,in which the parameterB1is the same as the parameterA1of the Chrastil model,defined asΔHtotal/R.
The results of the solubility data correlation using the KJ model were shown in
Tab.2.
Fig.5 exhibited the plots oflnxversusρas the result of correlation. The values of AARD were in the range 5.52%-9.12%.
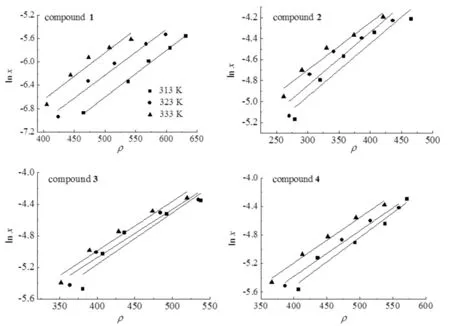
Fig.5 Plots oflnx versus ρ for compounds 1-4 using the KJ model at 313,323,333 K图5 313,323,333 K下用KJ模型拟合化合物1~4的lnx和 ρ 值的关联曲线
2.4 The SS model
The temperature effects on the solubility were discussed by Sung and Shim[4],the SS model pointed out that in the log-log plot the solubility isotherms were linear,whereas their slopes were decreasing with increasing temperature. Therefore,the KJ model was modified by taking the temperature effects into account as the following equation(7):
(7)
whereC1-C4are adjustable parameters.
The results of the solubility data correlation using the SS model were also shown in
Tab.2.
Fig.3 exhibited the plots oflnxversus lnρas the result of correlation. The values of AARD were in the range 4.49%-7.21%.
2.5 The MST model
Méndez-Santiago and Teja[2]proposed a density-based model,which comes from the linear relationship betweenTlnDandρ,derived from the theory of dilute solutions:
(8)

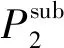
Tln(xP)=D1ρ+D2T+D3,
(9)
whereD1-D3are adjustable parameters.
The results of the solubility data correlation using the MST model were shown in
Tab.2.
Fig.5 exhibited the plots ofTln(xP)versusρas the result of correlation. The values of AARD were in the range 5.26%-9.17%.

Fig.6 Plots of T ln(xP)versus ρ for compounds 1-4 using the MST model at 313,323,333 K图6 313,323,333 K下用MST模型拟合化合物1~4的T ln(xP)和 ρ 值的关联曲线
2.6 The JCF model
Considering the nonlinear relationship betweenlnxand the pressure in isothermal conditions,the nonlinear relationship between lnxand the temperature in isobaric conditions,and the linear relationship between lnxand lnρin a certain range of pressure and temperature,Jouyban et al[14]proposed another density-based model,which can be written as equation(10):
(10)
whereE1-E6are adjustable parameters.
The results of the solubility data correlation using the JCF model were shown in
Tab.2.
Fig.3 exhibited the plots oflnxversus lnρas the result of correlation. The values of AARD were in the range 3.13%-6.22%. The results showed that the JCF model was in good agreement with experimental solubility data although it is rarely used[18-20].
2.7 Comparison of five semi-empirical models
As shown in
Tab.2,all the AARD values of these models are below 10%,which indicated that the application of these models to correlate the solubility were successful. Among these models,the JCF model gave the best fit to the solubility data with the AARD value of 4.23(compound1),3.13(compound2),6.22(compound3),3.46(compound4). The JCF model has six parameters,and that is why it provides the best results and has not been more often used now.
In order to better compare different models,the mean AARD values of each model and their corresponding standard deviation(SD)have been calculated in this work,and the results can be observed in
Fig.7.

Fig.7 Mean AARD(solid box)of each model with their corresponding SD图7 每种模型的平均AARD(实体箱)及对应的SD
As a result,the number of added parameters in the JCF model has a significant effect on the predictive capability of the model,which is consistent with the result in
Fig.7. The KJ model(lnxvsρ)is similar to the Chrastil model with three adjustable parameters. Compared with the Chrastil model,the fitness is worse. Nevertheless,it can be improved by the SS model,which reduces mean AARD from 7.13% to 5.66% and SD from 1.33% to 0.98%(Fig.7). The SS model(lnxvs lnρ+ lnρ/T)has four adjustable parameters,which also improve the accuracy of the expression of the density′s function in the KJ model. The MST model [Tln(xP)vsρ] is shown to give the relatively poor correlated results with mean AARD of 6.77% and SD of 1.49%(Fig.7),perhaps because of the oversimplified treatments used to derive this model.
In general,the results indicated that the modified models gave out better fitness than the original models. Moreover,the models that have similar semi-logarithmic solubility-density relationships(the KJ and MST models)showed worse correlated results,while the models that have the log-log solubility-density relationships(the JCF and SS models)had better correlated results,except the Chrastil model.
2.8 Estimation of the partial molar volumes of the solutes
The partial molar volumes of the solutes are crucial parameters for the solubility evaluation of solutes inSCFs. Since the corresponding data of partial molar volumes of our compounds1-4have not been found from the previous reports,it is useful and meaningful for other scientists to refer to our data in the future. The partial molar volumes of the four compounds can be calculated with the theory reported by Kumar and Johnston[11]:
(11)
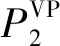
(12)

Tab.3 indicated that the partial molar volume for each solute decreased as temperature increased. Moreover,the partial molar volumes of solute in scCO2were negative values,possibly ascribed to the reason that a mole solute molecule was aggregated by a multitude of solvent molecules,which can be expressed by the following equation(13):
Solute +nsolventSolute(solvent)n,
(13)
wherenranges from 10 to 50.

Tab.3 Results of the calculation of partial molar using Kumar-Johnston theory for compounds 1-4a
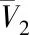

Fig.8 Plots of lnx versus lnρr for compounds 1-4 at 313,323,333 K图8 313,323,333 K下化合物1~4的lnx和lnρr 值的关联曲线
3 Conclusion
This study presented novel solubility data fordiacetone-D-glucose and three synthesized diacetone-D-glucose derivatives at the temperature(313,323,333 K)over the pressure range(8.0 to 13.6 MPa). The solubilities of the four compounds increased with the rising of pressure in isothermal conditions,whereas decreased with the increase of temperature in isobaric conditions. The experimental data of the four compounds were correlated using five different theoretical semi-empirical models(Chrastil,KJ,SS,MST,and JCF). Good agreements between the calculated and the experimental values were obtained for all the models. In general,the SS and JCF models as the modification of models correlated the solubility data were better than the other three original models. The results indicated that in any case the JCF model is shown to be the best model because it has more adjustable parameters of temperature and pressure. In addition,the partial molar volumes of the four compounds in scCO2were calculated by employing solubility data which use the theory developed by Kumar and Johnston.
